-
PDF
- Split View
-
Views
-
Cite
Cite
Peggy L. Porter, William E. Barlow, I-Tien Yeh, Ming Gang Lin, Xiaopu P. Yuan, Elizabeth Donato, George W. Sledge, Charles L. Shapiro, James N. Ingle, Charles M. Haskell, Kathy S. Albain, James M. Roberts, Robert B. Livingston, Daniel F. Hayes, p27 Kip1 and Cyclin E Expression and Breast Cancer Survival After Treatment With Adjuvant Chemotherapy , JNCI: Journal of the National Cancer Institute, Volume 98, Issue 23, 6 December 2006, Pages 1723–1731, https://doi.org/10.1093/jnci/djj467
Close - Share Icon Share
Abstract
Background: Abnormal expression of the cell cycle regulatory proteins p27 Kip1 (p27) and cyclin E may be associated with breast cancer survival and relapse. We studied these markers in a clinical trial setting with patients with breast cancer treated by a uniform drug regimen so that treatment was not associated with variability in outcome. Methods: We used tissue microarrays to evaluate the expression of p27 and cyclin E proteins by immunohistochemistry in tumor tissue from 2123 (68%) of 3122 patients with moderate-risk primary breast cancer who were enrolled in Southwest Oncology Group–Intergroup Trial S9313, in which patients were assigned to receive doxorubicin and cyclophosphamide administered concurrently (n = 1595) or sequentially (n = 1527). Disease-free and overall survival were equivalent in the two arms. Expression of the proteins was rated on a scale of 1–7, and the median value was used as the cut point. Log-rank tests and Cox regression analyses were used to assess associations with survival. Overall survival was defined as time to death from all causes; disease-free survival was defined as time to recurrence or death. All P values were from two-sided statistical tests. Results: Lower p27 expression was associated with worse overall survival (unadjusted hazard ratio [HR] = 1.50, 95% confidence interval [CI] = 1.21 to 1.86) and disease-free survival (unadjusted HR = 1.31, 95% CI = 1.10 to 1.57) than higher p27 expression. Among hormone receptor–positive patients, lower p27 expression was associated with worse overall survival (HR = 1.42, 95% CI = 1.05 to 1.94) and worse disease-free survival (HR = 1.27, 95% CI = 0.99 to 1.63) than higher p27 expression after adjustment for treatment, menopausal status, tumor size, and number of positive lymph nodes. Among these patients, 5-year overall survival associated with higher p27 expression (0.91, 95% CI = 0.89 to 0.93) was similar to that associated with lower p27 expression (0.85, 95% CI = 0.82 to 0.87). No association between p27 expression and survival was found in hormone receptor–negative patients. Cyclin E expression was not statistically significantly associated with overall survival (HR = 1.12, 95% CI = 0.91 to 1.38) or disease-free survival (HR = 1.09, 95% CI = 0.92 to 1.29). Conclusions: Low p27 expression appears to be associated with poor prognosis, especially among patients with steroid receptor–positive tumors.
Mutations in genes that regulate the cell cycle are found in most human cancers ( 1 ) . In addition, there are other cell cycle genes, including those encoding cyclin E and p27 Kip1 (p27), that contribute to tumor progression but are rarely mutated ( 2 – 8 ) . Cyclin E and p27 proteins are key regulators of the G 1 - to S-phase transition in the cell cycle. p27 is a member of the Cip/Kip family of cyclin-dependent kinase inhibitors, it is present in high levels in quiescent cells, but levels decline when cells proliferate in response to mitogenic signals, such as growth factors and cytokines ( 9 , 10 ) . Cyclin E protein is expressed in mid- to late-G 1 phase; along with its catalytic subunit, cyclin-dependent kinase 2, cyclin E stimulates cells to enter S phase ( 11 , 12 ) . Expression of these proteins is controlled largely by posttranslational mechanisms, indicating that the levels of p27 and cyclin E proteins in tumor cells may be the most accurate measure of their involvement in tumor progression ( 13 , 14 ) .
Data from several population-based and clinical studies indicate that abnormal expression of p27 and cyclin E protein, as measured immunohistochemically, is associated with poor clinical outcome in many human cancers, including breast cancer ( 15 – 19 ) . In a series of patients, overexpression of low–molecular weight forms of cyclin E, as measured by western blot analysis, was related to an almost 13-fold increase in mortality from breast cancer ( 20 ) . However, few of these studies accounted for systemic treatment of the patients. The prognostic effects of cyclin E and p27 could depend on how patients were treated because it is likely that the products of these genes may affect the patients' response to various therapeutic agents ( 21 – 24 ). Thus, the relationship of cyclin E and p27 expression and survival should be tested in patients with breast cancer who were treated similarly, for example, in a prospective clinical trial.
In this study, we evaluated the association of p27 and cyclin E protein expression with disease-free and overall survival in patients with moderate-risk breast cancer who were enrolled in an Intergroup randomized clinical trial of doxorubicin and cyclophosphamide ( 25 , 26 ) (protocol S9313, “Phase III Comparison of Adjuvant Chemotherapy with High-Dose Cyclophosphamide plus Doxorubicin versus Sequential Doxorubicin followed by Cyclophosphamide in High-Risk Breast Cancer Patients with 0–3 Positive Nodes”).
P ATIENTS AND M ETHODS
Patient Population
Patients enrolled in the Southwest Oncology Group (SWOG)–Intergroup (Eastern Cooperative Group [ECOG], North Central Cancer Treatment Group [NCCTG], and Cancer and Leukemia Group B [CALGB]) trial S9313 between April 1, 1994, and May 31, 1997, were eligible for testing and analysis in this study. The protocol compared disease-free survival and overall survival in 3122 women with moderate-risk primary breast cancer who received equivalent doses of either concurrent adjuvant high-dose chemotherapy with doxorubicin plus cyclophosphamide (n = 1595) or high-dose, sequential chemotherapy with doxorubicin followed by cyclophosphamide (n = 1527) for an 18-week period. Patients were eligible for treatment if they had tumors that were estrogen receptor (ER) negative and progesterone receptor (PR) negative and greater than 1 cm in diameter, greater than 2 cm regardless of hormone receptor status, or lymph node positive with fewer than four positive axillary lymph nodes. Five years of tamoxifen treatment was prescribed for all postmenopausal women and all hormone-responsive premenopausal women after chemotherapy. Subjects ranged in age from 21 to 76 years (median age = 47 years).
Patients with ductal carcinoma in situ or lobular carcinoma in situ in addition to invasive disease, metaplastic carcinoma, and bilateral synchronous tumors were eligible. As reported, the two arms of the study showed equivalent disease-free survival and overall survival ( 25 , 26 ) .
Demographic data included age, race, and menopausal status. Tumor data included stage, size, lymph node status (0, 1, 2, or 3 positive lymph nodes), ER status, and PR status. ER status and PR status were determined by methods and standards used by the local institutions and were not centrally reviewed. Median length of follow-up was 7 years. All patients provided written informed consent to participate in the clinical trial. Tissue blocks were collected prospectively concurrently with enrollment to the trial with patient consent. Permission to perform the studies described in this report was provided by the Fred Hutchinson Cancer Research Center Institutional Review Board.
Tissue Specimens and Tissue Microarray
Tumor tissue samples that were available and adequate for our experiments were provided by the SWOG, ECOG, CALBG, and NCCTG trial groups for 2123 (68.0%) of the 3122 women enrolled in the trial. These tissue samples were incorporated into tissue microarrays in Seattle.
For tissue microarray construction and immunohistochemistry testing, a histology slide from each subject's archival tumor block was reviewed to identify and mark the location of tumor and normal components. The markings were transferred by video merged to the corresponding tissue block. Marked donor blocks were cored into the recipient master block according to a grid map with 0.8-mm spacing from the center of one core to the center of the next core by use of an automated Beecher ATA 27 Tissue Arrayer (Beecher Instruments, Inc, Silver Spring, MD).
The 28 master tissue microarray blocks constructed from the study tumors incorporated up to three tumor and three normal cores (each 0.6 mm in diameter) from an archival block for each subject. Three tumor cores were obtained from 99% of the tumors. Cores were arrayed into grids of up to 480 cores in master blocks with three cores of normal lymph node tissue placed in the upper-left quadrant of the grid for orientation and as controls for the immunohistochemistry assay. Cores from each tumor were placed in the same master block. After construction, tissue microarray blocks were sealed with paraffin and stored at 4 °C in a low-oxygen cabinet filled with N 2 gas until sectioning. No more than 48 hours before immunohistochemistry testing, sections (5 μm thick) were cut from the tissue microarray master blocks, mounted on superfrost slides, and assayed for cyclin E [anti-cyclin E polyclonal antibody; Roberts laboratory, Fred Hutchinson Cancer Research Center ( 27 ) ] and for p27 (anti-p27 monclonal antibody; Neomarkers, Fremont, CA) expression as previously described ( 18 ) .
For tissue microarray image and data capture, sections of the array blocks stained with hematoxylin–eosin and by immunohistochemistry were scanned, and images were generated with the Bliss Imaging System (Bacus Laboratories, Inc, Lombard, IL), which is a Web-based platform for image acquisition. The x and y coordinates of each core within the grid were determined by the software and included as part of the unique identifier, which was linked to the SWOG–Intergroup clinical database for the S9313 trial.
Data were collected and verified from the digitized images by the study pathologists, all of whom were blinded to patient and other tumor characteristics. Expression of p27 and cyclin E proteins was scored by the staining intensity and the percentage of positive tumor cells on a scale of 1–7, as follows: 1 = −; 2 = −/+; 3 = +; 4 = +/++; 5 = ++; 6 = ++/+++; 7 = +++. Two pathologists (XPY and MGL) independently (and blinded to each other's scores) scored every core on the tissue microarray slides (6360 cores for cyclin E and 6360 cores for p27). Data were collectable (spots with tumor tissue present) for an average of 2.5 spots per tumor specimen (85% of tumors had at least three spots with data and only 4% of tumors had only one spot). The two primary pathologists' scores were in agreement for 87% of the cores on the cyclin E slides and 91% of the cores on the p27 slides. Those scores that were discrepant between the primary pathologists were reviewed (not blinded to the original scores) and resolved by a third pathologist (PLP).
To assign a composite score for each tumor, the values (1 through 7) for each independently scored core for that tumor were compared. If all the scores were in agreement, then that score became the composite score. If the majority of the scores were in agreement and were the highest score, that score became the composite score. If there was no majority score or if the majority was not the highest score, the cores were reviewed by a study pathologist (PLP), and a composite score was subjectively assigned that was representative of the tumor's highest staining.
Statistical Analysis
Disease-free survival was defined as the time to first recurrence (local, regional, or distant), new primary cancer in the contralateral breast, or death due to any cause. Overall survival was defined as the time to death from any cause. Patients were censored on the date of last contact if a treatment failure event had not been observed. Unadjusted survival was assessed by the Kaplan–Meier method (see Figs. 1 , 2 , and 3 ). Cox regression analysis was used to estimate hazard ratios (HRs) and their 95% confidence intervals (CIs). Analyses included unadjusted analyses of cyclin E and p27 expression; analyses adjusted for treatment assignment and prespecified stratifying variables (tumor size [<2, 2–5, or >5 cm], number of positive lymph nodes [0, 1, 2, or 3], and menopausal status); and additional analyses adjusted for hormone receptor status, which was determined post hoc to be an important predictor. It should be noted that, although we do not routinely test to confirm that the proportional hazards assumptions hold for our data, we assess graphically whether the relationships hold over the entire time studied.
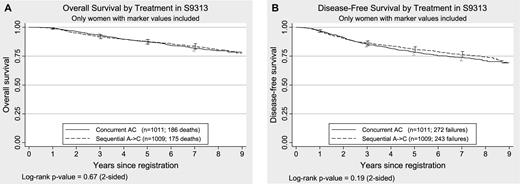
Kaplan–Meier plots of overall survival and disease-free survival by treatment assignment in 2020 women enrolled in S9313 and tested for this study. A ) Overall survival. Overall survival was defined as the time to death from any cause. B ) Disease-free survival. Disease-free survival was defined as the time to first recurrence (local, regional, or distant), new primary cancer in the contralateral breast, or death due to any cause. All statistical tests were two-sided. The 95% confidence intervals are shown at 1, 3, 5, and 7 years for both treatment groups. The numbers of patients at risk for death due to any cause (overall survival) at 0, 1, 3, 5, and 7 years were 2020, 1995, 1833, 1706, and 1037, respectively. The numbers of patients at risk for death or recurrence (disease-free survival) at 0, 1, 3, 5, and 7 years were 2020, 1951, 1699, 1551, and 940, respectively, for the entire sample.
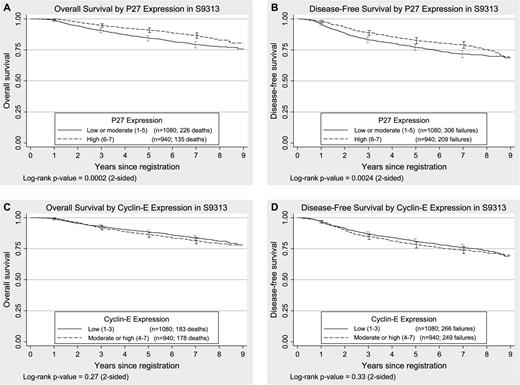
Kaplan–Meier plots of overall survival and disease-free survival by p27 Kip1 (p27) and cyclin E expression in tumors from women enrolled in S9313. A and B ) p27 expression. C and D ) Cyclin E expression. Overall survival was defined as the time to death from any cause. Disease-free survival was defined as the time to first recurrence (local, regional, or distant), new primary cancer in the contralateral breast, or death due to any cause. All statistical tests were two-sided. The 95% confidence intervals are shown at 1, 3, 5, and 7 years for both treatment groups. The numbers of patients at risk for death due to any cause (overall survival) at 0, 1, 3, 5, and 7 years were 2020, 1995, 1833, 1706, and 1037, respectively. The numbers of patients at risk for death or recurrence (disease-free survival) at 0, 1, 3, 5, and 7 years were 2020, 1951, 1699, 1551, and 940, respectively, for the entire sample.
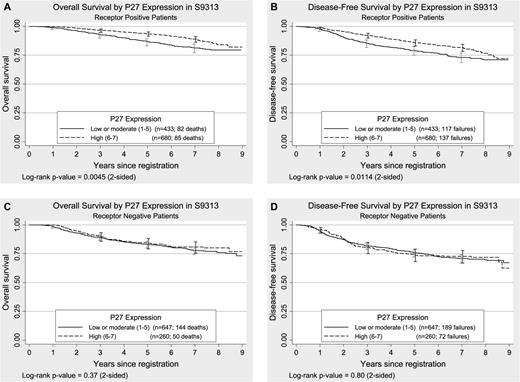
Kaplan–Meier plots of overall survival and disease-free survival by p27 Kip1 (p27) expression in steroid receptor–positive and steroid receptor–negative tumors from women enrolled in S9313. A and B ) p27 expression in steroid receptor–positive tumors. C and D ) p27 expression in steroid receptor–negative tumors. Overall survival was defined as the time to death from any cause. Disease-free survival was defined as the time to first recurrence (local, regional, or distant), new primary cancer in the contralateral breast, or death due to any cause. All statistical tests were two-sided. The 95% confidence intervals are shown at 1, 3, 5, and 7 years for both treatment groups. The numbers of patients at risk for death due to any cause (overall survival) at 0, 1, 3, 5, or 7 years were 2020, 1995, 1833, 1706, and 1037, respectively. The numbers of patients at risk for death or recurrence (disease-free survival) at 0, 1, 3, 5, or 7 years were 2020, 1951, 1699, 1551, and 940, respectively, for the entire sample.
All reported P values and confidence intervals are from two-sided tests. Statistical testing was done with different cutoffs as described below to determine the sensitivity of the results to the choice of cut point. Because well-established and replicated cutoffs for the expression status of these proteins were not available, we made the a priori choice to analyze and report the scores dichotomized at their median (cyclin E divided as low [scores of 1–3] versus high [scores of 4–7]; p27 divided as low [scores of 1–5] and high [scores of 6 or 7]). We also did statistical testing with previously reported cutoffs for high cyclin E expression (scores of 5–7) and low or intermediate p27 expression (scores of 1–3 versus 4 or 5) ( 18 ) and with marker expression as an ordinal value ranging from 1 to 7. For p27 expression, the median cutoff corresponded to the previously reported cutoff between low or intermediate and high expression for p27 ( 18 ) . We report results by use of the dichotomized median value and comment on results obtained by use of other cut points.
R ESULTS
Of the 3122 patients enrolled in clinical trial S9313, cyclin E and p27 expression was evaluated in 2032 (65.1%) and 2031 (65.1%) patients, respectively, and the expression of both proteins was evaluated in 2020 (64.7%) patients. The expression of both cyclin E and p27 could also be evaluated in 2020 (95.1%) of the 2123 tumor cores in the tissue microarray. Using the median as the cut point resulted in approximately equal distributions of tumors with low and high expression of both proteins, as expected, with 46% of cyclin E values scored as 4–7 and with 46% of p27 values scored as 6 or 7.
Neither overall survival ( P = .67) nor disease-free survival ( P = .19) was statistically significantly different between the randomized arms by use of a log-rank test for subjects for whom both markers were available ( Fig. 1 ). However, participants for whom marker values were available were recruited earlier into the trial and tended to have slightly worse prognostic indicators (i.e., larger tumor size, older age, more positive lymph nodes) than those without marker values, and they were also more likely to be hormone receptor negative (data not shown). After adjustment for registration year, tumor size, number of positive lymph nodes, ER status, PR status, and menopausal status, disease-free survival did not statistically significantly differ between those with markers and those without (HR = 1.07, 95% CI = 0.92 to 1.24; P = .40), and overall survival was marginally, but not statistically significantly, different (HR = 1.20, 95% CI = 0.99 to 1.46; P = .062).
Relationship Between Cyclin E Expression and Survival
High expression of cyclin E protein was not statistically significantly associated with either overall survival ( P = .27) or disease-free survival ( P = .33) ( Fig. 2 ). In unadjusted models, high cyclin E expression was not associated with overall survival (HR = 1.12, 95% CI = 0.91 to 1.38) or with disease-free survival (HR = 1.09, 95% CI = 0.92 to 1.29) as compared with low cyclin E expression ( Table 1 ). After adjustment for tumor size (<2, 2–5, or >5 cm), number of positive lymph nodes (0, 1, 2, or 3 lymph nodes), menopausal status, and randomized treatment, cyclin E remained nonstatistically significantly associated with either type of survival. When we used the cut point between a score of 1–4 and a score of 5–7, as previously described ( 18 ) , the expression of cyclin E was not associated with either type of survival (data not shown). If cyclin E was analyzed as a continuous variable with values of 1–7 in unadjusted analyses, neither overall survival per unit increase of cyclin E (HR = 1.05, 95% CI = 0.98 to 1.12; P = .17) nor disease-free survival per unit increase of cyclin E (HR = 1.04, 95% CI = 0.98 to 1.10; P = .18) was statistically significant.
Cox regression analysis of disease-free and overall survival in relation to median p27 and cyclin E expression (n = 2020) *
| Marker . | Unadjusted HR (95% CI) . | Adjusted † HR (95% CI) . | Adjusted ‡ HR (95% CI) . | Adjusted/negative receptors § HR (95% CI) . | Adjusted/positive receptors § HR (95% CI) . | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Disease-free survival | ||||||||||
| p27 | 1.31 (1.10 to 1.57) | 1.32 (1.10 to 1.57) | 1.18 (0.98 to 1.42) | 1.06 (0.81 to 1.39) | 1.27 (0.99 to 1.63) | |||||
| Cyclin E | 1.09 (0.92 to 1.29) | 1.13 (0.95 to 1.34) | 1.02 (0.85 to 1.22) | 0.95 (0.74 to 1.21) | 1.13 (0.88 to 1.46) | |||||
| Overall survival | ||||||||||
| p27 | 1.50 (1.21 to 1.86) | 1.51 (1.22 to 1.87) | 1.31 (1.05 to 1.64) | 1.19 (0.86 to 1.64) | 1.42 (1.05 to 1.94) | |||||
| Cyclin E | 1.12 (0.91 to 1.38) | 1.17 (0.95 to 1.44) | 1.03 (0.83 to 1.27) | 0.87 (0.65 to 1.16) | 1.25 (0.92 to 1.71) | |||||
| Marker . | Unadjusted HR (95% CI) . | Adjusted † HR (95% CI) . | Adjusted ‡ HR (95% CI) . | Adjusted/negative receptors § HR (95% CI) . | Adjusted/positive receptors § HR (95% CI) . | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Disease-free survival | ||||||||||
| p27 | 1.31 (1.10 to 1.57) | 1.32 (1.10 to 1.57) | 1.18 (0.98 to 1.42) | 1.06 (0.81 to 1.39) | 1.27 (0.99 to 1.63) | |||||
| Cyclin E | 1.09 (0.92 to 1.29) | 1.13 (0.95 to 1.34) | 1.02 (0.85 to 1.22) | 0.95 (0.74 to 1.21) | 1.13 (0.88 to 1.46) | |||||
| Overall survival | ||||||||||
| p27 | 1.50 (1.21 to 1.86) | 1.51 (1.22 to 1.87) | 1.31 (1.05 to 1.64) | 1.19 (0.86 to 1.64) | 1.42 (1.05 to 1.94) | |||||
| Cyclin E | 1.12 (0.91 to 1.38) | 1.17 (0.95 to 1.44) | 1.03 (0.83 to 1.27) | 0.87 (0.65 to 1.16) | 1.25 (0.92 to 1.71) | |||||
p27 Kip1 = p27; HR = hazard ratio; CI = confidence interval. Overall survival was defined as the time to death from any cause. Disease-free survival was defined as the time to first recurrence (local, regional, or distant), new primary cancer in the contralateral breast, or death due to any cause.
Analyses were adjusted for tumor size (<2, 2–5, or >5 cm), number of positive lymph nodes (0, 1, 2, or 3), menopausal status, and randomized treatment but not receptor status.
Analyses were adjusted for tumor size (<2, 2–5, or >5 cm), number of positive lymph nodes (0, 1, 2, or 3), menopausal status, randomized treatment, and receptor status (both estrogen receptor and progesterone receptor negative versus either positive).
Subset analyses by receptor status were adjusted for tumor size, number of positive lymph nodes, menopausal status, and treatment.
Cox regression analysis of disease-free and overall survival in relation to median p27 and cyclin E expression (n = 2020) *
| Marker . | Unadjusted HR (95% CI) . | Adjusted † HR (95% CI) . | Adjusted ‡ HR (95% CI) . | Adjusted/negative receptors § HR (95% CI) . | Adjusted/positive receptors § HR (95% CI) . | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Disease-free survival | ||||||||||
| p27 | 1.31 (1.10 to 1.57) | 1.32 (1.10 to 1.57) | 1.18 (0.98 to 1.42) | 1.06 (0.81 to 1.39) | 1.27 (0.99 to 1.63) | |||||
| Cyclin E | 1.09 (0.92 to 1.29) | 1.13 (0.95 to 1.34) | 1.02 (0.85 to 1.22) | 0.95 (0.74 to 1.21) | 1.13 (0.88 to 1.46) | |||||
| Overall survival | ||||||||||
| p27 | 1.50 (1.21 to 1.86) | 1.51 (1.22 to 1.87) | 1.31 (1.05 to 1.64) | 1.19 (0.86 to 1.64) | 1.42 (1.05 to 1.94) | |||||
| Cyclin E | 1.12 (0.91 to 1.38) | 1.17 (0.95 to 1.44) | 1.03 (0.83 to 1.27) | 0.87 (0.65 to 1.16) | 1.25 (0.92 to 1.71) | |||||
| Marker . | Unadjusted HR (95% CI) . | Adjusted † HR (95% CI) . | Adjusted ‡ HR (95% CI) . | Adjusted/negative receptors § HR (95% CI) . | Adjusted/positive receptors § HR (95% CI) . | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Disease-free survival | ||||||||||
| p27 | 1.31 (1.10 to 1.57) | 1.32 (1.10 to 1.57) | 1.18 (0.98 to 1.42) | 1.06 (0.81 to 1.39) | 1.27 (0.99 to 1.63) | |||||
| Cyclin E | 1.09 (0.92 to 1.29) | 1.13 (0.95 to 1.34) | 1.02 (0.85 to 1.22) | 0.95 (0.74 to 1.21) | 1.13 (0.88 to 1.46) | |||||
| Overall survival | ||||||||||
| p27 | 1.50 (1.21 to 1.86) | 1.51 (1.22 to 1.87) | 1.31 (1.05 to 1.64) | 1.19 (0.86 to 1.64) | 1.42 (1.05 to 1.94) | |||||
| Cyclin E | 1.12 (0.91 to 1.38) | 1.17 (0.95 to 1.44) | 1.03 (0.83 to 1.27) | 0.87 (0.65 to 1.16) | 1.25 (0.92 to 1.71) | |||||
p27 Kip1 = p27; HR = hazard ratio; CI = confidence interval. Overall survival was defined as the time to death from any cause. Disease-free survival was defined as the time to first recurrence (local, regional, or distant), new primary cancer in the contralateral breast, or death due to any cause.
Analyses were adjusted for tumor size (<2, 2–5, or >5 cm), number of positive lymph nodes (0, 1, 2, or 3), menopausal status, and randomized treatment but not receptor status.
Analyses were adjusted for tumor size (<2, 2–5, or >5 cm), number of positive lymph nodes (0, 1, 2, or 3), menopausal status, randomized treatment, and receptor status (both estrogen receptor and progesterone receptor negative versus either positive).
Subset analyses by receptor status were adjusted for tumor size, number of positive lymph nodes, menopausal status, and treatment.
Relationship Between p27 Expression and Survival
The expression of p27 protein was statistically significantly associated with overall survival ( P <.001) and with disease-free survival ( P = .002) ( Fig. 2 ), with lower expression associated with poorer survival. In unadjusted models, lower levels of p27 were associated with worse overall survival (HR = 1.50, 95% CI = 1.21 to 1.86) and worse disease-free survival (HR = 1.31, 95% CI = 1.10 to 1.57) than higher levels. After adjustment for tumor size, number of positive lymph nodes, menopausal status, and treatment, lower p27 expression remained statistically significantly associated with worse overall survival (HR = 1.51, 95% CI = 1.22 to 1.87) and worse disease-free survival (HR = 1.32, 95% CI = 1.10 to 1.57). When p27 expression was used as a continuous variable with values of 1–7, the strength of the statistically significant association between p27 expression and survival decreased per unit decrease of p27 expression for overall survival (for each unit decrease in p27 expression, HR = 1.11, 95% CI = 1.04 to 1.17; P = .001) and for disease-free survival (for each unit decrease in p27 expression, HR = 1.08, 95% CI = 1.02 to 1.13; P = .004) (data not shown).
Relationship to Cell Cycle Protein Expression and Steroid Receptor Status
There was strong association between both p27 and cyclin E expression and ER and/or PR status. High cyclin E expression was associated with negative ER status and negative PR status (both P <.001), and high p27 expression was associated with positive ER status and positive PR status (both P <.001). These relationships suggested that adjusting for ER and/or PR status might distinguish between the effects of cyclin E and p27 expression. Because ER and PR expression is highly associated (χ 2 [1 df] = 985.9, P <.001), we carried out an unplanned subset analysis in which we divided the patients with both markers into a receptor-positive group (i.e., ER-positive and/or PR-positive tumors; n = 1113) and a receptor-negative group (i.e., ER-negative and PR-negative tumors; n = 907) and analyzed cyclin E and p27 expression separately by receptor status.
Among receptor-positive patients, p27 expression was highly statistically significantly associated with overall survival ( P = .005) and with disease-free survival ( P = .011) ( Fig. 3 ). Among patients with receptor-positive tumors, lower p27 expression was associated with poorer overall survival (HR = 1.42, 95% CI = 1.05 to 1.94) and with poorer disease-free survival (HR = 1.27, 95% CI = 0.99 to 1.63) than higher p27 expression after adjustment for tumor size, number of positive lymph nodes, menopausal status, and treatment ( Table 1 ). Among these patients, 5-year overall survival associated with higher p27 expression (0.91, 95% CI = 0.89 to 0.93) was similar to that associated with lower p27 expression (0.85, 95% CI = 0.82 to 0.87). Among patients with receptor-negative tumors, no association was observed between p27 expression and either overall survival (HR = 1.19, 95% CI = 0.86 to 1.64; P = .37) or disease-free survival (HR = 1.06, 95% CI = 0.81 to 1.39; P = .80) ( Table 1 and Fig. 3 ). Despite the apparently different outcomes among groups with different receptor status according to p27 expression, the interaction of p27 and receptor status was not statistically significant for overall survival ( P = .44) or for disease-free survival ( P = .29).
Cyclin E expression was not statistically significantly associated with either overall survival or disease-free survival among either receptor-negative or receptor-positive groups ( Table 1 ). There was a slight difference in the effect of cyclin E expression on survival between those with receptor-positive tumors and those with receptor-negative tumors, although the interactions were not statistically significant for overall survival ( P = .076) or for disease-free survival ( P = .27). High cyclin E expression was associated with somewhat poorer overall survival among patients with receptor-positive tumors (HR = 1.25, 95% CI = 0.92 to 1.71) than among those with receptor-negative tumors and with better overall survival among patients with receptor-negative tumors (HR = 0.87, 95% CI = 0.65 to 1.16) than among those with receptor-positive tumors, although neither finding was statistically significant. High cyclin E expression was associated with slightly poorer disease-free survival for patients with receptor-positive tumors than low cyclin E expression (HR = 1.13, 95% CI = 0.88 to 1.46) but not for those with receptor-negative tumors (HR = 0.95, 95% CI = 0.74 to 1.21), although neither association was statistically significant.
D ISCUSSION
Among patients with breast cancer treated with essentially uniform anthracycline-based adjuvant chemotherapy, we found an association between low levels of p27 protein expression and poor clinical outcome. These findings are in keeping with those of most previous studies ( 16 , 18 , 19 , 28 – 30 ) that showed decreased overall or disease-free survival among patients whose tumors lack p27 expression, even though these studies differed in the age of the specimens, the antibodies used, the interpretation of results, and the composition of the patient populations. In some studies, p27 expression was not an independent prognostic marker in multivariable analyses ( 28 , 29 , 31 ) or did not maintain statistical significance after long-term follow-up ( 29 ) . The association between reduced p27 and poor clinical outcome has been found among breast cancer subgroups, including young patients ( 18 ) , Chinese and Japanese patients ( 30 , 32 ) , patients with inherited BRCA mutations ( 33 ) , and patients with lymph node–negative disease ( 16 , 18 , 31 ) . In all these studies ( 16 , 18 , 30 – 33 ) , the most important limitation in assessing prognostic value was the lack of uniform treatment.
Two studies other than this study evaluated the expression of p27 retrospectively in a treatment trial setting: the Austrian Breast and Colorectal Cancer Study Group (ABCSG) (patients were diagnosed from 1990 through 1999) ( 21 ) and the International Breast Cancer Study Group (IBCSG) (patients were diagnosed from 1981 through 1985) ( 22 ) . In the ABCSG study, high levels of p27 expression were associated with improved survival in patients treated with tamoxifen or with combined hormonal therapy, including goserelin ( 21 ) . In the older IBCSG trial, no association between p27 expression and survival was observed except in the group of lymph node–negative patients who were given one course of perioperative cyclophosphamide, methotrexate, and 5-fluorouracil (CMF), where low p27 expression and c-erbB-2 overexpression was associated with better survival ( 22 ) . In the IBCSG trial, age of the specimens, differences in treatment regimens, and low availability of tumors for testing (18% of the subjects enrolled in the trial) may have contributed to the difference between results in that study compared with those in other studies that report worse prognosis associated with reduced p27 ( 16 , 18 , 19 , 28 – 30 ) .
In addition to being associated with poor survival, low levels of p27 expression have been associated with factors that predict poor survival, including high tumor grade ( 18 , 19 , 28 , 31 ) , elevated cyclin E expression ( 18 ) , overexpression of c-erbB-2 ( 22 , 34 ) , and a low or negative ER status ( 19 , 22 , 28 , 31 ) . Our findings strongly support an association between p27 expression and low or negative steroid receptor expression, and our unplanned subset analyses by ER and PR status found a differential association of p27 expression with survival between steroid receptor–positive and steroid receptor–negative groups. This observation is in keeping with in vitro studies that indicate estrogen receptor signaling modulates the p27 inhibition of cell cycle progression ( 24 ) . For example, in the ER-positive MCF-7 breast cancer cell line, estrogen stimulates cell cycle progression, in part, through the decreased expression of cell cycle inhibitors p27 and p21, whereas blockade by pure estrogen receptor antagonists results in elevated levels of p27 and cell cycle arrest through binding with cyclin E complexes ( 24 ) . There is evidence that p27 is essential for cell cycle arrest by tamoxifen ( 24 , 35 ) ; in the ABCSG trial, high p27 expression was independently associated with better disease-free survival and better overall survival among patients treated with hormone therapy than among those treated with CMF chemotherapy ( 21 ) . In another small study ( 36 ) , low p27 expression was one of a panel of markers that was associated with poor survival in tamoxifen-treated patients but not in patients treated with surgery alone. The S9313 trial was not designed to address treatment response or resistance to tamoxifen. However, as part of the trial, 5 years of tamoxifen treatment was prescribed for all postmenopausal women and all hormone-responsive premenopausal women after chemotherapy. Our data in patients with receptor-positive tumors, therefore, indicated that p27 expression was at least prognostic and might also be predictive of response to hormonal therapy.
Tumors from the S9313 trial should be evaluated quantitatively for the expression and amplification of the estrogen receptor, the progesterone receptor, and c-erbB-2. With this information, we should be able to determine the relationships among these related molecules and breast cancer. For example, results of several studies ( 37 – 39 ) have supported an association between c-erbB-2 overexpression and/or amplification and anthracycline-based adjuvant therapy for breast cancer. However, c-erbB-2 overexpression alone did not predict prognosis in tamoxifen-treated patients who received cyclophosphamide, doxorubicin, and fluorouracil adjuvant therapy in a study reported by the CALGB ( 40 ) . In preclinical studies, amplification of c-erbB-2 results in the redistribution and decreased expression of p27 and the subsequent deregulation of cell cycle progression ( 41 ) . This activity may limit the effect of tamoxifen, which requires the expression of p27 ( 24 ) . Low levels of p27 protein expression are associated with c-erbB-2 amplification and with chemotherapy response in lymph node–negative patients ( 22 , 34 ) . Recently, in vitro studies have demonstrated that the dual ErbB1 and ErbB2 inhibitor lapatinib can abrogate tamoxifen resistance ( 42 ) . With completion of c-erbB-2 assays on tumors from this trial, we should have the opportunity to assess the effect of c-erbB-2 amplification on the relationship of p27 and survival in patients who received doxorubicin-based adjuvant therapy, especially in the receptor-positive subgroup pf patients treated with tamoxifen.
In this group of patients treated with doxorubicin plus cyclophosphamide, overexpression of cyclin E was associated with a slightly higher risk of recurrence or death (although not statistically significantly so) among steroid receptor–positive patients but not overall or among receptor-negative patients. Support for the role of cyclin E in human tumor progression comes from its known activities in controlling both cell cycle progression and genome stability ( 27 , 43 , 44 ) and from studies that have directly measured protein expression in clinical tumor samples ( 18 , 20 , 45 , 46 ) . The most compelling evidence comes from a study of 395 women in which overexpression of low–molecular weight forms of cyclin E (measured by western blot analysis) was associated with a 13-fold increase in mortality from breast cancer ( 20 ) . Groups that have evaluated cyclin E expression by immunohistochemistry have failed to find such a dramatic effect on survival, except in some studies of BRCA mutation carriers ( 18 , 33 , 47 ) . The polyclonal antibody used in this study and in previous studies recognizes both the low–molecular weight and full-length forms of cyclin E (data not shown). However, the relative quantity of the forms cannot be determined by immunohistochemistry, and thus, our findings cannot be compared with findings that are based on western blot analysis. Because it is the low–molecular weight forms of cyclin E that appear to be more resistant to antiestrogen-induced cell cycle arrest in ER-positive MCF-7 cells ( 48 ) , and possibly to other agents, studies that can assess the relative contribution of full-length and low–molecular weight forms of cyclin E are required to determine the prognostic and predictive value of the protein. Additionally, the makeup of the group of patients under study may influence the prognostic value of cyclin E: in an immunohistochemistry study of cyclin E expression in tumors of BRCA mutation carriers, a statistically significant ninefold increased risk of mortality was associated with cyclin E overexpression ( 33 ) .
This study has several limitations. Although it consisted of the largest group of patients tested to date for p27 and cyclin E expression, we were limited by the tissue samples available and were only able to test 68% of the patients enrolled in the main treatment trial. The patients whose tumors were available for testing had worse prognostic factors and slightly worse overall survival (although not statistically significantly so), but equivalent disease-free survival, compared with patients whose markers were unavailable. The effect of treatment was the same in both groups, and it is likely that the estimated effect of p27 and cyclin E expression on survival was not biased by tissue availability. Our results have not yet been validated in other studies, so they still need to be confirmed before establishing p27 expression as a prognostic factor in patients treated with chemotherapy. Another limitation that decreases the generalizability of our results is the lack of centralized and uniform assessment of steroid receptors in this clinical trial.
In conclusion, we have shown that low p27 expression is associated with a worse prognosis in patients newly diagnosed with breast cancer who received doxorubicin plus cyclophosphamide adjuvant chemotherapy, especially among steroid receptor–positive, tamoxifen-treated patients. We have also validated the value of tissue microarray for testing promising markers of prognosis in the Cooperative Group setting. Additional studies are needed to determine the relationship of p27 and response to other agents and to assess its value in selecting patients for hormonal therapy. Future studies should help to elucidate the interaction of p27 and c-erbB-2 expression in relation to survival in patients treated with doxorubicin plus cyclophosphamide.
Drs P. L. Porter and J. M. Roberts are named on a patented technology (Dr Roberts is an inventor of it) that is related to assays described in this article. The patent is assigned to their employer, the Fred Hutchinson Cancer Research Center. Both could receive a portion of any income received by their employer from the license, sale, or transfer of this technology.
Supported by the following Public Health Service (PHS) Cooperative Agreement grant numbers awarded by the National Cancer Institute, Department of Health and Human Services: CA38926, CA32102, CA49883, CA21115, CA25224, CA31946, CA32291, CA37981, CA35431, CA45377, CA58416, CA22433, CA58686, CA46113, CA04919, CA46441, CA58861, CA46282, CA35261, CA27057, CA76132, CA35192, CA76447, CA76462, CA45450, CA76429, CA63845, CA12644, CA20319, CA63844, CA45560, CA58415, CA14028, CA58658, CA42777, CA35119, CA35090, CA35117, CA13612, CA16385, CA67575, CA68183, CA46368, CA04920, CA74647, CA52654; by the Breast Cancer Research Foundation, New York, NY; and by The Expedition Inspiration Fund for Breast Cancer Research, Ketchum, Idaho. The authors had full responsibility for the design of the study, the collection of the data, the analysis and interpretation of the data, the decision to submit the manuscript for publication, and the writing of the manuscript.
The authors would like to thank Ann Yoder and Kelly Wirtala for management and processing of the tissue specimens and Stephanie Stafford and Peter Lin for laboratory and TMA database development and support.
References
Pietenpol J, Bohlander S, Sato Y, Papadopoulos N, Liu B, Friedman C, et al. Assignment of the human p27Kip1 gene to 12p13 and its analysis in leukemias.
Ponce-Castaneda MV, Lee MH, Latres E, Polyak K, Lacombe L, Montgomery K, et al. p27Kip1: chromosomal mapping to 12p1212p13.1 and absence of mutations in human tumors.
Konstantin S, Simpson J, Takeuchi S, Kawamata N, Miller C, Koeffler H. p27/Kip1 mutation found in breast cancer.
Leach F, Ellredge S, Willson J, Markowitz S, Kinzler K, Vogelstein B. Amplification of cyclin genes in colorectal carcinomas.
Keyomarsi K, O'Leary N, Molnar G, Lees E, Fingert HJ, Pardee AB. Cyclin E, a potential prognostic marker for breast cancer.
Said T, Medina D. Cell cyclins and cyclin-dependent kinase activities in mouse mammary tumor development.
Fero ML, Rivkin M, Tasch M, Porter P, Carow CE, Firpo E, et al. A syndrome of multiorgan hyperplasia with features of gigantism, tumorigenesis, and female sterility in p27(Kip1)-deficient mice.
Nourse J, Firpo E, Flanagan M, Coats S, Polyak C, Lee M, et al. Interleukin-2-mediated elimination of p27Kip1 cyclin-dependent kinase inhibitor prevented by rapamycin.
Coats S, Flannagan W, Nourse J, Roberts J. Requirement of p27Kip1 for restriction point control of the fibroblast cell cycle.
Dulic V, Lees E, Reed S. Association of human cyclin E with a periodic G1-S phase protein kinase.
Koff A, Cross F, Fisher A, Schumacher J, Leguellec K, Philippe M, et al. Human cyclin E, a new cyclin that interacts with two members of the CDC2 gene family.
Clurman B, Sheaff R, Thress K, Groudine M, Roberts J. Turnover of cyclin E by the ubiquitin-proteosome pathway is regulated by CDK2 binding and cyclin phosphorylation.
Pagano M, Tam S, Theodoras A, Beer-Romero P, Del Sal G, Chau V, et al. Role of the ubiquitin-proteosome pathway in regulating abundance of the cyclin-dependent kinase inhibitor p27.
Tsihlias J, Kapusta LR, DeBoer G, Morava-Protzner I, Zbieranowski I, Bhattacharya N, et al. Loss of cyclin-dependent kinase inhibitor p27Kip1 is a novel prognostic factor in localized human prostate adenocarcinoma.
Tan P, Cady B, Wanner M, Worland P, Cukor B, Magi-Galluzzi C, et al. The cell cycle inhibitor p27 is an independent prognostic marker in small (T1a,b) invasive breast carcinomas.
Singh SP, Lipman J, Goldman H, Ellis FH Jr, Aizenman L, Cangi MG, et al. Loss or altered subcellular localization of p27 in Barrett's associated adenocarcinoma.
Porter PL, Malone KE, Heagerty PJ, Alexander GM, Gatti LA, Firpo EJ, et al. Expression of cell-cycle regulators p27Kip1 and cyclin E, alone and in combination, correlate with survival in young breast cancer patients.
Catzavelos C, Bhattacharya N, Ung YC, Wilson JA, Roncari L, Sandhu C, et al. Decreased levels of the cell-cycle inhibitor p27Kip1 protein: prognostic implications in primary breast cancer.
Keyomarsi K, Tucker S, Buchholz T, Callister M, Ding Y, Hortobagyi G, et al. Cyclin E and survival in patients with breast cancer.
Pohl G, Rudas M, Dietze O, Lax S, Markis E, Pirker R, et al. High p27Kip1 expression predicts superior relapse-free and overall survival for premenopausal women with early-stage breast cancer receiving adjuvant treatment with tamoxifen plus goserelin.
Spataro V, Litman H, Viale G, Maffini F, Masullo M, Golouh R, et al. Decreased immunoreactivity for p27 protein in patients with early-stage breast carcinoma is correlated with HER-2/neu overexpression and with benefit from one course of perioperative chemotherapy in patients with negative lymph node status: results from International Breast Cancer Study Group Trial V.
Le X-F, Pruefer F, Bast R. Her2-targeting antibodies modulate the cyclin-dependent kinase inhibitor p27 via multiple signaling pathways.
Cariou S, Donovan J, Flanagan W, Milic A, Bhattacharya N, Slingerland J. Down-regulation of p21WAF1/CIP1 or p27Kip1 abrogates antiestrogen-mediated cell cycle arrest in human breast cancer cells.
Haskell C. Phase III comparison of adjuvant high-dose doxorubicin plus cyclophosphamide (AC) versus sequential doxorubicin followed by cyclophosphamide (A->C) in breast cancer patients with 0-3 positive nodes (intergroup 0137).
Linden H, Haskell C, Green S, Osborne C, Sledge GJS, Shapiro CL, et al. Sequenced vs. simultaneous anthracycline and cyclophosphamide in high risk stage I–II breast cancer: final analysis from INT-0137 (S9313). J Clin Oncol. In press
Ohtsubo M, Theodoras A, Schumacher J, Roberts J, Pagano M. Human cyclin E, a nuclear protein essential for the G1-to-S phase transition.
Barnes A, Pinder S, Bell J, Paish E, Wencyk P, Robertson J, et al. Expression of p27kip1 in breast cancer and its prognostic significance.
Leivonen M, Nordling S, Lundin J, von Boguslawski K, Haglund C. p27 expression correlates with short-term, but not with long-term prognosis in breast cancer.
Tsuchiya A, Zhang G, Kanno M. Prognostic impact of cyclin-dependent kinase inhibitor p27kip1 in node-positive breast cancer.
Barbareschi M, van Tinteren H, Mauri F, Veronese S, Peterse H, Maisonneuve P, et al. p27kip1 expression in breast carcinomas: an immunohistochemical study on 512 patients with long-term follow-up.
Wu J, Shen Z, Shao Z. Prognostic significance of cyclin-dependent kinase inhibitor p27kip1 expression in human breast cancer.
Chappuis P, Donato E, Goffin J, Wong N, Begin L, Kapusta L, et al. Cyclin E expression in breast cancer: predicting germline BRCA1 mutations, prognosis and response to treatment.
Newman L, Xia W, Yang H, Sahin A, Bondy M, Lukmanji F, et al. Correlation of p27 protein expression with HER-2/neu expression in breast cancer.
Donovan J, Milic A, Slingerland J. Constitutive MEK/MAPK activation leads to p27(Kip1) deregulation and antiestrogen resistance in human breast cancer cells.
McCallum M, Baker C, Gillespie K, Cohen B, Stewart H, Leonard R, et al. A prognostic index for operable, node-negative breast cancer.
Thor AD, Berry DA, Budman DR, Muss HB, Kute T, Henderson IC, et al. erbB-2, p53, and efficacy of adjuvant therapy in lymph node-positive breast cancer.
Yamauchi H, Stearns V, Hayes D. The role of c-erbB-2 as a predictive factor in breast cancer.
Dressler L, Berry D, Broadwater G, Cowan D, Cox K, Griffin S, et al. Comparison of HER2 status by fluorescence in situ hybridization and immunohistochemistry to predict benefit from dose escalation of adjuvant doxorubicin-based therapy in node-positive breast cancer patients.
Berry D, Muss H, Thor A, Dressler L, Liu E, Broadwater G, et al. HER-2/neu and p53 expression versus tamoxifen resistance in estrogen receptor-positive, node-positive breast cancer.
Lane H, Beuvink I, Motoyama A, Daly J, Neve R, Hynes N. ErbB2 potentiates breast tumor proliferation through modulation of p27(Kip1)-Cdk2 complex formation: receptor overexpression does not determine growth dependency.
Chu I, Blackwell K, Chen S, Slingerland J. The dual ErbB1/ErbB2 inhibitor, lapatinib (GW572016), cooperates with tamoxifen to inhibit both cell proliferation- and estrogen-dependent gene expression in antiestrogen-resistant breast cancer.
Spruck C, Won K, Reed S. Deregulated cyclin E induces chromosome instability.
Loeb K, Kostner H, Firpo E, Norwood T, Tsuchiya K, Clurman B, et al. A mouse model for cyclin E-dependent genetic instability and tumorigenesis.
Nielsen N, Arnerlov C, Emdin S, Landberg G. Cyclin E overexpression, a negative prognostic factor in breast cancer with strong correlation to oestrogen receptor status.
Dutta A, Chandra R, Leiter LM, Lester S. Cyclins as markers of tumor proliferation: immunocytochemical studies in breast cancer.
Rudolph P, Kuhling H, Alm P, Ferno M, Baldetorp B, Olsson H, et al. Differential prognostic impact of the cyclins E and B in premenopausal and postmenopausal women with lymph node-negative breast cancer.