-
PDF
- Split View
-
Views
-
Cite
Cite
Stefan B. Eichmüller, Wolfram Osen, Ofer Mandelboim, Barbara Seliger, Immune Modulatory microRNAs Involved in Tumor Attack and Tumor Immune Escape, JNCI: Journal of the National Cancer Institute, Volume 109, Issue 10, October 2017, djx034, https://doi.org/10.1093/jnci/djx034
Close - Share Icon Share
Abstract
Current therapies against cancer utilize the patient’s immune system for tumor eradication. However, tumor cells can evade immune surveillance of CD8+ T and/or natural killer (NK) cells by various strategies. These include the aberrant expression of human leukocyte antigen (HLA) class I antigens, co-inhibitory or costimulatory molecules, and components of the interferon (IFN) signal transduction pathway. In addition, alterations of the tumor microenvironment could interfere with efficient antitumor immune responses by downregulating or inhibiting the frequency and/or functional activity of immune effector cells and professional antigen-presenting cells. Recently, microRNAs (miRNAs) have been identified as major players in the post-transcriptional regulation of gene expression, thereby controlling many physiological and also pathophysiological processes including neoplastic transformation. Indeed, the cellular miRNA expression pattern is frequently altered in many tumors of distinct origin, demonstrating the tumor suppressive or oncogenic potential of miRNAs. Furthermore, there is increasing evidence that miRNAs could also influence antitumor immune responses by affecting the expression of immune modulatory molecules in tumor and immune cells. Apart from their important role in tumor immune escape and altered tumor-host interaction, immune modulatory miRNAs often exert neoplastic properties, thus representing promising targets for future combined immunotherapy approaches. This review focuses on the characterization of miRNAs involved in the regulation of immune surveillance or immune escape of tumors and their potential use as diagnostic and prognostic biomarkers or as therapeutic targets.
Tumors have developed strategies to evade immune surveillance by cytotoxic T lymphocytes (CTL) and natural killer (NK) cells. These escape mechanisms could result from structural alterations or deregulated expression of genes/proteins in tumor cells and immune cells important for tumor cell recognition and killing, or by changes of the cellular components and physical factors within the tumor microenvironment (TME), leading to the induction of immune suppression. With the recent identification of small noncoding microRNAs (miRNAs) controlling the expression of immune modulatory molecules as well as the function of tumor and immune cell populations, the molecular mechanisms enabling tumors to escape from immune surveillance have gained even higher complexity. Here, we review the expression, function, and clinical relevance of immune modulatory miRNAs in the tumors and immune cells identified so far. In addition, their role in tumor-host interaction, cross-presentation, and neoplastic transformation, thereby altering antitumor immune responses, will be discussed. Elucidating the precise mechanisms of immune modulation by miRNAs is critical for an improved understanding of immune escape mechanisms, thus providing an opportunity for their use as prognostic biomarkers or as therapeutic targets to improve the prediction of patients’ clinical responses or to enhance the efficacy of immunotherapy approaches against cancer.
Immune Escape Strategies of Tumors
Tumor-mediated escape mechanisms include alterations in 1) the generation, processing, and presentation of T cell epitopes derived from tumor-associated antigens by human leukocyte antigen (HLA) class I and/or class II molecules, 2) signal transduction pathways, 3) the expression of costimulatory and co-inhibitory molecules, 4) the expression of apoptosis-related molecules, and 5) the secretion of immune-suppressive mediators as well as highly specialized double-membrane vesicles called exosomes, which play a crucial role in conditioning the local microenvironment and intercellular communication (1,2). The frequency of these escape mechanisms varies among different tumor (sub)types and is often correlated with worse prognosis and shorter survival of tumor patients.
The frequency and functional activity of effector cells and of professional antigen-presenting cells (APCs), such as dendritic cells (DCs), B cells, and macrophages, is often downregulated in the peripheral blood of tumor patients, while the number of immune suppressive myeloid-derived suppressor cells (MDSCs), NKT cells, regulatory T cells (Tregs), and tumor-associated macrophages (TAMs) is upregulated (3,4).
The TME orchestrated by T and B lymphocytes, TAMs, Tregs, MDSCs, DCs, and stromal cells, like cancer-associated fibroblasts (CAFs) and endothelial cells together with various soluble and physical factors, constitutes an immune-suppressive compartment. The extracellular matrix of the TME contains immune-suppressive cytokines, for example, interleukin (IL)-10 and transforming growth factor (TGF)–β, pro-angiogenic factors, chemokines, proteases, metabolites, arginase, and prostaglandins, creating a habitat that negatively interferes with antitumor immune responses (4–8).
Cancer-driven inflammation mediated by TAMs of the functional M2 type promote tumor angiogenesis, growth, metastasis, and immune suppression (9). In addition, MDSCs can stimulate tumor growth by enhancing angiogenesis or suppressing innate and adaptive immune responses including NK cell cytotoxicity, reducing priming activity of mature DCs, and suppressing T cell responses by induction of apoptosis, secretion of immune modulatory factors, modulation of amino acid metabolism, restriction of T cell homing, and induction of Tregs (10,11). CD4+CD25+FoxP3+ Tregs suppress the activity of immune cells, thereby maintaining immune tolerance to self-antigens, and effectively migrate to tumor sites (12). Soluble factors, like metabolites, but also hypoxia and low pH, could lead to reduced antitumor activity by impairing immune cell function.
Features of microRNAs in Tumors
miRNAs are small noncoding regulatory RNAs with a length of approximately 20 nucleotides, which are encoded by the genome. They have emerged as key players in the post-transcriptional control of gene expression by binding to the 3′ untranslated region (UTR) of targeted mRNA molecules, thereby affecting mRNA stability and/or translation. Based on bioinformatics analyses of their predictive binding profile, miRNAs are directly involved in the expression of at least 50% of all protein-coding genes in mammals (13,14). While an individual miRNA could target numerous cellular mRNAs, single mRNAs can be regulated by several miRNAs (15).
During the past years, high-throughput analyses have allowed the comparison of miRNA expression patterns between tumors and corresponding healthy tissues, demonstrating global changes within the miRNA expression profiles in different malignancies. Interestingly, miRNA genes were frequently located at fragile sites of the genome and in the vicinity of cancer-associated chromosomal regions (16). Deregulated biogenesis and expression of miRNAs is involved in the initiation and progression of tumors, metastasis formation, and therapy resistance, suggesting the existence of oncogenic and tumor-suppressive miRNAs (17–21). miRNAs have been shown to link inflammation with cancer (22) and have emerged as critical modulators of immune homeostasis and T cell immunity by controlling T cell activation, differentiation, and effector function (23), as well as the efficacy of DC-mediated cross-presentation (24). In order to promote tumorigenicity, differentially expressed miRNAs have also been identified in distinct immune populations, for example, T cells, NK cells, TAMs, CAFs, and MDSCs, which regulate their protumorigenic potential and participate in reprogramming cellular components of the TME (25).
miRNAs in Exosomes and Microvesicles
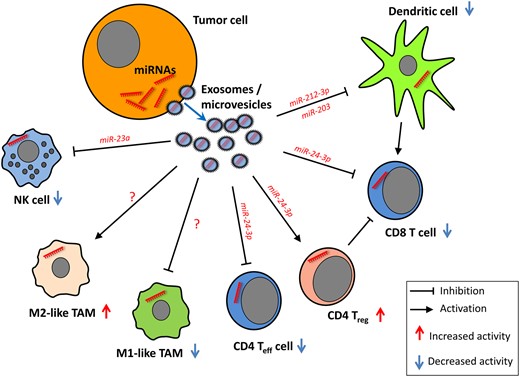
It is noteworthy that miRNAs do not necessarily exert their main functions within cells, but also through microvesicles (size 50–500 nm) and exosomes (size 30–100 nm), respectively (26). Exosomes represent bioactive vesicles derived from endosomal membranes and are involved in intercellular communication because of their specific cargo of proteins, mRNAs, lipids, and miRNAs (27,28). In cancer, exosomes play a role in metastasis, tumor progression, multidrug resistance, radiation-induced bystander effects, and epithelial-mesenchymal transition (29–31). Tumor-derived exosomes can facilitate the immune escape of cancer cells by direct interaction with immune cells, resulting in the suppression of T cells, DCs, and NK cells as well as the induction of immune-suppressive cells like MDSCs, Tregs, and regulatory B cells (30). The modulating effects of tumor-derived microvesicles on immune cell function within the TME have been comprehensively reviewed (30). The functional roles of tumor-derived miRNAs shuttled through microvesicles into various types of immune cells, as described in recent reports discussed below, are summarized in Figure 1.
Tumor-derived miRNAs shuttled through extracellular vesicles alter the function of immune cells. Natural killer (NK) cell function is impaired upon transfer of miR-23a (36). T cell proliferation and differentiation are impaired by tumor-derived miR-24-3p (26), whereas RNA-212-3p and miR-203 exert inhibitory effects on dendritic cell function (32,33). No tumor microvesicle–derived miRNAs affecting tumor-associated macrophage differentiation have been described so far. NK = natural killer cell; TAM = tumor-associated macrophage; Teff = effector T cell; Treg = regulatory T cell.
miRNAs can be transferred from pancreatic tumor cells to immature DCs by exosomes, thereby altering the function of DCs. miR-212-3p isolated from PANC-1-derived exosomes targets the MHC class II TF RFXAP, resulting in reduced expression of HLA-DR, -DP, and -DQ molecules (32). Cross-presentation by DCs can be modulated by tumor-derived miRNAs delivered via exosomes (24), and miR-203 from pancreatic cancer–derived exosomes was able to diminish expression of TLR4 as well as TNF-α and IL-12 in DCs (33). Coculture of T cells with miR-24-3p-containing exosomes from nasopharyngeal cancer cell lines impaired T cell proliferation and differentiation into Th17 cells, while the induction of Tregs was promoted due to targeting of fibroblast growth factor 1 (26).
The concept of miRNA delivery by exosomes has been recently challenged by Chevillet and coworkers, who analyzed the miRNA content in exosomes and supernatants of blood from cancer patients and healthy donors (34). It has been suggested that microvesicles, rather than exosomes derived from epithelial cells, contain substantial amounts of specific miRNAs, which in turn were able to activate macrophages (35). Moreover, the miRNA cargo of microvesicles derived from tumor cell lines is altered by physical factors of the TME, such as hypoxia, which negatively interferes with NK cell function by the delivery of TGF-β and miR-23a (36).
Because the number of publications on exosome- or microvesicle-derived miRNAs is still limited, it remains unclear whether different tumor types will release similar miRNA repertoires through their exosomes. As a given mRNA molecule can be targeted by multiple miRNAs, it appears conceivable that even different miRNAs derived from various tumor entities may result in regulation of the same pathways in targeted immune cells.
The following sections describe miRNAs as powerful regulators of immune modulatory genes involved in tumor immune escape, their use as potential biomarkers, and the possibility to manipulate miRNA expression for therapeutic intervention.
miRNAs Modulating Tumor Immune Escape
As reported, miRNAs not only have oncogenic or tumor suppressive function, but might also affect the immunogenicity of tumors and/or antitumor immune responses (37) and were thus termed immune modulatory miRNAs (im-miRNAs). While tumor-suppressive im-miRNAs improve immunogenicity and enhance immune responses, thereby promoting immune surveillance, oncogenic im-miRNAs have an immune suppressive potential and are involved in immune escape (Figure 2). im-miRNAs affecting tumor immune escape/evasion and expression of respective target genes are listed in Table 1 and described in detail below.
miRNAs involved in tumor immune escape and their respective target genes*
| Target mRNA . | miRNA . | Host cells . | Reference . |
|---|---|---|---|
| β2-microglobulin | miR-9 | NPC | Gao etal. 2013 (38) |
| TAP-1 | miR-9 | NPC | Gao etal. 2013 (38) |
| miR-346 | HAEC | Bartoszewski etal. (46) | |
| PSMB8 | miR-9 | NPC | Gao etal. 2013 (38) |
| miR-451 | LC | Yin etal. 2015 (47) | |
| PSMB10 | miR-9 | NPC | Gao etal. 2013 (38) |
| HLA-A | miR-181a | HBV-infected cells | Liu etal. 2009 (49) |
| HLA-B | miR-9 | NPC | Gao etal. 2013 (38) |
| HLA-C | miR-9 | NPC | Gao etal. 2013 (38) |
| miR-148a | PBLs | Kulkarni etal. 2013 (48) | |
| HLA-F | miR-9 | NPC | Gao etal. 2013 (38) |
| HLA-G | miR-152 | NSCLC | Cheng etal. 2014 (51) |
| miR-133 | RCC | Jasinski-Bergner etal. 2015(52) | |
| miR-148a | RCC | Jasinski-Bergner etal. 2015 (52) | |
| miR-548 | RCC | Jasinski-Bergner etal. 2016 (59) | |
| miR-628-5p | RCC | Jasinski-Bergner etal. 2016 (59) | |
| HLA-H | miR-9 | Nasopharyngeal cancer | Gao etal. 2013 (38) |
| HLA class I | miR-US4-1† | HCMV-infected HFF cells | Kim etal. 2011 (50) |
| ERAP1 | miR-US4-1† | HCMV-infected HFF cells | Kim etal. 2011 (50) |
| MICA | miR-25 | HCC | Kishikawa etal. 2013 (65) |
| miR-93 | HCC | Kishikawa etal. 2013 (65) | |
| miR-106b | HCC | Kishikawa etal. 2013 (65) | |
| MICB | miR-376 | Melanoma | Paschen etal. 2014 (64) |
| miR-433 | Melanoma | Paschen etal. 2014 (64) | |
| miR-10b | Various | Paschen etal. 2014 (64) | |
| MICA/MICB | miR-20a | BCSC, OC | Wang etal. 2014; Xie etal. 2014 (66,183) |
| ULBP2 | miR-34a/c | Melanoma | Paschen etal. 2014 (64) |
| miR-140-5p | Hela, Jurkat | Himmelreich etal. 2011 (69) | |
| miR-302c | Kasumi-1 | Min etal. 2013 (71) | |
| miR-409-3p | Hela, Jurkat | Himmelreich etal. 2011 (69) | |
| miR-433-p | Hela, Jurkat | Himmelreich etal. 2011 (69) | |
| miR-520c | Kasumi-1 | Min etal. 2013 (71) | |
| B7/CD28 | miR-21-3p | CRC | Wu etal. 2015 (86) |
| miR-186-5p | CRC | Wu etal. 2015 (86) | |
| miR-323b-5p | CRC | Wu etal. 2015 (86) | |
| miR-1207-5p | CRC | Wu etal. 2015 (86) | |
| miR-1279 | CRC | Wu etal. 2015 (86) | |
| miR-2117 | CRC | Wu etal. 2015 (86) | |
| miR-3692-3p | CRC | Wu etal. 2015 (86) | |
| PD-L1 | miR-513 | Cholangiocytes | Gong etal. 2009 (79) |
| miR-570 | Gastric cancer | Wang etal. 2013 (82) | |
| miR-34a | AML | Wang etal. 2015 (80) | |
| miR-200 | NSCLC | Chen etal. 2014 (81) | |
| miR-138-5p | CRC | Zhao etal. 2016 (83) | |
| miR-424 | Ovarian cancer | Xu etal. 2016 (84) | |
| B7-H2 | miR-24 | Gastric cancer | Yang etal. 2013 (88) |
| B7-H3 | miR-29a | Neuroblastoma | Cheung etal. 2014 (89) |
| miR-29c | BC | Nygren etal. 2014 (90) | |
| miR-187 | RCC | Zhao etal. 2013 (91) | |
| SOCS3, IRF2 | miR-221 | Prostate cancer | Kneitz etal. 2014 (104) |
| IFNγ | miR-155 | NK cells | Trotta etal. 2012 (106) |
| IFNγR | miR-155 | T cells | Banerjee etal. 2010 (102) |
| JAK2 | miR-375 | Gastric cancer | Ding etal. 2010 (108) |
| miR-135a | Gastric cancer | Wu etal. 2012 (109) | |
| miR-216a | Pancreatic cancer | Wang etal. 2014 (110) | |
| miR-101 | Breast cancer | Wang etal. 2014 (111) | |
| STAT1 | miR-145 | CRC | Gregersen etal. 2010 (112) |
| miR-150 | Transformed T cells | Moles etal. 2015 (119) | |
| miR-223 | Transformed T cells | Moles etal. 2015 (119) | |
| STAT3 | miR-124-3p | NPC | Xu etal. 2016 (134) |
| IRF-1 | miR-29b | Colorectal cancer | Yuan etal. 2015 (114) |
| IRF-4 | miR-125b | Macrophages | Chaudhuri etal. 2011 (184) |
| PTEN | miR-494 | MDSC | Liu etal. 2012 (143) |
| CYLD | miR-181-1b | Breast cancer | Iliopoulos etal. 2010 (172) |
| miR-362-5p | NK cells | Ni etal. 2015 (141) |
| Target mRNA . | miRNA . | Host cells . | Reference . |
|---|---|---|---|
| β2-microglobulin | miR-9 | NPC | Gao etal. 2013 (38) |
| TAP-1 | miR-9 | NPC | Gao etal. 2013 (38) |
| miR-346 | HAEC | Bartoszewski etal. (46) | |
| PSMB8 | miR-9 | NPC | Gao etal. 2013 (38) |
| miR-451 | LC | Yin etal. 2015 (47) | |
| PSMB10 | miR-9 | NPC | Gao etal. 2013 (38) |
| HLA-A | miR-181a | HBV-infected cells | Liu etal. 2009 (49) |
| HLA-B | miR-9 | NPC | Gao etal. 2013 (38) |
| HLA-C | miR-9 | NPC | Gao etal. 2013 (38) |
| miR-148a | PBLs | Kulkarni etal. 2013 (48) | |
| HLA-F | miR-9 | NPC | Gao etal. 2013 (38) |
| HLA-G | miR-152 | NSCLC | Cheng etal. 2014 (51) |
| miR-133 | RCC | Jasinski-Bergner etal. 2015(52) | |
| miR-148a | RCC | Jasinski-Bergner etal. 2015 (52) | |
| miR-548 | RCC | Jasinski-Bergner etal. 2016 (59) | |
| miR-628-5p | RCC | Jasinski-Bergner etal. 2016 (59) | |
| HLA-H | miR-9 | Nasopharyngeal cancer | Gao etal. 2013 (38) |
| HLA class I | miR-US4-1† | HCMV-infected HFF cells | Kim etal. 2011 (50) |
| ERAP1 | miR-US4-1† | HCMV-infected HFF cells | Kim etal. 2011 (50) |
| MICA | miR-25 | HCC | Kishikawa etal. 2013 (65) |
| miR-93 | HCC | Kishikawa etal. 2013 (65) | |
| miR-106b | HCC | Kishikawa etal. 2013 (65) | |
| MICB | miR-376 | Melanoma | Paschen etal. 2014 (64) |
| miR-433 | Melanoma | Paschen etal. 2014 (64) | |
| miR-10b | Various | Paschen etal. 2014 (64) | |
| MICA/MICB | miR-20a | BCSC, OC | Wang etal. 2014; Xie etal. 2014 (66,183) |
| ULBP2 | miR-34a/c | Melanoma | Paschen etal. 2014 (64) |
| miR-140-5p | Hela, Jurkat | Himmelreich etal. 2011 (69) | |
| miR-302c | Kasumi-1 | Min etal. 2013 (71) | |
| miR-409-3p | Hela, Jurkat | Himmelreich etal. 2011 (69) | |
| miR-433-p | Hela, Jurkat | Himmelreich etal. 2011 (69) | |
| miR-520c | Kasumi-1 | Min etal. 2013 (71) | |
| B7/CD28 | miR-21-3p | CRC | Wu etal. 2015 (86) |
| miR-186-5p | CRC | Wu etal. 2015 (86) | |
| miR-323b-5p | CRC | Wu etal. 2015 (86) | |
| miR-1207-5p | CRC | Wu etal. 2015 (86) | |
| miR-1279 | CRC | Wu etal. 2015 (86) | |
| miR-2117 | CRC | Wu etal. 2015 (86) | |
| miR-3692-3p | CRC | Wu etal. 2015 (86) | |
| PD-L1 | miR-513 | Cholangiocytes | Gong etal. 2009 (79) |
| miR-570 | Gastric cancer | Wang etal. 2013 (82) | |
| miR-34a | AML | Wang etal. 2015 (80) | |
| miR-200 | NSCLC | Chen etal. 2014 (81) | |
| miR-138-5p | CRC | Zhao etal. 2016 (83) | |
| miR-424 | Ovarian cancer | Xu etal. 2016 (84) | |
| B7-H2 | miR-24 | Gastric cancer | Yang etal. 2013 (88) |
| B7-H3 | miR-29a | Neuroblastoma | Cheung etal. 2014 (89) |
| miR-29c | BC | Nygren etal. 2014 (90) | |
| miR-187 | RCC | Zhao etal. 2013 (91) | |
| SOCS3, IRF2 | miR-221 | Prostate cancer | Kneitz etal. 2014 (104) |
| IFNγ | miR-155 | NK cells | Trotta etal. 2012 (106) |
| IFNγR | miR-155 | T cells | Banerjee etal. 2010 (102) |
| JAK2 | miR-375 | Gastric cancer | Ding etal. 2010 (108) |
| miR-135a | Gastric cancer | Wu etal. 2012 (109) | |
| miR-216a | Pancreatic cancer | Wang etal. 2014 (110) | |
| miR-101 | Breast cancer | Wang etal. 2014 (111) | |
| STAT1 | miR-145 | CRC | Gregersen etal. 2010 (112) |
| miR-150 | Transformed T cells | Moles etal. 2015 (119) | |
| miR-223 | Transformed T cells | Moles etal. 2015 (119) | |
| STAT3 | miR-124-3p | NPC | Xu etal. 2016 (134) |
| IRF-1 | miR-29b | Colorectal cancer | Yuan etal. 2015 (114) |
| IRF-4 | miR-125b | Macrophages | Chaudhuri etal. 2011 (184) |
| PTEN | miR-494 | MDSC | Liu etal. 2012 (143) |
| CYLD | miR-181-1b | Breast cancer | Iliopoulos etal. 2010 (172) |
| miR-362-5p | NK cells | Ni etal. 2015 (141) |
Controversially discussed miRNAs are found as tumor suppressors in some cancer types while exhibiting oncogenic properties in other cancer types. AML = acute myeloid leukemia; BC = bladder cancer; BCSC = breast cancer stem-like cell; CRC = colorectal cancer; HAEC = human airway epithelial cell; HBV = hepatitis B virus; HCC = hepatocellular cancer; HCMV = human cytomegalovirus; HFF = human foreskin fibroblast; LC = lung cancer; MDSC = myeloid derived suppressor cell; NPC = nasopharyngeal cancer; NSCLC = non–small cell lung cancer; OC = ovarian cancer; PBL = peripheral blood lymphocytes; RCC = renal cell cancer.
Virus-derived miRNA.
miRNAs involved in tumor immune escape and their respective target genes*
| Target mRNA . | miRNA . | Host cells . | Reference . |
|---|---|---|---|
| β2-microglobulin | miR-9 | NPC | Gao etal. 2013 (38) |
| TAP-1 | miR-9 | NPC | Gao etal. 2013 (38) |
| miR-346 | HAEC | Bartoszewski etal. (46) | |
| PSMB8 | miR-9 | NPC | Gao etal. 2013 (38) |
| miR-451 | LC | Yin etal. 2015 (47) | |
| PSMB10 | miR-9 | NPC | Gao etal. 2013 (38) |
| HLA-A | miR-181a | HBV-infected cells | Liu etal. 2009 (49) |
| HLA-B | miR-9 | NPC | Gao etal. 2013 (38) |
| HLA-C | miR-9 | NPC | Gao etal. 2013 (38) |
| miR-148a | PBLs | Kulkarni etal. 2013 (48) | |
| HLA-F | miR-9 | NPC | Gao etal. 2013 (38) |
| HLA-G | miR-152 | NSCLC | Cheng etal. 2014 (51) |
| miR-133 | RCC | Jasinski-Bergner etal. 2015(52) | |
| miR-148a | RCC | Jasinski-Bergner etal. 2015 (52) | |
| miR-548 | RCC | Jasinski-Bergner etal. 2016 (59) | |
| miR-628-5p | RCC | Jasinski-Bergner etal. 2016 (59) | |
| HLA-H | miR-9 | Nasopharyngeal cancer | Gao etal. 2013 (38) |
| HLA class I | miR-US4-1† | HCMV-infected HFF cells | Kim etal. 2011 (50) |
| ERAP1 | miR-US4-1† | HCMV-infected HFF cells | Kim etal. 2011 (50) |
| MICA | miR-25 | HCC | Kishikawa etal. 2013 (65) |
| miR-93 | HCC | Kishikawa etal. 2013 (65) | |
| miR-106b | HCC | Kishikawa etal. 2013 (65) | |
| MICB | miR-376 | Melanoma | Paschen etal. 2014 (64) |
| miR-433 | Melanoma | Paschen etal. 2014 (64) | |
| miR-10b | Various | Paschen etal. 2014 (64) | |
| MICA/MICB | miR-20a | BCSC, OC | Wang etal. 2014; Xie etal. 2014 (66,183) |
| ULBP2 | miR-34a/c | Melanoma | Paschen etal. 2014 (64) |
| miR-140-5p | Hela, Jurkat | Himmelreich etal. 2011 (69) | |
| miR-302c | Kasumi-1 | Min etal. 2013 (71) | |
| miR-409-3p | Hela, Jurkat | Himmelreich etal. 2011 (69) | |
| miR-433-p | Hela, Jurkat | Himmelreich etal. 2011 (69) | |
| miR-520c | Kasumi-1 | Min etal. 2013 (71) | |
| B7/CD28 | miR-21-3p | CRC | Wu etal. 2015 (86) |
| miR-186-5p | CRC | Wu etal. 2015 (86) | |
| miR-323b-5p | CRC | Wu etal. 2015 (86) | |
| miR-1207-5p | CRC | Wu etal. 2015 (86) | |
| miR-1279 | CRC | Wu etal. 2015 (86) | |
| miR-2117 | CRC | Wu etal. 2015 (86) | |
| miR-3692-3p | CRC | Wu etal. 2015 (86) | |
| PD-L1 | miR-513 | Cholangiocytes | Gong etal. 2009 (79) |
| miR-570 | Gastric cancer | Wang etal. 2013 (82) | |
| miR-34a | AML | Wang etal. 2015 (80) | |
| miR-200 | NSCLC | Chen etal. 2014 (81) | |
| miR-138-5p | CRC | Zhao etal. 2016 (83) | |
| miR-424 | Ovarian cancer | Xu etal. 2016 (84) | |
| B7-H2 | miR-24 | Gastric cancer | Yang etal. 2013 (88) |
| B7-H3 | miR-29a | Neuroblastoma | Cheung etal. 2014 (89) |
| miR-29c | BC | Nygren etal. 2014 (90) | |
| miR-187 | RCC | Zhao etal. 2013 (91) | |
| SOCS3, IRF2 | miR-221 | Prostate cancer | Kneitz etal. 2014 (104) |
| IFNγ | miR-155 | NK cells | Trotta etal. 2012 (106) |
| IFNγR | miR-155 | T cells | Banerjee etal. 2010 (102) |
| JAK2 | miR-375 | Gastric cancer | Ding etal. 2010 (108) |
| miR-135a | Gastric cancer | Wu etal. 2012 (109) | |
| miR-216a | Pancreatic cancer | Wang etal. 2014 (110) | |
| miR-101 | Breast cancer | Wang etal. 2014 (111) | |
| STAT1 | miR-145 | CRC | Gregersen etal. 2010 (112) |
| miR-150 | Transformed T cells | Moles etal. 2015 (119) | |
| miR-223 | Transformed T cells | Moles etal. 2015 (119) | |
| STAT3 | miR-124-3p | NPC | Xu etal. 2016 (134) |
| IRF-1 | miR-29b | Colorectal cancer | Yuan etal. 2015 (114) |
| IRF-4 | miR-125b | Macrophages | Chaudhuri etal. 2011 (184) |
| PTEN | miR-494 | MDSC | Liu etal. 2012 (143) |
| CYLD | miR-181-1b | Breast cancer | Iliopoulos etal. 2010 (172) |
| miR-362-5p | NK cells | Ni etal. 2015 (141) |
| Target mRNA . | miRNA . | Host cells . | Reference . |
|---|---|---|---|
| β2-microglobulin | miR-9 | NPC | Gao etal. 2013 (38) |
| TAP-1 | miR-9 | NPC | Gao etal. 2013 (38) |
| miR-346 | HAEC | Bartoszewski etal. (46) | |
| PSMB8 | miR-9 | NPC | Gao etal. 2013 (38) |
| miR-451 | LC | Yin etal. 2015 (47) | |
| PSMB10 | miR-9 | NPC | Gao etal. 2013 (38) |
| HLA-A | miR-181a | HBV-infected cells | Liu etal. 2009 (49) |
| HLA-B | miR-9 | NPC | Gao etal. 2013 (38) |
| HLA-C | miR-9 | NPC | Gao etal. 2013 (38) |
| miR-148a | PBLs | Kulkarni etal. 2013 (48) | |
| HLA-F | miR-9 | NPC | Gao etal. 2013 (38) |
| HLA-G | miR-152 | NSCLC | Cheng etal. 2014 (51) |
| miR-133 | RCC | Jasinski-Bergner etal. 2015(52) | |
| miR-148a | RCC | Jasinski-Bergner etal. 2015 (52) | |
| miR-548 | RCC | Jasinski-Bergner etal. 2016 (59) | |
| miR-628-5p | RCC | Jasinski-Bergner etal. 2016 (59) | |
| HLA-H | miR-9 | Nasopharyngeal cancer | Gao etal. 2013 (38) |
| HLA class I | miR-US4-1† | HCMV-infected HFF cells | Kim etal. 2011 (50) |
| ERAP1 | miR-US4-1† | HCMV-infected HFF cells | Kim etal. 2011 (50) |
| MICA | miR-25 | HCC | Kishikawa etal. 2013 (65) |
| miR-93 | HCC | Kishikawa etal. 2013 (65) | |
| miR-106b | HCC | Kishikawa etal. 2013 (65) | |
| MICB | miR-376 | Melanoma | Paschen etal. 2014 (64) |
| miR-433 | Melanoma | Paschen etal. 2014 (64) | |
| miR-10b | Various | Paschen etal. 2014 (64) | |
| MICA/MICB | miR-20a | BCSC, OC | Wang etal. 2014; Xie etal. 2014 (66,183) |
| ULBP2 | miR-34a/c | Melanoma | Paschen etal. 2014 (64) |
| miR-140-5p | Hela, Jurkat | Himmelreich etal. 2011 (69) | |
| miR-302c | Kasumi-1 | Min etal. 2013 (71) | |
| miR-409-3p | Hela, Jurkat | Himmelreich etal. 2011 (69) | |
| miR-433-p | Hela, Jurkat | Himmelreich etal. 2011 (69) | |
| miR-520c | Kasumi-1 | Min etal. 2013 (71) | |
| B7/CD28 | miR-21-3p | CRC | Wu etal. 2015 (86) |
| miR-186-5p | CRC | Wu etal. 2015 (86) | |
| miR-323b-5p | CRC | Wu etal. 2015 (86) | |
| miR-1207-5p | CRC | Wu etal. 2015 (86) | |
| miR-1279 | CRC | Wu etal. 2015 (86) | |
| miR-2117 | CRC | Wu etal. 2015 (86) | |
| miR-3692-3p | CRC | Wu etal. 2015 (86) | |
| PD-L1 | miR-513 | Cholangiocytes | Gong etal. 2009 (79) |
| miR-570 | Gastric cancer | Wang etal. 2013 (82) | |
| miR-34a | AML | Wang etal. 2015 (80) | |
| miR-200 | NSCLC | Chen etal. 2014 (81) | |
| miR-138-5p | CRC | Zhao etal. 2016 (83) | |
| miR-424 | Ovarian cancer | Xu etal. 2016 (84) | |
| B7-H2 | miR-24 | Gastric cancer | Yang etal. 2013 (88) |
| B7-H3 | miR-29a | Neuroblastoma | Cheung etal. 2014 (89) |
| miR-29c | BC | Nygren etal. 2014 (90) | |
| miR-187 | RCC | Zhao etal. 2013 (91) | |
| SOCS3, IRF2 | miR-221 | Prostate cancer | Kneitz etal. 2014 (104) |
| IFNγ | miR-155 | NK cells | Trotta etal. 2012 (106) |
| IFNγR | miR-155 | T cells | Banerjee etal. 2010 (102) |
| JAK2 | miR-375 | Gastric cancer | Ding etal. 2010 (108) |
| miR-135a | Gastric cancer | Wu etal. 2012 (109) | |
| miR-216a | Pancreatic cancer | Wang etal. 2014 (110) | |
| miR-101 | Breast cancer | Wang etal. 2014 (111) | |
| STAT1 | miR-145 | CRC | Gregersen etal. 2010 (112) |
| miR-150 | Transformed T cells | Moles etal. 2015 (119) | |
| miR-223 | Transformed T cells | Moles etal. 2015 (119) | |
| STAT3 | miR-124-3p | NPC | Xu etal. 2016 (134) |
| IRF-1 | miR-29b | Colorectal cancer | Yuan etal. 2015 (114) |
| IRF-4 | miR-125b | Macrophages | Chaudhuri etal. 2011 (184) |
| PTEN | miR-494 | MDSC | Liu etal. 2012 (143) |
| CYLD | miR-181-1b | Breast cancer | Iliopoulos etal. 2010 (172) |
| miR-362-5p | NK cells | Ni etal. 2015 (141) |
Controversially discussed miRNAs are found as tumor suppressors in some cancer types while exhibiting oncogenic properties in other cancer types. AML = acute myeloid leukemia; BC = bladder cancer; BCSC = breast cancer stem-like cell; CRC = colorectal cancer; HAEC = human airway epithelial cell; HBV = hepatitis B virus; HCC = hepatocellular cancer; HCMV = human cytomegalovirus; HFF = human foreskin fibroblast; LC = lung cancer; MDSC = myeloid derived suppressor cell; NPC = nasopharyngeal cancer; NSCLC = non–small cell lung cancer; OC = ovarian cancer; PBL = peripheral blood lymphocytes; RCC = renal cell cancer.
Virus-derived miRNA.
Tumor immune escape mechanisms. The figure shows mechanisms based on (A) loss of tumor antigen expression; (B) altered epitope generation and processing; (C) deficient peptide import into the endoplasmatic reticulum; (D) impaired expression of major histocompatibility complex class I heavy chain and β2-microglobulin; (E) release of anti-inflammatory cytokines; (F) downregulation, loss, or altered phosphorylation status of signal pathway components including the MAP kinase cascade as well as the interferon/ janus kinase/ signal transducer and activator of transcription 1 pathway; (G) aberrant expression of immune inhibitory molecules (eg, human leukocyte antigen–G, PD ligand, major histocompatibility complex class I–related molecule [MIC] A, MICB, human cytomegalovirus UL16-binding protein 2); (H) protection from T cell–mediated apoptosis; and (I) secretion of immune-suppressive mediators and exosomes. Impact of miRNAs at different steps within these pathways is indicated by stylized miRNA molecules. Translation of mRNAs has been mostly omitted for clarity. β2-m = β2-microglobulin; CD95 = cluster of differentiation 29 (FAS); CD95L = CD95 ligand (FAS ligand); CXCL12 = C-X-C motif chemokine ligand 12; ER = endoplasmatic reticulum; ERAP1 = endoplasmic reticulum aminopeptidase; FADD = Fas associated via death domain; FLIP = FLICE-inhibitory protein; HLA = human leukocyte antigen; IAP = inhibitor of apoptosis; IFN = interferon; JAK = janus kinase; IL = interleukin; MAPK = MAP kinase; MHC = major histocompatibility complex; MIC = major histocompatibility complex class I-related molecule; PD-1 = programmed cell death 1; PD-L1 = PD ligand 1; STAT = signal transducer and activator of transcription; TCR = T cell receptor; TAP = transporter associated with antigen processing; TGFβ = transforming growth factor β; ULBP = human cytomegalovirus UL16-binding protein.
HLA Class I APM Components
Based on in silico analysis, miRNA arrays, RNA sequencing, and/or functional screening with miRNA libraries, various mi-miRNAs affecting the expression of components of the MHC class I antigen processing machinery (APM) (Figure 2) have been identified; this includes for example, miR-9, modulating the expression of APM components and IFN-induced genes, in particular the proteasome subunits PSMB8 and PSMB10, TAP1, β2-microglobulin, HLA-B, HLA-C, and the nonclassical HLA-F and HLA-H molecules (38). However, the binding of miR-9 to the 3′-UTR of mRNAs encoding these molecules has not been analyzed (38). This miRNA can exert oncogenic as well as tumor-suppressive activity (38) as miR-9 was found to be overexpressed in brain tumors (39) or Hodgkin’s lymphoma (40), whereas in other tumor entities, such as breast cancer (41), colon cancer (42), nasopharyngeal carcinoma (43), and melanoma (44), miR-9 appeared underexpressed. The oncogenic capacity of miR-9 was further confirmed by its ability to promote tumor outgrowth and metastasis formation (40). Conversely, the tumor-suppressive potential of this miRNA was demonstrated by its inhibitory effects on tumor cell proliferation (44) and invasion, as well as on metastasis formation (45).
The ER stress–induced miR-346 also modulates the expression of HLA class I APM components and IFN-induced genes. Functional studies performed by overexpression and RNAi knockdown using miRNA mimics and miRNA inhibitors revealed TAP1 as a direct target of miR-346 (46).
Using bioinformatics and dual luciferase reporter assays, the proteasome subunit PSMB8 was identified as a direct target of miR-451 (47). Proliferation, invasion, and metastatic potential of lung cancer cells were affected after overexpression of miR-451 in these cells, suggesting that high levels of miR-451 expression are associated with the carcinogenesis of lung cancer (47).
Allelic variations in the 3′-UTR of HLA-C have been shown to modulate the binding capacity of miR-148a and, consequently, the extent of HLA-C surface expression (48). Overexpression of miR-148a is involved in neoplastic as well as in viral transformation, for example, in the control of HIV (48). Another miRNA binding to the 3′-UTR of the HLA-A mRNA is miR-181a, which is upregulated in hepatitis B virus (HBV)–infected cells (49), while miR-US4-1 derived from human cytomegalovirus could target HLA class I molecules and the ER-resident aminopeptidase (ERAP)1, thereby blocking CTL recognition (50).
HLA-G and MHC Class I–Related Proteins
Recently, a number of HLA-G-targeting miRNAs have been identified that belong to the miR-148 family, consisting of miR-148a, miR-148b, and miR-152. These miRNAs have been shown to act as tumor suppressors in many tumors including bladder cancer, glioblastoma, gastric cancer, lung cancer, hepatocellular carcinoma, and renal cell cancer (RCC) (51–56). While a role for miRNAs in the IFN-γ-mediated upregulation of HLA-G remains to be described, a link between TGF-β-induced HLA-G expression and reduced miR-152 expression has been shown in gastric cancer (57). HLA-G expression could be upregulated by the long noncoding RNA HOTAIR, which inhibits miR-152 (58). miR-133, miR-548, and miR-628-5p have been shown to inhibit HLA-G expression (59,60), and for some miRNAs, an effect on the cellular immune response has been demonstrated (52): miR-152 and miR-628-5p overexpression enhanced T and/or NK cell responses and exhibited tumor-suppressive capacity. Polymorphisms in the 3′-UTR of HLA-G might influence miRNA binding, as suggested for miR-139-3p and miR-608 (61).
The cytotoxic activity of NK cells is determined by the integration of activating and inactivating signals. The ligands of the activating NK cell receptor NKG2D, the major histocompatibility complex class I–related molecules (MICs) A and B and the human cytomegalovirus UL16-binding proteins (ULBPs), are aberrantly expressed in tumors of distinct origin (62–64). MICA and MICB expression can be controlled by oncogenic miRNAs, like miR-10b, miR-17-5p, miR-20a, miR-25, miR-93, miR-106b, and/or miR-433, which increase the proliferative, invasive, and angiogenic potential of tumor cells and negatively interfere with their susceptibility to NK cell–mediated cytotoxicity (37,65–67). MICA/B-targeting miRNAs are epigenetically regulated, and their expression could be altered by histone deacetylase inhibitors (68). Overexpression of miR-20a induces the downregulation of MICA/MICB expression in breast cancer stem cells, which enhances their metastatic potential and reduces their sensitivity to NK cell–mediated lysis (66). ULBP expression has been suggested to be regulated by tumor-suppressive miRNAs, for example, miR-34a/c, miR-140-5p, miR-302c, miR-409-3p, miR-433, and miR-530c (69–71), which might also modulate NK cell responses.
However, the mechanisms controlling the expression of HLA-G, MICs, and ULBPs are more complex and do not exclusively involve im-miRNAs. Recently, RNA-binding proteins have been found to be involved in the regulation of HLA-G (72), MIC, and ULBP expression (73,74).
Comodulatory Molecules of the B7 Family
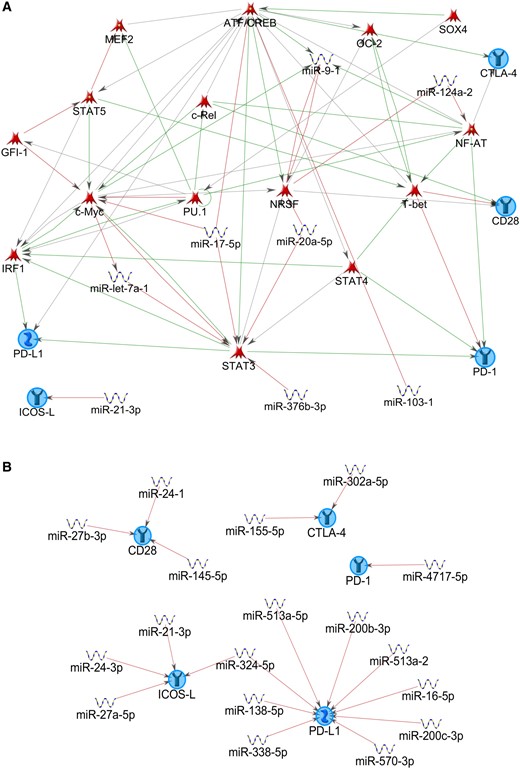
During recent years, the number of known B7 family members has grown, consisting of both co-inhibitory and costimulatory molecules, including B7.1 (CD80), B7.2 (CD86), CD28, CTLA-4 (cytotoxic T lymphocyte–associated protein 4), PD-1 (programmed cell death 1), PD-1 ligand (PD-L1), PDL2, ICOSL (inducible T cell costimulator ligand), B7-H3, B7-H4, B7-H6, and VISTA (V-domain Ig suppressor of T cell activation) (75). Interestingly, B7 family members may be subject to regulatory control by miRNAs (Figure 2). Bioinformatics MetaCore analysis (see the Supplementary Materials, available online) performed on the checkpoint molecules PD-1, PD-L1, CTLA-4, CD28, and ICOS-L revealed a complex regulatory network established between miRNAs, TFs, and the selected input molecules (Figure 3A). The majority of the checkpoint molecules included in the analysis appear to be regulated by multiple miRNAs, with PD-L1 showing the greatest number of regulating miRNAs (Figure 3B). However, as MetaCore represents a manually curated data base, the latter observation might simply reflect the preponderance of studies performed on PD-L1.
MetaCore analysis on selected T cell checkpoint molecules. Network plots were generated using MetaCore with programmed cell death 1, PD ligand 1, cluster of differentiation 28, cytotoxic T lymphocyte–associated protein 4, and inducible T cell costimulatory ligand as preset network nodes. A) This network was generated using the “auto expand” algorithm with 50 nodes. Object types included miRNAs and transcription factors. This network is primarily built on transcription factors (TFs) and includes TF-targeting miRNAs. B) The second network plot is based on “expand by one interaction” algorithm with the omission of TFs from the Object types. Thus, miRNAs directly targeting checkpoint molecules are depicted. For details on parameter settings, see the Supplementary Materials (available online). ATF/CREB = activating transcription factor/cAMP responsive element binding protein; CD = cluster of differentiation; c-Myc = human v-myc avian myelocytomatosis viral oncogene homolog (transcription factor); c-Rel = REL proto-oncogene, NF-kB subunit; CTLA-4 = cytotoxic T lymphocyte–associated protein 4; GFI-1 = growth factor independent 1 transcriptional repressor; ICOS-L = inducible T cell costimulatory ligand; IRF1 = interferon regulatory factor 1; MEF2 = myocyte enhancer factor 2; NF-AT = nuclear factor of activated T-cells; NRSF = neuron-restrictive silencing factor; OC-2 = one cut homeobox 2; PD-1 = programmed cell death 1; PD-L1 = PD ligand 1; PU.1 = transcription factor of the ETS family also known as Spi-1; SOX4 = sex determining region Y box 4; STAT = signal transducer and activator of transcription; T-bet = T-box 21, transcription factor.
PD-L1 expressed on B cells, T cells, macrophages, TAMs, and DCs (76–78) has been shown to be targeted by miR-513, leading to its translational repression (79). PD-L1 expression on tumor cells has been shown to be controlled by miR-34a (80), miR-200 (81), miR-570 (82), miR-138-5p (83), and miR-424 (84), which might be associated with the clinical outcome of tumor patients. High levels of miR-424 are not only inversely correlated with expression of PD-L1/PD-1, but also with CD80/CTLA-4 expression, and are associated with the progression-free survival of ovarian cancer patients. The miR-424-mediated inhibition of PD-L1 expression reverted chemoresistance (84). Another mechanism altering PD-L1 expression consists of a highly conserved guanine-to-cytosine mutation in its 3’-UTR leading to PD-L1 overexpression by disruption of miR-570 binding. This mutation was significantly associated with PD-L1 overexpression in gastric cancer and with pathological parameters including differentiation grade, depth of tumor invasion, lymph node metastasis, and TNM stage (85).
Single nucleotide polymorphisms (SNPs) within the 3′-UTR of 11 B7/CD28 genes have been determined in a large series of colorectal cancer patients and in healthy controls. These polymorphisms disrupt the regulatory function of miR-21-3p, miR-186-5p, miR-323b-5p, miR-1207-5p, miR-1279, miR-2117, and miR-3692-3p, resulting in the aberrant expression of co-inhibitory molecules of the B7 family (86).
A correlation between low B7-H2 expression on tumor cells and tumor immune escape was shown, indicating a potential role of B7-H2 in tumorigenesis (87). B7-H2 expression is directly controlled by miR-24, which could be disrupted by an SNP in the 3′-UTR of the B7-H2 gene, thereby contributing to the development of gastric cancer (88). Expression of the immune regulatory molecule B7-H3, frequently overexpressed in cancer and associated with metastasis and poor prognosis, is post-transcriptionally regulated by miR-29a (89), miR-29c, and miR-187 (89–91). miR-29c-mediated downregulation of B7-H3 expression was found in breast cancer (90), while reduced miR-187 expression has been identified in clear cell (RCC), which is associated with a lower survival (91). The co-inhibitor T cell immunoglobulin and mucin domain (TIM)–3 functions as an immunosuppressive mediator (92) and was shown to be highly expressed on tumor cells including acute myeloid leukemia (AML). Using in silico analysis, miR-330-5p was predicted to inhibit TIM-3 expression, which was confirmed by overexpressing this miRNA in AML cell lines (93). To date, no information exists about the role of miRNAs in the regulation of B7-H4, B7-H6, and VISTA expression.
IFN-γ Signal Transduction Pathway
The regulation of IFN-γ signaling includes kinases and phosphatases, histone acetylases, suppressors of cytokine signaling molecules (SOCS), and phosphokinases, such as mitogen activated protein kinase (MAPK), protein kinase C (PKC), and phosphoinositide 3-kinase/protein kinase B (PI3K/AKT) (94). IFN-γ treatment can alter the expression of miRNAs, thereby modulating the expression of genes involved in proliferation, differentiation, signal transduction, immune response, and carcinogenesis (95–97). Conversely, the expression of IFN signaling components can be either directly or indirectly influenced by miRNAs (98–101). In addition, studies have confirmed that miRNAs are able to target components of the IFN-γ signaling pathway (102). Simultaneously, components of the janus kinase, signal transducer and activator of transcription (JAK/STAT) pathway can regulate miRNAs as found by analyzing miRNA expression levels that are controlled by transcription factors, such as c-myc, hypoxia inducible factor (HIF), and STATs (103). The contribution and regulatory role of miRNAs in IFN-γ signaling resemble an emerging research area. A link between miR-221 expression controlling cell growth, invasiveness, and apoptosis in prostate cancer and activation of the JAK/STAT signaling pathway has been demonstrated by the miR-221-mediated inhibition of SOCS3 and interferon regulatory factor (IRF) 2 expression, which are known to be negative regulators of this pathway (104). As reviewed elsewhere, miR-155 derived from the non-protein-coding transcript of the BIC gene is required for the normal function of B cells, T cells, and DCs (105). miR-155 expression is increased during B cell, T cell, macrophage, and DC activation, and it has been shown to regulate IFN-γ production in NK cells (106) and to downregulate IFN-γ-R expression (102). STAT1 upregulates miR-155, which in turn downregulates SOCS1, a negative inhibitor of JAK1, as discussed elsewhere (107). These findings illustrate that a single miRNA can regulate several target mRNAs of the IFN signaling cascade and that miRNAs can be regulated by various targets.
miRNAs regulating components of the IFN-γ signaling pathway mainly act as tumor suppressors. The antiproliferative effect of miR-375 affecting JAK2 protein expression has been described for gastric cancer (108). Similarly, miR-135a expression was downregulated in gastric cancer cell lines, while its overexpression resulted in the inhibition of gastric cancer cell proliferation through targeting of JAK2, thus qualifying miR-135a as a tumor suppressor at least in this tumor entity (109). Several studies confirmed further JAK2-targeting miRNAs, including miR-216a, known to inhibit cell growth and promote apoptosis of pancreatic cancer cells by regulating the JAK2/STAT3 signaling pathway (110), as well as miR-101, which promotes apoptosis of breast cancer cells upon targeting of JAK2 (111). Similar results were found for miR-145, a direct target of the 3′-UTR of STAT1, known to be downregulated in several cancer types (112). Notably, STAT1 is able to upregulate miR-29 family members in melanoma cells, inhibiting their proliferation by downregulating CDK6 expression (113). Interestingly, IRF1 binds to miR-29b, which was associated with an increased sensitivity to IFN-γ-induced apoptosis and reduced cell growth in colorectal cancer (114).
Further studies confirmed that miR-223 and miR-150 are equally involved in IFN-γ signaling, but their functional role in cancer cells is still controversial as both miRNAs could exert oncogenic or tumor-suppressive activity, respectively. In hepatocellular carcinoma (115), AML (116), and the E2A-negative subtype of gastric mucosa–associated lymphoid tissue lymphoma (117), miR-223 expression is repressed, while an upregulation of miR-223 has been recently described in acute T cell lymphocytic leukemia (118). It was found that both miR-223 and miR-150 target the STAT1 3’-UTR and reduce STAT1 expression (119), which in turn reduced the expression of IFN-γ-regulated genes. The expression of miR-150 is upregulated in CD19+ B cells from chronic lymphocytic leukemia, while miR-150 is downregulated in chronic myeloid leukemia, acute lymphoblastic leukemia, and mantel cell lymphoma (120), whereas miR-150 is upregulated in adult T cell leukemia/lymphoma cells (121). These inverse expression patterns of miR-223 and miR-150 suggest that both miRNAs could act as oncogenic as well as tumor-suppressor miRNAs depending on the cellular context.
miRNAs in Antitumor Immune Cell Function
Recently, miRNAs have emerged as critical regulators of virtually all immune cell types (122–124), and some miRNAs, for example, miR-24, miR-30b, and miR-142-3p, have been shown to pair innate and adaptive immunity (125). While some miRNAs basally expressed at low levels in leukocytic subtypes, such as B cells (126,127), T cells (128), macrophages (122), DCs (126), and progenitor/stem cell populations (128), are important for the induction of an effective immune response, other miRNAs expressed by TAMs, MDSCs, NK cells, and T cells regulate their protumorigenic potential. Altered miRNA expression patterns within tumor cells or stromal cells can modulate the immunosuppressive tumor micromilieu and the immune cell recruitment, as outlined in recent reviews (25,129–131), thereby limiting antitumor responses.
Lymphoid Immune Cell Compartment
miRNAs have been demonstrated to affect the function of peripheral and tumor-infiltrating lymphocytes. In healthy mice, members of the miR-17-92 cluster were found overexpressed in TH1 cells relative to TH2 like cells, whereas T cells from tumor-bearing mice and from glioma patients showed reduced miR-17-92 expression levels, which were associated with impaired IFN-γ production in these cells. In the same study, miR-17-92-overexpressing CD4+ T cells from transgenic mice exhibited an “enhanced” TH1 phenotype marked by increased IFN-γ secretion compared with wild-type mice, thus strongly suggesting that miR-17-92 can drive TH1 differentiation of mature CD4+ T cells (132).
Using genetically modified mouse strains, a recent study described the opposing effects of miR-155 and miR-146a on tumor growth and on the recruitment of IFN-γ+/CD4+ T cells into the tumor site. Whereas miR-155-deficient mice suffered from rapid tumor outgrowth and minimal intratumoral T cell infiltration, mice lacking miR-146a showed reduced tumor growth, accompanied by enhanced infiltration with IFN-γ+/CD4+ T cells. Of note, double knock-out mice lacking miR-155 and miR-146a failed to control tumor growth and showed low infiltration by IFN-γ+/CD4+ T cells, comparable with that of miR-155 KO mice. Thus, in the tumor model described, loss of miR-146a was dominated by miRNA-155 deficiency (133).
The expression pattern of miRNAs in T cells can be modulated by tumor cells, thereby counteracting tumor-directed T cell responses. During tumor immune escape, cancer cells alter the expression of miRNAs to suppress the immune system. Downregulation of miR-124-3p increases Treg infiltration and reduces cytokine production through an altered expression of STAT3, which represents a target of miR-124-3p (134). In contrast, tumor-derived miR-214 transferred through microvesicles to CD4+ T cells was shown to target PTEN and led to expansion of Tregs (135). Tregs were also recruited into the TME of HBV-positive hepatocellular carcinoma through CCL2 secreted by tumor resident CD4+ T cells in response to TGF-β-mediated suppression of miR-34. Consistently, tumors with ectopic miR-34 expression contained fewer infiltrating Tregs and showed reduced metastasis formation (136). In human melanoma, upregulation of miR-30b/-30d correlates with tumor stage, metastatic potential, shorter time to recurrence, and reduced overall survival (137). Increased miR-30d expression in immune-competent mice triggered the immune-suppressive properties of lung metastases marked by enhanced infiltration with Tregs (137). Thus, Tregs exhibit an altered miRNA expression pattern, which reflects their immune-suppressive activities.
So far, little is known about the expression and regulation of miRNAs in CTLs. It has been shown that the TGF-β-induced suppression of effector function is mediated by an upregulated expression of miR-23a (138), thereby affecting the expression of BLIMP-1 and Bcl-xL, respectively. Similarly, enhanced expression of miR-491 induced by TGF-β suppressed CTL function via targeting of CDK4 and TCF1 (139). It was furthermore shown that inhibition of miR-23 in CTLs reversed immune suppression, thereby limiting tumor aggressiveness (138).
Concerning NK cells, TGF-β was shown to upregulate miR-183 expression, which in turn reduced the expression of the adaptor protein DAP12, resulting in impaired NK cell activity (140). Furthermore, miR-362-5p was found to be upregulated in peripheral NK cells targeting the inhibitor of the NF-kB signaling pathway CYLD, leading to enhanced expression of cytotoxic effector molecules (141).
In hepatocellular carcinoma positive for the hepatitis B virus, suppressed levels of miR-34a resulted in enhanced production of the chemokine CCL22, thereby recruiting Tregs into the TME to facilitate immune escape (136). In human melanoma, upregulation of miR-30b/-30d correlates with tumor stage, metastatic potential, shorter time to recurrence, and reduced overall survival (137). Upregulation of miR-30d in immune-competent mice triggered the immune-suppressive properties of lung metastases visible by the enhanced infiltration of Tregs (137). Bezman and co-authors suggested that miR-150 differentially controls the development of NK and invariant NKT (iNKT) cells by targeting c-Myb. Mice with miR-150 deletions have a defect in their ability to develop mature NK cells (142). Overexpression of miR-150 promotes the development of mature NK cells, which are highly susceptible to activation, and reduced iNKT cells (142). miR-155 has been shown to modulate the functional activity of T cells, NK cells, B cells, and APCs, such as macrophages and DCs (105). Concerning human NK cells, miR-155 is involved in the regulation of IFN-γ production by downregulating SH2 domain containing inositol phosphatase-1 (SHIP1), suggesting it to be a potential target for the treatment of neoplastic disease (106).
Myeloid-Derived Suppressor Cells
Tumor cells can promote tumorigenesis by altering the miRNA expression profiles in myeloid-derived suppressor cells (MDSCs) that regulate immune suppression within the TME. miRNA array analysis of MDSCs identified a number of deregulated miRNAs, for example, miR-494, that suppress the antitumor CD8+ T cell responses mediated through TGF-β by targeting PTEN, leading to an activation of the STAT3 and AKT pathways (143). Moreover, a number of further miRNAs are downregulated in MDSCs, including miR-17-5p and miR-20a (144,145), which promote the differentiation of myeloid cells and regulate immune-suppressive signaling pathways. MDSCs suppress the antitumor functions of CD4+ and CD8+ T cells and inhibit the functional activities of NK cells (146). miR-155 and miR-21 are reported to be the most upregulated miRNAs in bone marrow–derived MDSCs, regulating PTEN and SHIP1 expression, respectively (147). Both miRNAs promote MDSC expansion, thereby enhancing tumor aggressiveness via immune suppression (147).
Tumor-Associated Macrophages
Tumor-associated macrophages (TAMs) are highlighted by their marked functional plasticity, allowing them to acquire immune-stimulating (M1-like) or immune-suppressive (M2-like) capacity, respectively, as well as a variety of functional phenotypes in between these extremes (148). With a few exceptions (149–151), TAMs that have accumulated in tumors largely display the M2 phenotype and have been generally associated with poor prognosis (152). Interestingly, miRNAs have been found to be involved in the activation and differentiation of macrophages, including TAMs (153). Squadrito and coworkers identified miR-511-3p as the first miRNA, highly and selectively expressed in M2-like TAMs (154). Notably, miR-511-3p represents an intronic miRNA encoded within the murine and human CD206 gene and thus is co-expressed with CD206 upon stimulation of the alternative activation pathway. Unexpectedly, overexpression of this miRNA results in the restricted expression of M2-associated gene products and reduces tumor growth in mice. miR-511-3p was found to target the Rho associated coiled–coil containing protein kinase 2 (ROCK2), thereby preventing ROCK2-mediated phosphorylation of the M2-inducing transcription factor IRF4. Thus, miR-511-3p might act as a fine tuner of tumor-promoting gene expression in M2-like macrophages (154).
In differentiated macrophages, miR-125a was determined acting as a Notch downstream mediator targeting factor-inhibiting hypoxia inducible factor 1 (FIH1) and IRF4, thereby promoting M1 differentiation. Macrophages overexpressing miR-125a suppressed tumor outgrowth and sustained the generation of an immune stimulatory micromilieu in transplantable tumor models (158).
In TAMs isolated from human glioma, reduced expression levels of miR-142-3p were detected compared with peripheral monocytes, as could be shown for in vitro differentiated M2-like macrophages. miR-142-3p overexpression resulted in TGF-β-mediated apoptosis induction of transfected M2, shifting the balance of functional TAMs toward M1. In animal experiments, local metronomic administration of miR-142-3p could control outgrowth of transplanted G261 glioma cells and prolong survival of C57BL/6 mice that had been challenged by intracranial tumor cell injection (155). Therefore, the modulating effect of miR-142-3p on the TAMs consisted of elimination of M2 rather than of a shift toward a more immune-stimulatory functional phenotype.
Li and colleagues revealed miR-146 and miR-222 as the most downregulated miRNA species in TAMs from explanted murine late-stage 4T1 breast tumors (156). They confirmed reduced expression of these miRNAs also in human breast cancer samples and detected decreased miR-146a levels in human colon and gastric cancer. Despite their common downregulation in TAMs, miR146a and miR-222 unexpectedly showed the opposite effects on M1/M2 marker gene expression and tumor growth control when overexpressed in macrophages: miR-146a-overexpressing macrophages admixed to transplanted 4T1 breast cancer cells promoted tumor growth in syngeneic BALB/c mice, whereas the enhanced expression of miR-222 inhibited macrophage migration and suppressed tumor outgrowth. Thus, the decision on the functional phenotype of TAMs seems to depend at least in part on an integral of the opposing effects caused by miR-146a and miR-222.
Expression of miR-155 has been widely associated with worse prognosis and unfavorable clinical outcome in various tumor entities (155–158). This might be due to the TLR9 ligand binding or IFN-β treatment–induced miR-155 expression in macrophages, which can dampen the pro-inflammatory activity of myeloid immune cells through the targeting of SHIP1. Notably, lethally irradiated mice reconstituted with hematopoietic stem cells overexpressing miR-155 showed reduced SHIP1 levels and developed myeloproliferative disorders, suggesting an oncogenic potential for miR-155 in this myeloid compartment. In contrast, transfection of miR-155 led to a redifferentiation of M2-like TAMs isolated from murine S180 tumors into M1-like TAMs resulting in increased tumoricidal capacity and enhanced inhibitory effects on tumor cell invasion (159). Similarly, in studies performed with the endogenous murine PyMT breast cancer model, miR-155 was found to be selectively overexpressed among pro-inflammatory TAMs. Stable knock-down of this miRNA within the myeloid compartment resulted in enhanced tumor growth, showing that miR-155 expressed by M1-like macrophages can have tumor suppressive potential (160). Thus, miR-155 appears polyfunctional, capable of inducing immunostimulatory as well as oncogenic signals depending on the entity and differentiation stage of the cell type; hence, it has been suggested that miR-155 be termed “immunomiR” rather than “oncomiR” (160).
Graff and colleagues observed that polarization of human monocyte–derived macrophages toward an M1-like type in vitro was accompanied by the increased intracellular expression of miR-155* and miRNA-125a-3p, but decreased miR-26a* expression (161). In the same study, polarization into M2a- or M2b-like macrophages resulted in upregulated expression of miR-193b or miR-27a*, miR-29b*, miR-132 and miR-222*, respectively (161). However, it has to be kept in mind that these experiments were performed with in vitro differentiated monocyte–derived macrophages; whether these results also hold true for TAMs remains to be proven.
Cancer-Associated Fibroblasts
Cancer-associated fibroblasts (CAFs), mainly originating from normal bone marrow–derived fibroblasts attracted to the tumor tissue, constitute a major cellular component of the tumor stroma. CAFs inhibit immune responses by promoting the recruitment of Treg, MDSC, and TAM. The transition from fibroblasts to CAFs is initiated by IL-1β released from tumor cells or immune cells resulting in activation of the NF-κB signaling pathway (162), which is accompanied by a specific miRNA expression profile. Comparative miRNA analysis of CAFs from endometrial cancer and fibroblasts from healthy control tissue showed an inverse miRNA expression pattern (163). For example, miR-31 and miR-148 were found to be highly expressed in normal fibroblasts but maximally downregulated in CAFs. The candidate target for miR-148a is WNT10B, and SATB2 for miR-31, suggesting that these miRNAs act as tumor suppressors (164,165). A downregulation of miR-15/-16 was also found in CAFs, interfering with the expression of FGF2 and FGFR1 (166). In ovarian cancer, miR-155 was found upregulated whereas miR-31 and miR-214 were downregulated during the differentiation of tumor resident fibroblasts into CAFs (167). Similarly, the transition from fibroblasts to CAFs was marked by increased miR-21 expression in colorectal and pancreatic cancer (168). In contrast, the diminished expression of miR-149 was observed in CAFs of gastric and colorectal cancer (169), and of miR-200c in CAFs of breast tumors (170).
Several miRNAs have been described to be upregulated in CAFs as well as in tumor cells. Such oncomiRs are, for example, miR-146a and miR-424, which were found strongly upregulated in the murine RIP-Tag2 model of pancreatic cancer and in a number of human endocrine tumors (171). Another group of miRNAs highly expressed in CAFs and in tumor cells are involved in the NF-κB signaling pathway: In human mammary carcinoma, miR-181-1b was found to enhance NF-κB activity by targeting the negative regulator CYLD, resulting in a positive feedback loop (172). Conversely, the NF-κB-induced miR-146 targets several key regulator molecules of the NF-κB pathway, thereby creating a negative feedback loop (173). Furthermore, the transfer of tumor-suppressive miRNAs from the producing CAFs to other cells of the TME has to be postulated. In fact, the transfer of miRNAs via exosomes (174,175) has been described and might explain the mechanism of miRNA spread among different cell types within the TME.
Perspectives
In tumors, the cellular miRNA expression pattern is often deregulated, typically marked by an overexpression of tumor-promoting oncomirs and/or the decreased expression of tumor-suppressive miRNAs, respectively. In addition, im-miRs have been identified in both tumor cells and different immune cell populations, as well as in extracellular vesicles, which shape the antitumor responses. These different miRNA sets often play an essential role in cancer initiation and progression as well as in tumor immune escape and response to immunotherapies, thus representing potential biomarkers and therapeutic targets in cancer therapy. Indeed, the therapeutic potential of miRNAs either substituting the effect of tumor suppressor miRNAs underrepresented in tumors or silencing the activity of overexpressed oncomirs, respectively, has been demonstrated in murine tumor models of distinct origin (176–179). For example, the intravenous injection of liposome-encapsulated miR-124 reduced the outgrowth of liver tumors (178), miR-125 complexed with nanoparticles inhibited tumor growth when intratumorally administered (179), and the administration of a miR-143-antagomir inhibited metastasis formation in a mouse model of human HCC (176). However, neither the prognostic nor the therapeutic potential of im-miRs has been systematically analyzed, and reports on the clinical application of miRNAs have remained scarce. Future miRNA-based treatment approaches have to meet several criteria, such as 1) efficient delivery of the applied miRNAs to their specific target cell population, 2) absent off-target effects within the recipient cell, and 3) in other cell types, for example, those expressing toll-like receptors (TLRs) known to bind single-stranded RNA, for example, TLR7 and TLR8. Indeed, it has been shown that miRNAs shuttled through tumor-derived extracellular vesicles can bind to TLRs within the endosomes of myeloid recipient cells, thereby stimulating the secretion of pro-inflammatory cytokines such as TNF-α and IL-6 (180), potentially counteracting the initial idea of therapeutic miRNA treatment. On the other hand, the first reports about clinical trials focused on therapeutic targeting or on the application of therapeutic miRNAs have started to emerge (clinicaltrials.gov: NCT01829971) (181). The inhibition of viral miR-122 mediated by an antisense oligonucleotide applied to chronic hepatitis C virus patients resulted in a prolonged drop in viral RNA load among the majority of treated patients without causing any side effects (181). miRNAs have gained relevance in clinical use as diagnostic, prognostic, or therapeutic markers (182). miR-21 and miR-34 have been suggested as promising targets for anti-miRNA or miRNA replacement therapy, respectively, against breast cancer (182). In fact, a multicenter phase I study evaluating the safety of miRNA-34 application in patients with primary liver cancer or other malignancies including breast cancer has just been recently completed (clinicaltrials.gov: NCT01829971).
Conclusions
During the past few years, the post-transcriptional control of gene expression by miRNAs has been established as a key regulator in a wide variety of physiological and pathophysiological processes because of the role of miRNA-mediated RNAi in differentiation, proliferation, apoptosis, and immune responses, as well as in viral and bacterial infections and neoplastic transformation. The relevance of im-miRNAs in mounting tumor immune escape or immune surveillance, respectively, by altering the communication between cancer cells, immune cells, and other components of the TME is being deciphered, reaching a further level of complexity in these processes. Some im-miRs have a dual function, either as an oncomiR or suppressive miRNA, and might provide new insights into the mechanisms steering tumor growth and progression and antitumor immune responses. Moreover, they might be confirmed as promising therapeutic targets for tumor (immuno)therapy.
Although the majority of altered miRNA expression patterns detected are dedicated to cancer cells, there is already evidence that miRNAs expressed by infiltrating immune cells particularly influence tumorigenicity as well. The identification of further im-miRs and their functional characterization might lead to a plethora of novel candidate biomarkers useful for immune monitoring and targeted RNAi therapy.
Funding
This paper was partially sponsored by the Deutsche Forschungsgemeinschaft (DFG) Se-581-21, the GRK1591 (BS), the German Israelian Foundation (GIF; BS and OM) and the Mildred Scheel project (BS).
Notes
The sponsor had no role in the writing of the manuscript or the decision to submit the manuscript for publication.
We are grateful to Theresa Kordaß for her advice during performance of the MetaCore analysis and Sylvi Magdeburg for secretarial help.


![Tumor immune escape mechanisms. The figure shows mechanisms based on (A) loss of tumor antigen expression; (B) altered epitope generation and processing; (C) deficient peptide import into the endoplasmatic reticulum; (D) impaired expression of major histocompatibility complex class I heavy chain and β2-microglobulin; (E) release of anti-inflammatory cytokines; (F) downregulation, loss, or altered phosphorylation status of signal pathway components including the MAP kinase cascade as well as the interferon/ janus kinase/ signal transducer and activator of transcription 1 pathway; (G) aberrant expression of immune inhibitory molecules (eg, human leukocyte antigen–G, PD ligand, major histocompatibility complex class I–related molecule [MIC] A, MICB, human cytomegalovirus UL16-binding protein 2); (H) protection from T cell–mediated apoptosis; and (I) secretion of immune-suppressive mediators and exosomes. Impact of miRNAs at different steps within these pathways is indicated by stylized miRNA molecules. Translation of mRNAs has been mostly omitted for clarity. β2-m = β2-microglobulin; CD95 = cluster of differentiation 29 (FAS); CD95L = CD95 ligand (FAS ligand); CXCL12 = C-X-C motif chemokine ligand 12; ER = endoplasmatic reticulum; ERAP1 = endoplasmic reticulum aminopeptidase; FADD = Fas associated via death domain; FLIP = FLICE-inhibitory protein; HLA = human leukocyte antigen; IAP = inhibitor of apoptosis; IFN = interferon; JAK = janus kinase; IL = interleukin; MAPK = MAP kinase; MHC = major histocompatibility complex; MIC = major histocompatibility complex class I-related molecule; PD-1 = programmed cell death 1; PD-L1 = PD ligand 1; STAT = signal transducer and activator of transcription; TCR = T cell receptor; TAP = transporter associated with antigen processing; TGFβ = transforming growth factor β; ULBP = human cytomegalovirus UL16-binding protein.](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/jnci/109/10/10.1093_jnci_djx034/4/m_djx034f2.jpeg?Expires=1716402077&Signature=Ydtb9m26cGWpzETNvng2uJLx81nxiUNXS3m9PPikaP3rrbz12tX3xYpN5C9C02cqAY-cKCYMKp5tN-t5DfVR4pCNnlfAjXW8L5MhjnHTcSGpUgZ24qHJsRTicbSxKg6TddL9F4H17xTLq6ymgP6mr-hTGm9tAOJK0497rTjRm62Whv~AQbHMmDosOVQPXfQssjvuLBv35ESrnIb6LUZ5PmkEz1gf-XujXGfNZsFZVue8sz5JfRD61BvSVLPU0lHG1S6Q4qPF5LTbajkjkvU7D5gJTV4AWM4tmd79Ca9zVwJVMf3SY9uiluhhkELpKlEDjz~CeasA6MZn0HSIJ4KRHw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)