-
PDF
- Split View
-
Views
-
Cite
Cite
Davide Mauri, Nicholas Pavlidis, Nikolaos P. Polyzos, John P. A. Ioannidis, Survival With Aromatase Inhibitors and Inactivators Versus Standard Hormonal Therapy in Advanced Breast Cancer: Meta-analysis, JNCI: Journal of the National Cancer Institute, Volume 98, Issue 18, 20 September 2006, Pages 1285–1291, https://doi.org/10.1093/jnci/djj357
Close - Share Icon Share
Abstract
Background: Aromatase inhibitors and inactivators have been extensively tested in patients with advanced breast cancer, but it is unclear whether they offer any survival benefits compared with standard hormonal treatment with tamoxifen or progestagens. We performed a meta-analysis of randomized controlled trials that compared several generations of aromatase inhibitors and inactivators with standard hormonal treatment in patients with advanced breast cancer. Methods: The endpoint that we assessed was survival. Trials were located through searches of PubMed and Cochrane Library (last update March 2006). Relative hazards (RHs) were summarized across trials through fixed- and random-effects analyses, and heterogeneity was assessed with the Q and I2 statistics. All statistical tests were two-sided. Results: Twenty-five different comparisons, with a total of 8504 patients, were included in the meta-analysis. We found statistically significant survival benefits with third-generation aromatase inhibitors and inactivators (vorozole, letrozole, examestane, and anastrazole) (RH = 0.87, 95% confidence interval [CI] = 0.82 to 0.93; P <.001) but not with first-generation (aminoglutethimide) or second-generation (formestane and fadrozole) agents. The difference in the summary effects between these two groups of trials was statistically significant ( P = .04). The survival benefit with third-generation agents in first-line trials, in which these agents were compared with tamoxifen (11% RH reduction, 95% CI = 1% to 19%; P = .03), was identical to their benefit in second- and subsequent-line trials in which these agents were compared with other treatments (14% RH reduction, 95% CI = 6% to 21%; P <.001). Conclusions: Inhibition of the aromatase system, in particular with third-generation aromatase inhibitors and inactivators, appears to be associated with statistically significant improved survival of patients with advanced breast cancer compared with standard hormonal treatments.
Breast cancer is the most common cancer in women ( 1 ) . Although most major advances have occurred in the treatment of early-stage disease, effective systemic therapies (chemotherapy or hormone therapy) would have even more impact if they could also prolong the life of patients with advanced breast cancer ( 2 ). Several regimens have been tested to identify treatments that may improve survival of patients with advanced breast cancer. Tamoxifen and several progestagen compounds with antiestrogenic action (e.g., medroxyprogesterone acetate and megestrol acetate) have been extensively used for first- and second-line treatments, respectively, of such patients. Aromatase inhibitors and inactivators, which have documented benefits in early-stage disease ( 3 ) , are also being used to treat patients with advanced breast cancer, but their effectiveness in these patients has been more controversial. Thirty randomized controlled trials have compared aromatase inhibitors with standard hormonal therapy in patients with advanced breast cancer ( 4 – 30 ) , but it is unclear whether these agents offer a survival benefit. A recent meta-analysis suggested that, as second-line therapy, these agents have similar outcomes to progestagen agents in terms of disease progression or overall response rates ( 31 ). However, giving priority to the use of these agents would be contentious unless a clear survival benefit can be documented. Moreover, different aromatase inhibitors and inactivators may have different survival effects. Various agents of this class have been developed over the last three decades that belong to different generations of drug development with different pharmacologic profiles. These differences reflect various chemical structures and pharmacodynamics and may also be clinically relevant.
To address whether aromatase inhibitors and inactivators of different generations offer survival benefits, we performed a meta-analysis of randomized trials among patients with advanced breast cancer, in which aromatase inhibitors or inactivators were compared with the standard hormonal treatments in a first-line or second-line (or subsequent-line) setting. We identified and systematically organized the cumulative evidence from randomized studies of the impact of systemic therapies on survival of patients with advanced breast cancer. We also evaluated whether specific aromatase inhibitors had superior efficacy to standard hormonal therapy and whether the benefits, if present, extended over the first-line treatment and subsequent lines of treatment, regardless of the hormonal therapy used as comparator.
M ATERIALS AND M ETHODS
Identification of Randomized Studies
We searched the Cochrane Central Trials Registry and PubMed without year and language restriction. The last search was updated in March 2006. We used the searching algorithm (breast OR mammary) AND (cancer OR carcinoma OR neopl*) AND (aromatase OR aromatase inhibitors OR AIs OR aminoglutethimide OR formestane OR fadrozole OR anastrazole OR letrozole OR exemestane OR vorozole) AND (clinical trial OR randomized controlled trial OR double-blind OR single-blind OR single-blind OR random OR randomized OR placebo).
Eligibility Criteria
We considered all randomized controlled trials to be eligible that compared an aromatase inhibitor or inactivator with tamoxifen or progestagens (such as medroxyprogesterone acetate, megestrol acetate, or fluoxymesterone) in patients with advanced breast cancer (i.e., metastatic and inoperable locally advanced or recurrent breast adenocarcinoma) in any line of treatment (first [front] line or second or subsequent line [in patients who had received such hormonal therapy in the past]). Trials were eligible regardless of the doses and schedules used for the regimens compared.
We excluded trials in which the randomization was limited to earlier stages of the disease (patients with less than stage IV disease) and trials that compared regimens in breast malignancies of histologic type other than adenocarcinoma (e.g., inflammatory breast cancer or sarcoma). We also excluded meeting abstracts (because they had not undergone full peer review and should be considered as preliminary reports open to modification), single-arm studies, dose-escalation studies, and nonrandomized and pseudorandomized trials (e.g., those with alternate allocation of subjects).
Trials that used other concomitant anticancer treatments (e.g., surgery, radiotherapy, or radioisotopic treatment) were eligible if these treatments did not differ systematically between the investigated arms. Trials in which the compared arms differed systematically in the use of these additional disease-related treatments were, however, excluded because the differences in survival could not necessarily be attributed to the comparison of aromatase inhibitor treatment with standard hormonal treatment.
Whenever multiple reports pertained to overlapping groups of patients, we retained only the report with longest follow-up (largest number of events) for the meta-analysis calculations to avoid duplication of information. Data from interim analyses were eligible if no further final data were available.
Data Extraction and Outcomes
From each eligible trial, we recorded the following items for both arms: authors' names; journal and year of publication; country of origin; years of patient enrollment; number of centers involved; number of patients randomly assigned and analyzed per arm, age, tumor stage, and menopausal status; hormonal receptor status; the exact regimens used and their dose and schedule; the line of treatment; and any additional treatments given to both arms. We recorded study design items, including whether there was a description of the mode of randomization, allocation concealment, the number of withdrawals per arm, and blinding ( 32 ) and whether any planned or unplanned interim analyses had been performed ( 33 ) . We also recorded the median survival by arm and whether any statistically significant difference had been detected between the compared arms at a P value of .05.
Statistical Analysis
We determined and combined relative hazards (RHs) of mortality for the comparison of aromatase inhibitors or inactivators against standard hormonal treatment across the eligible studies. The natural logarithms of the relative hazards were combined by use of general variance models that weighed each study by the inverse of its variance ( 34 ) . We assessed the statistical significance of between-study heterogeneity with the chi-square–based Q statistic (considered statistically significant for P <.10) and used the I2 statistic to examine the extent of between-study heterogeneity (considered large for I2 values of 50%–74% and very large for I2 values of 75% and higher) ( 35 ) . Data were combined with both fixed- and random-effects models. In the absence of between-study heterogeneity, the two models give identical results. With between-study heterogeneity, the random effects tend to give wider confidence intervals (CIs) because they also incorporate a between-study variance in the within-study variance of each study.
We used estimates of relative hazard derived from Cox proportional hazards models, whenever these values were reported in analyses of the individual-level data done by the primary investigators. The standard deviation of logarithms of the relative hazard was estimated as the difference of the upper minus the lower 95% confidence interval divided by 3.92. The variance was then estimated as the square of the standard error. Whenever relative hazard estimates from Cox models were not provided, we estimated the logarithms of the relative hazard and its variance from presented information with the P value from the log-rank test and events by patients, by arm, and/or by median survival by arm. When the number of events per arm ( E1 and E2 ) was provided, we calculated the variance of the logarithm of the relative hazard by the sum of 1/ E1 and 1/ E2 ( 36 ) ; we then calculated the logarithm of the relative hazard, so that its P value would be the same as the P value from the log-rank test. If the P value from a log-rank test was not available, we calculated the relative hazard as the inverse of the ratio of the two median survival times by assuming exponential survival curves and proportional hazards.
We considered trials in which two arms with different doses of aromatase inhibitor were compared with a third arm of an antiestrogen agent as including two comparisons, unless the investigators only presented data merging the two aromatase inhibitor arms. The same strategy was applied when investigators presented results from two similar trials in the same report.
We analyzed data separately according to the generation of the agent (first-generation [aminoglutethimide] and second-generation [formestane and fadrozole] inhibitors and inactivators versus third-generation inhibitors and inactivators [vorozole, letrozole, examestane, and anastrazole]) because typically third-generation agents are currently used. We then performed subgroup analyses according to type of comparison (tamoxifen versus other) and line of treatment (first versus second or subsequent line). These two subgroup analyses are identical because tamoxifen was always the agent used for comparison in first-line treatment trials and progestagens were always the agents used for comparisons in subsequent-line trials.
We evaluated whether summary effect sizes changed over time in cumulative meta-analysis and recursive cumulative meta-analysis ( 37 ) and whether there was any evidence that the results of studies with more precision differed from those of studies with less precision ( 38 ) . Finally, we examined the quality characteristics of the combined trials and investigated whether any studies with statistically significant results had been stopped early as part of planned or unplanned interim analyses ( 33 ) and whether there was any evidence for time-dependent survival differences in any trials. In sensitivity analyses, we excluded such trials from the calculations. Analyses were performed with SPSS version 13.0 (SPSS Inc, Chicago, IL) and with Comprehensive Meta-Analysis version 2 (Biostat, Englewood, NJ). All statistical tests were two-sided.
R ESULTS
Eligible Trials
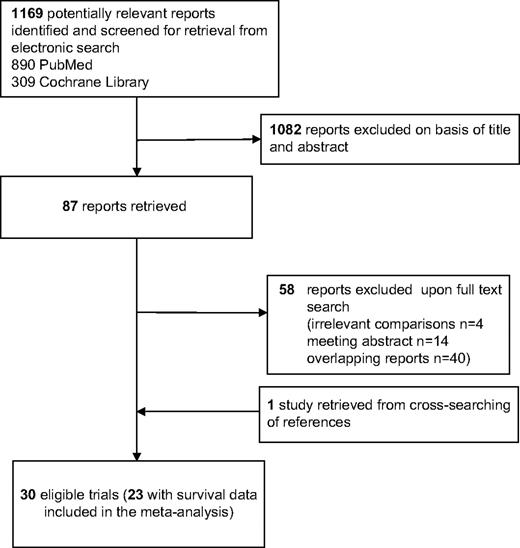
We identified 30 different trials that were potentially eligible for our study ( Fig. 1 ). Of these 30 trials, seven with a total of 1005 patients did not address survival because they were typically phase I/II or II trials that had not collected survival information. Thus, the meta-analysis included a total of 23 eligible trials and 8504 patients ( 4 – 24 ) , of whom 4559 had been randomly assigned to receive aromatase inhibitors or inactivators and 3945 had been assigned to receive standard hormonal treatments. Of these 23 trials, two had been jointly analyzed, and we used data from the combined analysis that were stratified by trial ( 10 ) . The designs of another three trials included three randomized arms, each with two doses of aromatase inhibitor, compared with an antiestrogen agent, and these trials were analyzed as two comparisons per trial ( 21 – 23 ). A total of 25 comparisons were thus evaluated in this meta-analysis.
Screened, excluded, and included articles and studies in the meta-analysis.
Trial Characteristics
Table 1 shows the key characteristics of the 23 included trials (25 comparisons). In all, we included six comparisons of the first-generation aromatase inhibitor aminoglutethimide, seven comparisons of second-generation inhibitors or inactivators (fadrozole or formestane), and 12 comparisons of third-generation inhibitors (anastrozole, letrozole, or vorozole) or inactivators (exemestane). Nine comparisons, all of which pertained to first-line treatment, used tamoxifen as the comparator, whereas the other 16 comparisons involved second- or even third-line treatment with progestagens (i.e., megestrol acetate or medroxyprogesterone acetate). One male patient was included in one early trial ( 4 ) , and a small number of perimenopausal women were included in some trials; otherwise, all trials included exclusively postmenopausal women ( Table 1 ). The median age was between 57 and 68 years across treatment arms. Hormone receptor status had been ascertained to various degrees across trials, but documented hormone receptor–negative cases were typically rare ( Table 1 ). Data on crossover to the alternative treatment arm were not always available, but crossover seemed to be substantial in some trials ( Table 1 ).
Characteristics of eligible trials *
| Author [trial] (reference) . | Year . | Regimen . | Line . | No. . | Median survival, mo . | Median age, y . | Receptor status (%) [+/−/unknown] . | MP, % . | RH (var) . | % Crossover . |
|---|---|---|---|---|---|---|---|---|---|---|
| Smith ( 4 ) | 1982 | AG (250 mg) × 4 | First | 57 | 20 | 57 | NA/NA/NA | 72 | 1.00 (.042) | 91 |
| TAM (10 mg) × 2 | 60 | 20 | 55 | NA/NA/NA | 80 | 85 | ||||
| Gale ( 5 ) | 1994 | AG (250 mg) × 4 | First | 122 | 24.5 | 61 † | 47/8/45 † | 100 | 0.90 (.020) | 35 |
| TAM (10 mg) × 2 | 119 | 21.6 | 100 | 37 | ||||||
| Thurlimann [SAKK 20/88] ( 6 ) | 1996 | Fadrozole (1 mg) × 2 | First | 105 | 38.4 | 65 | 81/12/7 | 100 | 0.91 (.036) | 78 |
| TAM (20 mg) × 1 | 107 | 39.6 | 65 | 76/13/11 | 100 | 62 | ||||
| Falkson ( 7 ) | 1996 | Fadrozole (1 mg) × 2 | First | 40 | 30.4 | 67 | 25/0/75 | 98 | 1.10 (.060) | NA |
| TAM (20 mg) × 1 | 40 | 33.7 | 57 | 40/3/58 | 100 | NA | ||||
| Perez-Carrion ( 8 ) | 1994 | Formestane (250 mg) q2wk | First | 203 | 33 | 63 | 40/0/59 | 98 | 1.02 (.019) | NA |
| TAM (30 mg) × 1 | 206 | 34 | 62 | 39/1/61 | 97 | NA | ||||
| Mouridsen ( 9 ) | 2001 | Letrozole (2.5 mg) × 1 | First | 458 | 34 | 65 | 65/1/34 | 100 | 0.90 (.006) | 43 |
| TAM (20 mg) × 1 | 458 | 30 | 64 | 67/0/33 | 100 | 42 | ||||
| Nabholtz [NA & TARGET] ( 10 ) | 2003 | Anastrozole (1 mg) × 1 | First | 511 | 39.2 | 67 | 60/0/40 | 100 | 0.97 (.007) | 27 |
| TAM (20 mg) × 1 | 510 | 40.1 | 67 | 60/0/40 | 100 | 26 | ||||
| Milla-Santos ( 11 ) | 2003 | Anastrozole (1 mg) × 1 | First | 121 | 17.4 | NA | 100/0/0 | 100 | 0.64 (.024) | NA |
| TAM (40 mg) × 1 | 117 | 16 | NA | 100/0/0 | 100 | NA | ||||
| Castelazo-Rico ( 12 ) | 2004 | Anastrozole (1 mg) × 1 | First | 10 | >24 ‡ | NA | 100/0/0 | 100 | 0.50 (3.00) | NA |
| TAM (20 mg) × 1 | 10 | >24 ‡ | NA | 100/0/0 | 100 | NA | ||||
| Garcia-Giralt ( 13 ) | 1992 | AG (250 mg) × 2 | Second | 131 | 19.2 | NA | NA/NA/NA | 100 | 1.04 (.023) | 45 |
| MPA (500 mg) × 2 | 119 | 17 | NA | NA/NA/NA | 100 | 58 | ||||
| Canney ( 14 ) | 1988 | AG (125 mg) × 2 | Second | 106 | 15.8 | 64 | NA/NA/NA | 99 | 0.99 (.026) | 22 |
| MPA (250 mg) × 4 | 112 | 15.7 | 64 | NA/NA/NA | 100 | 29 | ||||
| Lundgren ( 15 ) | 1989 | AG (250 mg) × 2 | Second | 86 | 22.4 | 62 | NA/NA/NA | 96 | 0.79 (.034) | NA |
| MA (160 mg) × 1 | 90 | 16.3 | 63 | NA/NA/NA | 97 | NA | ||||
| Russell [SWOG] ( 16 ) | 1997 | AG (250 mg) × 2 | Second | 80 | 27 | 65 | 100/0/0 | 100 | 1.04 (.024) | 46 |
| MA (40 mg) × 4 | 75 | 26 | 65 | 100/0/0 | 100 | 61 | ||||
| Buzdar [Fadrozole 03] ( 17 ) | 1996 | Fadrozole (1 mg) × 2 | Second | 195 | 27.1 | 65 | NA/NA/NA | 100 | 0.85 (.020) | NA |
| MA (40 mg) × 4 | 184 | 23 | 68 | NA/NA/NA | 100 | NA | ||||
| Buzdar [Fadrozole 06] ( 17 ) | 1996 | Fadrozole (1 mg) × 2 | Second | 150 | 25.8 | 67 | NA/NA/NA | 100 | 1.08 (.029) | NA |
| MA (40 mg) × 4 | 148 | 27.9 | 65 | NA/NA/NA | 100 | NA | ||||
| Bezwoda ( 18 ) | 1998 | Fadrozole (1 mg) × 2 | Second | 46 | 19.1 | NA | NA/NA/NA | 98 | 1.14 (.073) | NA |
| MA (40 mg) × 4 | 50 | 24.4 | NA | NA/NA/NA | 100 | NA | ||||
| Freue ( 20 ) | 2000 | Formestane (250 mg) q2wk | Second | 276 | 18.7 | NA | 42/2/57 | 100 | 1.06 (.015) | NA |
| MA (160 mg) × 1 | 271 | 19.9 | NA | 42/3/55 | 100 | NA | ||||
| Goss [NA vorozole] ( 19 ) | 1999 | Vorozole (2.5 mg) × 1 | Second | 225 | 26.3 | 66 | 86/1/13 | 100 | 1.01 (.028) | NA |
| MA (40 mg) × 4 | 227 | 28.8 | 67 | 82/1/17 | 100 | NA | ||||
| Dombernowsky [AR/BC2] ( 21 ) | 1998 | Letrozole (0.5 mg) × 1 | Second | 188 | 21.5 | NA | 55/0/45 | 100 | 1.12 (.017) | NA |
| Letrozole (2.5 mg) × 1 | 174 | 25.3 | NA | 58/0/43 | 100 | 0.82 (.019) | NA | |||
| MA (160 mg) × 1 | 189 | 21.5 | NA | 59/0/41 | 100 | NA | ||||
| Buzdar ( 22 ) | 2001 | Letrozole (0.5 mg) × 1 | Second | 202 | 33.1 | 67 | 83/1/16 | 100 | 0.79 (.015) | NA |
| Letrozole (2.5 mg) × 1 | 199 | 28.6 | 66 | 80/0/20 | 100 | 0.92 (.014) | NA | |||
| MA (40 mg) × 4 | 201 | 26.2 | 66 | 80/0/20 | 100 | NA | ||||
| Buzdar [Arimidex 04/05] ( 23 ) | 1998 | Anastrozole (1 mg) × 1 | Second | 263 | 26.7 | 65 | 74/2/24 | 100 | 0.78 (.012) | NA |
| Anastrozole (10 mg) × 1 | 248 | 25.5 | 66 | 68/5/27 | 100 | 0.83 (.012) | NA | |||
| MA (40 mg) × 4 | 253 | 22.5 | 65 | 71/4/25 | 100 | NA | ||||
| Kaufmann ( 24 ) | 2000 | Exemestane (25 mg) × 1 | Second | 366 | >29.5 ‡ | 65 | 67/1/32 | 100 | 0.77 (.016) | NA |
| MA (40 mg) × 4 | 403 | 28.7 | 65 | 68/0/32 | 100 | NA |
| Author [trial] (reference) . | Year . | Regimen . | Line . | No. . | Median survival, mo . | Median age, y . | Receptor status (%) [+/−/unknown] . | MP, % . | RH (var) . | % Crossover . |
|---|---|---|---|---|---|---|---|---|---|---|
| Smith ( 4 ) | 1982 | AG (250 mg) × 4 | First | 57 | 20 | 57 | NA/NA/NA | 72 | 1.00 (.042) | 91 |
| TAM (10 mg) × 2 | 60 | 20 | 55 | NA/NA/NA | 80 | 85 | ||||
| Gale ( 5 ) | 1994 | AG (250 mg) × 4 | First | 122 | 24.5 | 61 † | 47/8/45 † | 100 | 0.90 (.020) | 35 |
| TAM (10 mg) × 2 | 119 | 21.6 | 100 | 37 | ||||||
| Thurlimann [SAKK 20/88] ( 6 ) | 1996 | Fadrozole (1 mg) × 2 | First | 105 | 38.4 | 65 | 81/12/7 | 100 | 0.91 (.036) | 78 |
| TAM (20 mg) × 1 | 107 | 39.6 | 65 | 76/13/11 | 100 | 62 | ||||
| Falkson ( 7 ) | 1996 | Fadrozole (1 mg) × 2 | First | 40 | 30.4 | 67 | 25/0/75 | 98 | 1.10 (.060) | NA |
| TAM (20 mg) × 1 | 40 | 33.7 | 57 | 40/3/58 | 100 | NA | ||||
| Perez-Carrion ( 8 ) | 1994 | Formestane (250 mg) q2wk | First | 203 | 33 | 63 | 40/0/59 | 98 | 1.02 (.019) | NA |
| TAM (30 mg) × 1 | 206 | 34 | 62 | 39/1/61 | 97 | NA | ||||
| Mouridsen ( 9 ) | 2001 | Letrozole (2.5 mg) × 1 | First | 458 | 34 | 65 | 65/1/34 | 100 | 0.90 (.006) | 43 |
| TAM (20 mg) × 1 | 458 | 30 | 64 | 67/0/33 | 100 | 42 | ||||
| Nabholtz [NA & TARGET] ( 10 ) | 2003 | Anastrozole (1 mg) × 1 | First | 511 | 39.2 | 67 | 60/0/40 | 100 | 0.97 (.007) | 27 |
| TAM (20 mg) × 1 | 510 | 40.1 | 67 | 60/0/40 | 100 | 26 | ||||
| Milla-Santos ( 11 ) | 2003 | Anastrozole (1 mg) × 1 | First | 121 | 17.4 | NA | 100/0/0 | 100 | 0.64 (.024) | NA |
| TAM (40 mg) × 1 | 117 | 16 | NA | 100/0/0 | 100 | NA | ||||
| Castelazo-Rico ( 12 ) | 2004 | Anastrozole (1 mg) × 1 | First | 10 | >24 ‡ | NA | 100/0/0 | 100 | 0.50 (3.00) | NA |
| TAM (20 mg) × 1 | 10 | >24 ‡ | NA | 100/0/0 | 100 | NA | ||||
| Garcia-Giralt ( 13 ) | 1992 | AG (250 mg) × 2 | Second | 131 | 19.2 | NA | NA/NA/NA | 100 | 1.04 (.023) | 45 |
| MPA (500 mg) × 2 | 119 | 17 | NA | NA/NA/NA | 100 | 58 | ||||
| Canney ( 14 ) | 1988 | AG (125 mg) × 2 | Second | 106 | 15.8 | 64 | NA/NA/NA | 99 | 0.99 (.026) | 22 |
| MPA (250 mg) × 4 | 112 | 15.7 | 64 | NA/NA/NA | 100 | 29 | ||||
| Lundgren ( 15 ) | 1989 | AG (250 mg) × 2 | Second | 86 | 22.4 | 62 | NA/NA/NA | 96 | 0.79 (.034) | NA |
| MA (160 mg) × 1 | 90 | 16.3 | 63 | NA/NA/NA | 97 | NA | ||||
| Russell [SWOG] ( 16 ) | 1997 | AG (250 mg) × 2 | Second | 80 | 27 | 65 | 100/0/0 | 100 | 1.04 (.024) | 46 |
| MA (40 mg) × 4 | 75 | 26 | 65 | 100/0/0 | 100 | 61 | ||||
| Buzdar [Fadrozole 03] ( 17 ) | 1996 | Fadrozole (1 mg) × 2 | Second | 195 | 27.1 | 65 | NA/NA/NA | 100 | 0.85 (.020) | NA |
| MA (40 mg) × 4 | 184 | 23 | 68 | NA/NA/NA | 100 | NA | ||||
| Buzdar [Fadrozole 06] ( 17 ) | 1996 | Fadrozole (1 mg) × 2 | Second | 150 | 25.8 | 67 | NA/NA/NA | 100 | 1.08 (.029) | NA |
| MA (40 mg) × 4 | 148 | 27.9 | 65 | NA/NA/NA | 100 | NA | ||||
| Bezwoda ( 18 ) | 1998 | Fadrozole (1 mg) × 2 | Second | 46 | 19.1 | NA | NA/NA/NA | 98 | 1.14 (.073) | NA |
| MA (40 mg) × 4 | 50 | 24.4 | NA | NA/NA/NA | 100 | NA | ||||
| Freue ( 20 ) | 2000 | Formestane (250 mg) q2wk | Second | 276 | 18.7 | NA | 42/2/57 | 100 | 1.06 (.015) | NA |
| MA (160 mg) × 1 | 271 | 19.9 | NA | 42/3/55 | 100 | NA | ||||
| Goss [NA vorozole] ( 19 ) | 1999 | Vorozole (2.5 mg) × 1 | Second | 225 | 26.3 | 66 | 86/1/13 | 100 | 1.01 (.028) | NA |
| MA (40 mg) × 4 | 227 | 28.8 | 67 | 82/1/17 | 100 | NA | ||||
| Dombernowsky [AR/BC2] ( 21 ) | 1998 | Letrozole (0.5 mg) × 1 | Second | 188 | 21.5 | NA | 55/0/45 | 100 | 1.12 (.017) | NA |
| Letrozole (2.5 mg) × 1 | 174 | 25.3 | NA | 58/0/43 | 100 | 0.82 (.019) | NA | |||
| MA (160 mg) × 1 | 189 | 21.5 | NA | 59/0/41 | 100 | NA | ||||
| Buzdar ( 22 ) | 2001 | Letrozole (0.5 mg) × 1 | Second | 202 | 33.1 | 67 | 83/1/16 | 100 | 0.79 (.015) | NA |
| Letrozole (2.5 mg) × 1 | 199 | 28.6 | 66 | 80/0/20 | 100 | 0.92 (.014) | NA | |||
| MA (40 mg) × 4 | 201 | 26.2 | 66 | 80/0/20 | 100 | NA | ||||
| Buzdar [Arimidex 04/05] ( 23 ) | 1998 | Anastrozole (1 mg) × 1 | Second | 263 | 26.7 | 65 | 74/2/24 | 100 | 0.78 (.012) | NA |
| Anastrozole (10 mg) × 1 | 248 | 25.5 | 66 | 68/5/27 | 100 | 0.83 (.012) | NA | |||
| MA (40 mg) × 4 | 253 | 22.5 | 65 | 71/4/25 | 100 | NA | ||||
| Kaufmann ( 24 ) | 2000 | Exemestane (25 mg) × 1 | Second | 366 | >29.5 ‡ | 65 | 67/1/32 | 100 | 0.77 (.016) | NA |
| MA (40 mg) × 4 | 403 | 28.7 | 65 | 68/0/32 | 100 | NA |
NA = not available data; × (followed by a number) = number of doses per day; q2wk = every 2 weeks; AG = aminoglutethimide; TAM = tamoxifen; MPA = medroxyprogesterone acetate; MA = megestrol acetate; RH (var) = relative hazard and variance of the natural logarithm of the relative hazard; OT = proportion of patients who stayed on the allocated treatment until the end of reported follow-up or death; SAKK = Swiss Group for Clinical Cancer Research; NA & TARGET = North American and Tamoxifen or Arimidex Randomized Group Efficacy and Tolerability trials; SWOG = South Western Oncology Group; NA vorozole = North American vorazole; AR/BC2 = Letrozole International Trial Group protocol. Crossover was to the alternative treatment arm.
Not specified by arm.
Characteristics of eligible trials *
| Author [trial] (reference) . | Year . | Regimen . | Line . | No. . | Median survival, mo . | Median age, y . | Receptor status (%) [+/−/unknown] . | MP, % . | RH (var) . | % Crossover . |
|---|---|---|---|---|---|---|---|---|---|---|
| Smith ( 4 ) | 1982 | AG (250 mg) × 4 | First | 57 | 20 | 57 | NA/NA/NA | 72 | 1.00 (.042) | 91 |
| TAM (10 mg) × 2 | 60 | 20 | 55 | NA/NA/NA | 80 | 85 | ||||
| Gale ( 5 ) | 1994 | AG (250 mg) × 4 | First | 122 | 24.5 | 61 † | 47/8/45 † | 100 | 0.90 (.020) | 35 |
| TAM (10 mg) × 2 | 119 | 21.6 | 100 | 37 | ||||||
| Thurlimann [SAKK 20/88] ( 6 ) | 1996 | Fadrozole (1 mg) × 2 | First | 105 | 38.4 | 65 | 81/12/7 | 100 | 0.91 (.036) | 78 |
| TAM (20 mg) × 1 | 107 | 39.6 | 65 | 76/13/11 | 100 | 62 | ||||
| Falkson ( 7 ) | 1996 | Fadrozole (1 mg) × 2 | First | 40 | 30.4 | 67 | 25/0/75 | 98 | 1.10 (.060) | NA |
| TAM (20 mg) × 1 | 40 | 33.7 | 57 | 40/3/58 | 100 | NA | ||||
| Perez-Carrion ( 8 ) | 1994 | Formestane (250 mg) q2wk | First | 203 | 33 | 63 | 40/0/59 | 98 | 1.02 (.019) | NA |
| TAM (30 mg) × 1 | 206 | 34 | 62 | 39/1/61 | 97 | NA | ||||
| Mouridsen ( 9 ) | 2001 | Letrozole (2.5 mg) × 1 | First | 458 | 34 | 65 | 65/1/34 | 100 | 0.90 (.006) | 43 |
| TAM (20 mg) × 1 | 458 | 30 | 64 | 67/0/33 | 100 | 42 | ||||
| Nabholtz [NA & TARGET] ( 10 ) | 2003 | Anastrozole (1 mg) × 1 | First | 511 | 39.2 | 67 | 60/0/40 | 100 | 0.97 (.007) | 27 |
| TAM (20 mg) × 1 | 510 | 40.1 | 67 | 60/0/40 | 100 | 26 | ||||
| Milla-Santos ( 11 ) | 2003 | Anastrozole (1 mg) × 1 | First | 121 | 17.4 | NA | 100/0/0 | 100 | 0.64 (.024) | NA |
| TAM (40 mg) × 1 | 117 | 16 | NA | 100/0/0 | 100 | NA | ||||
| Castelazo-Rico ( 12 ) | 2004 | Anastrozole (1 mg) × 1 | First | 10 | >24 ‡ | NA | 100/0/0 | 100 | 0.50 (3.00) | NA |
| TAM (20 mg) × 1 | 10 | >24 ‡ | NA | 100/0/0 | 100 | NA | ||||
| Garcia-Giralt ( 13 ) | 1992 | AG (250 mg) × 2 | Second | 131 | 19.2 | NA | NA/NA/NA | 100 | 1.04 (.023) | 45 |
| MPA (500 mg) × 2 | 119 | 17 | NA | NA/NA/NA | 100 | 58 | ||||
| Canney ( 14 ) | 1988 | AG (125 mg) × 2 | Second | 106 | 15.8 | 64 | NA/NA/NA | 99 | 0.99 (.026) | 22 |
| MPA (250 mg) × 4 | 112 | 15.7 | 64 | NA/NA/NA | 100 | 29 | ||||
| Lundgren ( 15 ) | 1989 | AG (250 mg) × 2 | Second | 86 | 22.4 | 62 | NA/NA/NA | 96 | 0.79 (.034) | NA |
| MA (160 mg) × 1 | 90 | 16.3 | 63 | NA/NA/NA | 97 | NA | ||||
| Russell [SWOG] ( 16 ) | 1997 | AG (250 mg) × 2 | Second | 80 | 27 | 65 | 100/0/0 | 100 | 1.04 (.024) | 46 |
| MA (40 mg) × 4 | 75 | 26 | 65 | 100/0/0 | 100 | 61 | ||||
| Buzdar [Fadrozole 03] ( 17 ) | 1996 | Fadrozole (1 mg) × 2 | Second | 195 | 27.1 | 65 | NA/NA/NA | 100 | 0.85 (.020) | NA |
| MA (40 mg) × 4 | 184 | 23 | 68 | NA/NA/NA | 100 | NA | ||||
| Buzdar [Fadrozole 06] ( 17 ) | 1996 | Fadrozole (1 mg) × 2 | Second | 150 | 25.8 | 67 | NA/NA/NA | 100 | 1.08 (.029) | NA |
| MA (40 mg) × 4 | 148 | 27.9 | 65 | NA/NA/NA | 100 | NA | ||||
| Bezwoda ( 18 ) | 1998 | Fadrozole (1 mg) × 2 | Second | 46 | 19.1 | NA | NA/NA/NA | 98 | 1.14 (.073) | NA |
| MA (40 mg) × 4 | 50 | 24.4 | NA | NA/NA/NA | 100 | NA | ||||
| Freue ( 20 ) | 2000 | Formestane (250 mg) q2wk | Second | 276 | 18.7 | NA | 42/2/57 | 100 | 1.06 (.015) | NA |
| MA (160 mg) × 1 | 271 | 19.9 | NA | 42/3/55 | 100 | NA | ||||
| Goss [NA vorozole] ( 19 ) | 1999 | Vorozole (2.5 mg) × 1 | Second | 225 | 26.3 | 66 | 86/1/13 | 100 | 1.01 (.028) | NA |
| MA (40 mg) × 4 | 227 | 28.8 | 67 | 82/1/17 | 100 | NA | ||||
| Dombernowsky [AR/BC2] ( 21 ) | 1998 | Letrozole (0.5 mg) × 1 | Second | 188 | 21.5 | NA | 55/0/45 | 100 | 1.12 (.017) | NA |
| Letrozole (2.5 mg) × 1 | 174 | 25.3 | NA | 58/0/43 | 100 | 0.82 (.019) | NA | |||
| MA (160 mg) × 1 | 189 | 21.5 | NA | 59/0/41 | 100 | NA | ||||
| Buzdar ( 22 ) | 2001 | Letrozole (0.5 mg) × 1 | Second | 202 | 33.1 | 67 | 83/1/16 | 100 | 0.79 (.015) | NA |
| Letrozole (2.5 mg) × 1 | 199 | 28.6 | 66 | 80/0/20 | 100 | 0.92 (.014) | NA | |||
| MA (40 mg) × 4 | 201 | 26.2 | 66 | 80/0/20 | 100 | NA | ||||
| Buzdar [Arimidex 04/05] ( 23 ) | 1998 | Anastrozole (1 mg) × 1 | Second | 263 | 26.7 | 65 | 74/2/24 | 100 | 0.78 (.012) | NA |
| Anastrozole (10 mg) × 1 | 248 | 25.5 | 66 | 68/5/27 | 100 | 0.83 (.012) | NA | |||
| MA (40 mg) × 4 | 253 | 22.5 | 65 | 71/4/25 | 100 | NA | ||||
| Kaufmann ( 24 ) | 2000 | Exemestane (25 mg) × 1 | Second | 366 | >29.5 ‡ | 65 | 67/1/32 | 100 | 0.77 (.016) | NA |
| MA (40 mg) × 4 | 403 | 28.7 | 65 | 68/0/32 | 100 | NA |
| Author [trial] (reference) . | Year . | Regimen . | Line . | No. . | Median survival, mo . | Median age, y . | Receptor status (%) [+/−/unknown] . | MP, % . | RH (var) . | % Crossover . |
|---|---|---|---|---|---|---|---|---|---|---|
| Smith ( 4 ) | 1982 | AG (250 mg) × 4 | First | 57 | 20 | 57 | NA/NA/NA | 72 | 1.00 (.042) | 91 |
| TAM (10 mg) × 2 | 60 | 20 | 55 | NA/NA/NA | 80 | 85 | ||||
| Gale ( 5 ) | 1994 | AG (250 mg) × 4 | First | 122 | 24.5 | 61 † | 47/8/45 † | 100 | 0.90 (.020) | 35 |
| TAM (10 mg) × 2 | 119 | 21.6 | 100 | 37 | ||||||
| Thurlimann [SAKK 20/88] ( 6 ) | 1996 | Fadrozole (1 mg) × 2 | First | 105 | 38.4 | 65 | 81/12/7 | 100 | 0.91 (.036) | 78 |
| TAM (20 mg) × 1 | 107 | 39.6 | 65 | 76/13/11 | 100 | 62 | ||||
| Falkson ( 7 ) | 1996 | Fadrozole (1 mg) × 2 | First | 40 | 30.4 | 67 | 25/0/75 | 98 | 1.10 (.060) | NA |
| TAM (20 mg) × 1 | 40 | 33.7 | 57 | 40/3/58 | 100 | NA | ||||
| Perez-Carrion ( 8 ) | 1994 | Formestane (250 mg) q2wk | First | 203 | 33 | 63 | 40/0/59 | 98 | 1.02 (.019) | NA |
| TAM (30 mg) × 1 | 206 | 34 | 62 | 39/1/61 | 97 | NA | ||||
| Mouridsen ( 9 ) | 2001 | Letrozole (2.5 mg) × 1 | First | 458 | 34 | 65 | 65/1/34 | 100 | 0.90 (.006) | 43 |
| TAM (20 mg) × 1 | 458 | 30 | 64 | 67/0/33 | 100 | 42 | ||||
| Nabholtz [NA & TARGET] ( 10 ) | 2003 | Anastrozole (1 mg) × 1 | First | 511 | 39.2 | 67 | 60/0/40 | 100 | 0.97 (.007) | 27 |
| TAM (20 mg) × 1 | 510 | 40.1 | 67 | 60/0/40 | 100 | 26 | ||||
| Milla-Santos ( 11 ) | 2003 | Anastrozole (1 mg) × 1 | First | 121 | 17.4 | NA | 100/0/0 | 100 | 0.64 (.024) | NA |
| TAM (40 mg) × 1 | 117 | 16 | NA | 100/0/0 | 100 | NA | ||||
| Castelazo-Rico ( 12 ) | 2004 | Anastrozole (1 mg) × 1 | First | 10 | >24 ‡ | NA | 100/0/0 | 100 | 0.50 (3.00) | NA |
| TAM (20 mg) × 1 | 10 | >24 ‡ | NA | 100/0/0 | 100 | NA | ||||
| Garcia-Giralt ( 13 ) | 1992 | AG (250 mg) × 2 | Second | 131 | 19.2 | NA | NA/NA/NA | 100 | 1.04 (.023) | 45 |
| MPA (500 mg) × 2 | 119 | 17 | NA | NA/NA/NA | 100 | 58 | ||||
| Canney ( 14 ) | 1988 | AG (125 mg) × 2 | Second | 106 | 15.8 | 64 | NA/NA/NA | 99 | 0.99 (.026) | 22 |
| MPA (250 mg) × 4 | 112 | 15.7 | 64 | NA/NA/NA | 100 | 29 | ||||
| Lundgren ( 15 ) | 1989 | AG (250 mg) × 2 | Second | 86 | 22.4 | 62 | NA/NA/NA | 96 | 0.79 (.034) | NA |
| MA (160 mg) × 1 | 90 | 16.3 | 63 | NA/NA/NA | 97 | NA | ||||
| Russell [SWOG] ( 16 ) | 1997 | AG (250 mg) × 2 | Second | 80 | 27 | 65 | 100/0/0 | 100 | 1.04 (.024) | 46 |
| MA (40 mg) × 4 | 75 | 26 | 65 | 100/0/0 | 100 | 61 | ||||
| Buzdar [Fadrozole 03] ( 17 ) | 1996 | Fadrozole (1 mg) × 2 | Second | 195 | 27.1 | 65 | NA/NA/NA | 100 | 0.85 (.020) | NA |
| MA (40 mg) × 4 | 184 | 23 | 68 | NA/NA/NA | 100 | NA | ||||
| Buzdar [Fadrozole 06] ( 17 ) | 1996 | Fadrozole (1 mg) × 2 | Second | 150 | 25.8 | 67 | NA/NA/NA | 100 | 1.08 (.029) | NA |
| MA (40 mg) × 4 | 148 | 27.9 | 65 | NA/NA/NA | 100 | NA | ||||
| Bezwoda ( 18 ) | 1998 | Fadrozole (1 mg) × 2 | Second | 46 | 19.1 | NA | NA/NA/NA | 98 | 1.14 (.073) | NA |
| MA (40 mg) × 4 | 50 | 24.4 | NA | NA/NA/NA | 100 | NA | ||||
| Freue ( 20 ) | 2000 | Formestane (250 mg) q2wk | Second | 276 | 18.7 | NA | 42/2/57 | 100 | 1.06 (.015) | NA |
| MA (160 mg) × 1 | 271 | 19.9 | NA | 42/3/55 | 100 | NA | ||||
| Goss [NA vorozole] ( 19 ) | 1999 | Vorozole (2.5 mg) × 1 | Second | 225 | 26.3 | 66 | 86/1/13 | 100 | 1.01 (.028) | NA |
| MA (40 mg) × 4 | 227 | 28.8 | 67 | 82/1/17 | 100 | NA | ||||
| Dombernowsky [AR/BC2] ( 21 ) | 1998 | Letrozole (0.5 mg) × 1 | Second | 188 | 21.5 | NA | 55/0/45 | 100 | 1.12 (.017) | NA |
| Letrozole (2.5 mg) × 1 | 174 | 25.3 | NA | 58/0/43 | 100 | 0.82 (.019) | NA | |||
| MA (160 mg) × 1 | 189 | 21.5 | NA | 59/0/41 | 100 | NA | ||||
| Buzdar ( 22 ) | 2001 | Letrozole (0.5 mg) × 1 | Second | 202 | 33.1 | 67 | 83/1/16 | 100 | 0.79 (.015) | NA |
| Letrozole (2.5 mg) × 1 | 199 | 28.6 | 66 | 80/0/20 | 100 | 0.92 (.014) | NA | |||
| MA (40 mg) × 4 | 201 | 26.2 | 66 | 80/0/20 | 100 | NA | ||||
| Buzdar [Arimidex 04/05] ( 23 ) | 1998 | Anastrozole (1 mg) × 1 | Second | 263 | 26.7 | 65 | 74/2/24 | 100 | 0.78 (.012) | NA |
| Anastrozole (10 mg) × 1 | 248 | 25.5 | 66 | 68/5/27 | 100 | 0.83 (.012) | NA | |||
| MA (40 mg) × 4 | 253 | 22.5 | 65 | 71/4/25 | 100 | NA | ||||
| Kaufmann ( 24 ) | 2000 | Exemestane (25 mg) × 1 | Second | 366 | >29.5 ‡ | 65 | 67/1/32 | 100 | 0.77 (.016) | NA |
| MA (40 mg) × 4 | 403 | 28.7 | 65 | 68/0/32 | 100 | NA |
NA = not available data; × (followed by a number) = number of doses per day; q2wk = every 2 weeks; AG = aminoglutethimide; TAM = tamoxifen; MPA = medroxyprogesterone acetate; MA = megestrol acetate; RH (var) = relative hazard and variance of the natural logarithm of the relative hazard; OT = proportion of patients who stayed on the allocated treatment until the end of reported follow-up or death; SAKK = Swiss Group for Clinical Cancer Research; NA & TARGET = North American and Tamoxifen or Arimidex Randomized Group Efficacy and Tolerability trials; SWOG = South Western Oncology Group; NA vorozole = North American vorazole; AR/BC2 = Letrozole International Trial Group protocol. Crossover was to the alternative treatment arm.
Not specified by arm.
Design and Quality Characteristics
Eleven trials were double blind ( 9 , 10 , 17 , 18 , 21 – 24 ) , 16 described in detail the mode of randomization ( 6 , 9 – 11 , 14 , 17 , 18 , 20 , 21 , 23 , 24 , 28 , 29 ) , 15 described some method for ensuring allocation concealment ( 6 , 9 , 10 , 14 , 17 , 18 , 20 , 21 , 23 , 24 , 28 , 29 ) , and 18 described withdrawals in sufficient detail ( 5 – 9 , 13 – 20 , 25 – 29 ) . No trials were stopped early because of statistically significant survival differences in an interim analysis.
Meta-analysis
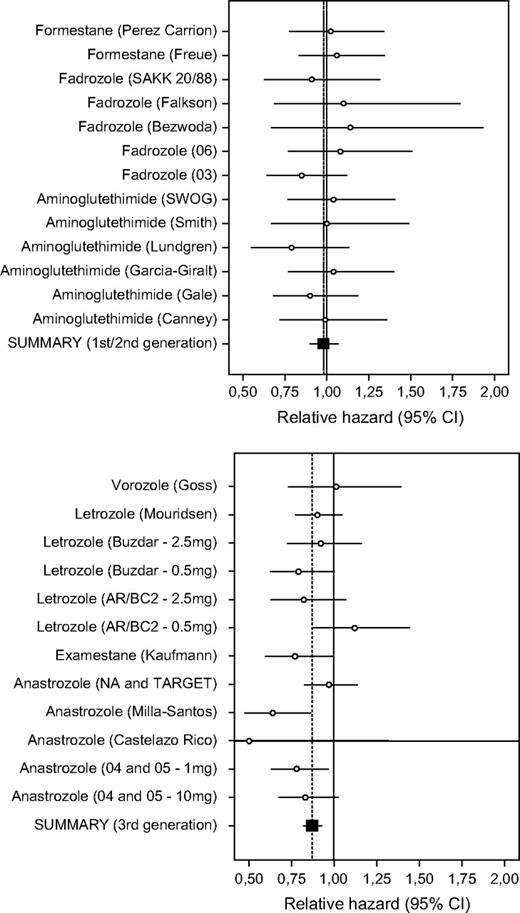
Meta-analysis according to generation of aromatase inhibitor ( Table 2 and Fig. 2 ) found that only third-generation aromatase inhibitors and inactivators (RH = 0.87, 95% CI = 0.82 to 0.93; P <.001) were statistically significantly associated with increased survival compared with standard hormone therapy. There was no evidence of any increased survival with aminoglutethimide and second-generation agents (RH = 0.98, 95% CI = 0.90 to 1.07). The difference in the summary effects between these two groups of trials was statistically significant ( P = .04). A combination of the data across all 25 available comparisons also provided a statistically significant summary effect for mortality (RH = 0.91, 95% CI = 0.86 to 0.96; P = .001). There was no statistically significant between-study heterogeneity when all studies were considered ( Q = 22.8 with 24 df), possibly because of the relatively wide confidence intervals of single trials.
Meta-analysis of survival for the comparison between aromatase inhibitors or inactivators and standard hormonal therapy. Upper ) First- and second-generation agents. Lower ) Third-generation agents. Each trial is identified by the name of the tested aromatase inhibitor or inactivator and the name of the first author or trial/protocol name/abbreviation. The point estimate for the relative hazard and its 95% confidence interval (CI) are indicated as a circle and whiskers, respectively. Also shown is the summary estimate and its 95% CI (results from fixed- and random-effects analyses were identical or very similar, as noted in Table 2 ).
Summary estimates and subgroup analyses *
| Data . | No. of comparisons . | RH (95% CI) . | Q . | I2 , % . |
|---|---|---|---|---|
| Generation | ||||
| First | 6 | 0.96 (0.84 to 1.09) | 2.0 | 0 |
| Second | 7 | 1.00 (0.89 to 1.13) | 2.5 | 0 |
| Third | 12 | 0.87 (0.82 to 0.93) | 13.8 | 20 |
| All | 25 | 0.91 (0.86 to 0.96) | 22.8 | 0 |
| Line/comparator | ||||
| First/tamoxifen | ||||
| First/second | 5 | 0.97 (0.84 to 1.13) | 0.9 | 0 |
| Third | 4 | 0.89 (0.80 to 0.99) | 5.8 | 48 |
| All | 9 | 0.92 (0.84 to 1.00) | 7.5 | 0 |
| Other/progestagen | ||||
| First/second | 8 | 0.99 (0.89 to 1.10) | 3.7 | 0 |
| Third | 8 | 0.86 (0.79 to 0.94) | 7.7 | 9 |
| All | 16 | 0.91 (0.85 to 0.97) | 15.2 | 1 |
| Data . | No. of comparisons . | RH (95% CI) . | Q . | I2 , % . |
|---|---|---|---|---|
| Generation | ||||
| First | 6 | 0.96 (0.84 to 1.09) | 2.0 | 0 |
| Second | 7 | 1.00 (0.89 to 1.13) | 2.5 | 0 |
| Third | 12 | 0.87 (0.82 to 0.93) | 13.8 | 20 |
| All | 25 | 0.91 (0.86 to 0.96) | 22.8 | 0 |
| Line/comparator | ||||
| First/tamoxifen | ||||
| First/second | 5 | 0.97 (0.84 to 1.13) | 0.9 | 0 |
| Third | 4 | 0.89 (0.80 to 0.99) | 5.8 | 48 |
| All | 9 | 0.92 (0.84 to 1.00) | 7.5 | 0 |
| Other/progestagen | ||||
| First/second | 8 | 0.99 (0.89 to 1.10) | 3.7 | 0 |
| Third | 8 | 0.86 (0.79 to 0.94) | 7.7 | 9 |
| All | 16 | 0.91 (0.85 to 0.97) | 15.2 | 1 |
RH = relative hazard; CI = confidence interval. Data were from fixed-effects calculations and are identical to random effects calculations except for minor differences for the third-generation compounds in the overall analysis (RH = 0.87, 95% CI = 0.81 to 0.94) and for first-line treatment (RH = 0.86, 95% CI = 0.73 to 1.02). No estimate of between-study heterogeneity was statistically significant.
Summary estimates and subgroup analyses *
| Data . | No. of comparisons . | RH (95% CI) . | Q . | I2 , % . |
|---|---|---|---|---|
| Generation | ||||
| First | 6 | 0.96 (0.84 to 1.09) | 2.0 | 0 |
| Second | 7 | 1.00 (0.89 to 1.13) | 2.5 | 0 |
| Third | 12 | 0.87 (0.82 to 0.93) | 13.8 | 20 |
| All | 25 | 0.91 (0.86 to 0.96) | 22.8 | 0 |
| Line/comparator | ||||
| First/tamoxifen | ||||
| First/second | 5 | 0.97 (0.84 to 1.13) | 0.9 | 0 |
| Third | 4 | 0.89 (0.80 to 0.99) | 5.8 | 48 |
| All | 9 | 0.92 (0.84 to 1.00) | 7.5 | 0 |
| Other/progestagen | ||||
| First/second | 8 | 0.99 (0.89 to 1.10) | 3.7 | 0 |
| Third | 8 | 0.86 (0.79 to 0.94) | 7.7 | 9 |
| All | 16 | 0.91 (0.85 to 0.97) | 15.2 | 1 |
| Data . | No. of comparisons . | RH (95% CI) . | Q . | I2 , % . |
|---|---|---|---|---|
| Generation | ||||
| First | 6 | 0.96 (0.84 to 1.09) | 2.0 | 0 |
| Second | 7 | 1.00 (0.89 to 1.13) | 2.5 | 0 |
| Third | 12 | 0.87 (0.82 to 0.93) | 13.8 | 20 |
| All | 25 | 0.91 (0.86 to 0.96) | 22.8 | 0 |
| Line/comparator | ||||
| First/tamoxifen | ||||
| First/second | 5 | 0.97 (0.84 to 1.13) | 0.9 | 0 |
| Third | 4 | 0.89 (0.80 to 0.99) | 5.8 | 48 |
| All | 9 | 0.92 (0.84 to 1.00) | 7.5 | 0 |
| Other/progestagen | ||||
| First/second | 8 | 0.99 (0.89 to 1.10) | 3.7 | 0 |
| Third | 8 | 0.86 (0.79 to 0.94) | 7.7 | 9 |
| All | 16 | 0.91 (0.85 to 0.97) | 15.2 | 1 |
RH = relative hazard; CI = confidence interval. Data were from fixed-effects calculations and are identical to random effects calculations except for minor differences for the third-generation compounds in the overall analysis (RH = 0.87, 95% CI = 0.81 to 0.94) and for first-line treatment (RH = 0.86, 95% CI = 0.73 to 1.02). No estimate of between-study heterogeneity was statistically significant.
Treatment Line
The survival benefit for third-generation agents was practically identical in both first-line trials, in which the comparator was tamoxifen (11% RH reduction, 95% CI = 1% to 19%; P = .03), and second-line (or subsequent-line) trials, in which progestagen comparators were used (14% RH reduction, 95% CI = 6% to 21%; P <.001). There was no between-study heterogeneity in either of these two subgroups. There was no statistically significant benefit observed with first- or second-generation agents (RH = 0.97 and 0.99, respectively, for first-line and second-line [or subsequent-line] trials) ( Table 2 ).
Bias Diagnostics
In cumulative meta-analysis of all trials, no statistically significant effect on survival was found in the trials published through 1997. A nominally statistically significant effect was detected in trials published through the end of 1998 (summary RH = 0.93, 95% CI = 0.86 to 1.00; P = .047); this effect was temporarily lost by the end of 1999 (summary RH = 0.94, 95% CI = 0.88 to 1.00; P = .054) and reappeared in 2000 with the publication of an exemestane trial ( 24 ) . The relative hazard has continued to remain in the range of 0.91–0.92 and to be statistically significant through 2006. This pattern is consistent with the later introduction of third-generation agents in clinical trials. There was no major change in the magnitude of the effect when cumulative meta-analyses were performed separately by generation of aromatase inhibitor.
There was no evidence that less precise or smaller trials gave different results from more precise or larger trials. This lack of significant difference between less and more precise trials was true across all trials (tau = 0.18 and P = .21) and when limited to trials of third-generation aromatase inhibitors and exemestane (tau = −0.26 and P = .24).
Four of the 25 comparisons showed statistically significant results on their own (three with third-generation aromatase inhibitors and one with exemestane). None of the trials with statistically significant differences had been stopped early in an interim analysis. Finally, one trial ( 9 ) suggested that the difference between the two compared arms varied according to the duration of follow-up: a survival benefit was seen between 6 and 20 months of follow-up but not over the total trial duration. No formal test of time dependence was performed in that trial. When we excluded this trial from our analysis, the summary relative hazard was unchanged.
D ISCUSSION
This meta-analysis showed that treatment with aromatase inhibitors and inactivators, in particular the newer third-generation agents, is associated with increased survival among patients with advanced breast cancer. This association was apparent in the first-, second-, and subsequent-line treatment settings. Tamoxifen and progestagens have been the agents used in standard hormonal treatment of advanced breast cancer in the first- and second-line settings, respectively ( 39 ) , even though aromatase inhibitors and inactivators are already widely used in the Western world. Our findings may challenge this standard of care, not only for second-line treatment—in which aromatase inhibitors are increasingly accepted on an equal or better standing than progestagens ( 31 ) —but also for first-line treatment, in which tamoxifen has largely remained the first choice to date. It is common practice for patients with hormone receptor–positive advanced breast cancer to receive both tamoxifen and an aromatase inhibitor, typically in sequence. Our results indicate that aromatase inhibitors and inactivators should be the first-line therapy for such patients. The association between increased survival and treatment with aromatase inhibitors and inactivators did not seem to extend to first- and second-generation agents. These earlier agents also tend to have less favorable tolerability profiles.
The estimated benefits reflect the intention-to-treat analyses, but we should caution that crossover in some trials ( 6 , 9 ) may have even diluted the treatment differences. The particular benefits of third-generation agents may be due to many reasons. Third-generation aromatase inhibitors and inactivators are more selective than first- and second-generation agents and are apparently less toxic ( 9 , 40 ) , and they also have a convenient pharmacologic profile that allows easier dosing ( 41 ). Given the demonstrated survival benefit of third-generation agents compared with standard hormonal therapy, our results may represent a departure from the standard management of advanced breast cancer with hormonal therapy that has been used for the last two decades. The standard of care may need to be reconsidered. Both efficacy and tolerability also need to be taken into account in clinical decision making. The available evidence suggests that aromatase inhibitors cause less weight gain, dyspnea, and peripheral edema than progestins but that they may cause more hot flushes ( 31 ) . The balance of toxicities is also not unfavorable when compared with the toxicities associated with tamoxifen, and, in some trials, tolerability is actually substantially better for aromatase inhibitors than for tamoxifen ( 9 , 10 ) . For some serious adverse events, such as thrombosis, more studies are required to determine the relative risk associated with aromatase inhibitors compared with tamoxifen, but the current evidence does not suggest that aromatase inhibitors are worse ( 42 ) . Increased quality of life has also been demonstrated with aromatase inhibitors compared with tamoxifen ( 43 ) .
The absolute magnitude of the survival benefit also needs to be considered. For a theoretical group of patients with an expected median survival of 30 months with standard hormonal treatment, we estimate from our data that the increased median survival conferred by a third-generation aromatase inhibitor or inactivator is about 4 months (13% RH reduction and assuming exponential mortality curves). These 4 months can be a considerable survival benefit for an advanced-stage patient ( 44 ). However, for women with median survival of 10 months, the expected benefit would slightly exceed only 1 month. These calculations make the assumption of a similar relative hazard reduction for patients at different levels of risk, and they would not be true if this hypothesis is violated.
Some limitations of our study need to be discussed. First, it may be useful to perform a meta-analysis of individual-level data that targets outcomes separately by subgroups of patients who have various risks of death ( 45 ). Second, our meta-analysis is based on data from trials whose results have published, and we note that publication bias is a potential threat to the validity of the results. Third, we did not obtain updated individual patient data, the use of such data might have further enhanced the accuracy and reduced the uncertainty of the estimates ( 46 , 47 ). However, we found no evidence of between-study heterogeneity and no hint of bias across several pertinent diagnostics. Allowing for these caveats, the meta-analysis offers strong evidence for the use of third-generation aromatase inhibitors and inactivators in the treatment of advanced breast cancer.
The authors have full responsibility for the design of the study, the collection of the data, the analysis and interpretation of the data, the decision to submit the manuscript for publication, and the writing of the manuscript.
References
Greenlee RT, Murray T, Bolden S, Wingo PA. Cancer statistics, 2000.
Stockler M, Wilcken NR, Ghersi D, Simes RJ. Systematic reviews of chemotherapy and endocrine therapy in metastatic breast cancer.
Baum M, Budzar AU, Cuzick J, Forbes J, Houghton JH, Klijn JG, et al. Anastrozole alone or in combination with tamoxifen versus tamoxifen alone for adjuvant treatment of postmenopausal women with early breast cancer: first results of the ATAC randomised trial.
Smith IE, Harris AL, Morgan M, Gazet JC, McKinna JA. Tamoxifen versus aminoglutethimide versus combined tamoxifen and aminoglutethimide in the treatment of advanced breast carcinoma.
Gale KE, Andersen JW, Tormey DC, Mansour EG, Davis TE, Horton J, et al. Hormonal treatment for metastatic breast cancer. An Eastern Cooperative Oncology Group phase III trial comparing aminoglutethimide to tamoxifen.
Thurlimann B, Beretta K, Bacchi M, Castiglione-Gertsch M, Goldhirsch A, Jungi WF, et al. First-line fadrozole HCI (CGS 16949A) versus tamoxifen in postmenopausal women with advanced breast cancer. Prospective randomised trial of the Swiss Group for Clinical Cancer Research SAKK 20/88.
Falkson CI, Falkson HC. A randomised study of CGS 16949A (fadrozole) versus tamoxifen in previously untreated postmenopausal patients with metastatic breast cancer.
Perez-Carrion R, Alberola Candel V, Calabresi F, Michel RT, Santos R, Delozier T, et al. Comparison of the selective aromatase inhibitor formestane with tamoxifen as first-line hormonal therapy in postmenopausal women with advanced breast cancer.
Mouridsen H, Gershanovich M, Sun Y, Perez-Carrion R, Boni C, Monnier A, et al. Superior efficacy of letrozole versus tamoxifen as first-line therapy for postmenopausal women with advanced breast cancer: results of a phase III study of the International Letrozole Breast Cancer Group.
Nabholtz JM, Bonneterre J, Buzdar A, Robertson JF, Thurlimann B. Anastrozole (Arimidex) versus tamoxifen as first-line therapy for advanced breast cancer in postmenopausal women: survival analysis and updated safety results.
Milla-Santos A, Milla L, Portella J, Rallo L, Pons M, Rodes E, et al. Anastrozole versus tamoxifen as first-line therapy in postmenopausal patients with hormone-dependent advanced breast cancer: a prospective, randomized, phase III study.
Castelazo Rico G, Molotla Xolalpa D, Basavilvazo Rodriguez MA, Angeles Victoria L, Zarate A, Hernandez Valencia M. Survival of breast cancer patients treated with inhibitors of the aromatase vs tamoxifen.
Garcia-Giralt E, Ayme Y, Carton M, Daban A, Delozier T, Fargeot P, et al. Second and third line hormonotherapy in advanced post-menopausal breast cancer: a multicenter randomized trial comparing medroxyprogesterone acetate with aminoglutethimide in patients who have become resistant to tamoxifen.
Canney PA, Priestman TJ, Griffiths T, Latief TN, Mould JJ, Spooner D. Randomized trial comparing aminoglutethimide with high-dose medroxyprogesterone acetate in therapy for advanced breast carcinoma.
Lundgren S, Gundersen S, Klepp R, Lonning PE, Lund E, Kvinnsland S. Megestrol acetate versus aminoglutethimide for metastatic breast cancer.
Russell CA, Green SJ, O'Sullivan J, Hynes HE, Budd GT, Congdon JE, et al. Megestrol acetate and aminoglutethimide/hydrocortisone in sequence or in combination as second-line endocrine therapy of estrogen receptor-positive metastatic breast cancer: a Southwest Oncology Group phase III trial.
Buzdar AU, Smith R, Vogel C, Bonomi P, Keller AM, Favis G, et al. Fadrozole HCL (CGS-16949A) versus megestrol acetate treatment of postmenopausal patients with metastatic breast carcinoma: results of two randomized double blind controlled multiinstitutional trials.
Bezwoda WR, Gudgeon A, Falkson G, Jordaan JP, Goedhals L. Fadrozole versus megestrol acetate: a double-blind randomised trial in advanced breast cancer.
Goss PE, Winer EP, Tannock IF, Schwartz LH. Randomized phase III trial comparing the new potent and selective third-generation aromatase inhibitor vorozole with megestrol acetate in postmenopausal advanced breast cancer patients. North American Vorozole Study Group.
Freue M, Kjaer M, Boni C, Joliver J, Janicke F, Willemse PH, et al. Open comparative trial of formestane versus megestrol acetate in postmenopausal patients with advanced breast cancer previously treated with tamoxifen.
Dombernowsky P, Smith I, Falkson G, Leonard R, Panasci L, Bellmunt J, et al. Letrozole, a new oral aromatase inhibitor for advanced breast cancer: double-blind randomized trial showing a dose effect and improved efficacy and tolerability compared with megestrol acetate.
Buzdar A, Douma J, Davidson N, Elledge R, Morgan M, Smith R, et al. Phase III, multicenter, double-blind, randomized study of letrozole, an aromatase inhibitor, for advanced breast cancer versus megestrol acetate.
Buzdar AU, Jonat W, Howell A, Jones SE, Blomqvist CP, Vogel CL, et al. Anastrozole versus megestrol acetate in the treatment of postmenopausal women with advanced breast carcinoma: results of a survival update based on a combined analysis of data from two mature phase III trials. Arimidex Study Group.
Kaufmann M, Bajetta E, Dirix LY, Fein LE, Jones SE, Zilembo N, et al. Exemestane is superior to megestrol acetate after tamoxifen failure in postmenopausal women with advanced breast cancer: results of a phase III randomized double-blind trial. The Exemestane Study Group.
Lipton A, Harvey HA, Santen RJ, Boucher A, White D, Bernath A, et al. Randomized trial of aminoglutethimide versus tamoxifen in metastatic breast cancer.
Samonis G, Margioris AN, Bafaloukos D, Razis DV. Prospective randomized study of aminoglutethimide (AG) versus medroxyprogesterone acetate (MPA) versus AG+MPA in generalized breast cancer.
Alonso-Munoz MC, Ojeda-Gonzalez MB, Beltran-Fabregat M, Dorca-Ribugent J, Lopez-Lopez L, Borras-Balada J, et al. Randomized trial of tamoxifen versus aminoglutethimide and versus combined tamoxifen and aminoglutethimide in advanced postmenopausal breast cancer.
Paridaens R, Dirix L, Lohrisch C, Beex L, Nooij M, Cameron D, et al. Mature results of a randomized phase II multicenter study of exemestane versus tamoxifen as first-line hormone therapy for postmenopausal women with metastatic breast cancer.
Thurlimann B, Castiglione M, Hsu-Schmitz SF, Cavalli F, Bonnefoi H, Fey MF, et al. Formestane versus megestrol acetate in postmenopausal breast cancer patients after failure of tamoxifen: a phase III prospective randomised cross over trial of second-line hormonal treatment (SAKK 20/90). Swiss Group for Clinical Cancer Research (SAKK).
Hultborn R, Johansson-Terje I, Bergh J, Glas U, Hallsten L, Hatschek T, et al. Second-line endocrine treatment of advanced breast cancer—a randomized cross-over study of medroxy-progesterone acetate and aminoglutethimide.
Carlini P, Bria E, Giannarelli D, Ferretti G, Felici A, Papaldo P, et al. New aromatase inhibitors as second-line endocrine therapy in postmenopausal patients with metastatic breast carcinoma: a pooled analysis of the randomized trials.
Cochrane Handbook.The Cochrane Handbook for Systematic Reviews of Interventions. Available at http://www.cochrane.org/resources/handbook/handbook.pdf . [Last assessed: March 20, 2006.]
Montori VM, Devereaux PJ, Adhikari NK, Burns KE, Eggert CH, Briel M, et al. Randomized trials stopped early for benefit: a systematic review.
Lau J, Ioannidis JP, Schmid CH. Quantitative synthesis in systematic reviews.
Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis.
Ioannidis JP, Cappelleri JC, Lau J, Skolnik PR, Melville B, Chalmers TC, et al. Early or deferred zidovudine therapy in HIV-infected patients without an AIDS-defining illness.
Ioannidis JP, Lau J. Evolution of treatment effects over time: empirical insight from recursive cumulative metaanalyses.
Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias.
Kataja VV, Colleoni M, Bergh J; ESMO Guidelines Task Force. ESMO Minimum Clinical Recommendations for diagnosis, treatment and follow-up of locally recurrent or metastatic breast cancer (MBC).
Nabholtz JM, Buzdar A, Pollak M, Harwin W, Burton G, Mangalik A, et al. Anastrozole is superior to tamoxifen as first-line therapy for advanced breast cancer in postmenopausal women: results of a North American multicenter randomized trial. Arimidex Study Group.
Buzdar AU, Robertson JF, Eiermann W, Nabholtz JM. An overview of the pharmacology and pharmacokinetics of the newer generation aromatase inhibitors anastrozole, letrozole, and exemestane.
Deitcher SR, Gomes MP. The risk of venous thromboembolic disease associated with adjuvant hormone therapy for breast carcinoma: a systematic review.
Irish W, Sherrill B, Cole B, Gard C, Glendenning GA, Mouridsen H. Quality-adjusted survival in a crossover trial of letrozole versus tamoxifen in postmenopausal women with advanced breast cancer.
Ioannidis JP, Pavlidis N. Levels of absolute survival benefit for systemic therapies of advanced cancer: a call for standards.
Trikalinos TA, Ioannidis JP. Predictive modeling and heterogeneity of baseline risk in meta-analysis of individual patient data.
Stewart LA, Tierney JF. To IPD or not to IPD? Advantages and disadvantages of systematic reviews using individual patient data.