-
PDF
- Split View
-
Views
-
Cite
Cite
Vit Hubka, Adela Cmokova, Magdalena Skorepova, Peter Mikula, Miroslav Kolarik, Trichophyton onychocola sp. nov. isolated from human nail, Medical Mycology, Volume 52, Issue 3, April 2014, Pages 285–292, https://doi.org/10.1093/mmy/myt010
Close - Share Icon Share
Abstract
A previously undescribed Trichophyton species was isolated from the nail of a 33-year-old man with a history of probable distal lateral subungual onychomycosis (without confirmation by mycological examination). The infection occurred for the first time five years earlier (in 2006) and affected the right great toenail, with complete clinical remission after treatment with ciclopirox olamine. This undescribed species was isolated during probable relapse in 2011, but its etiological significance was not confirmed, that is, direct microscopy was negative and additional clinical samples were not collected. The species is probably geophilic based on phylogenetic analysis (internal transcribed spacer [ITS] rDNA) and is most closely related to the anamorphic T. thuringiense, homothallic Arthroderma ciferrii (anamorph T. georgiae), and heterothallic A. melis. The new species is characterized by yellowish colonies, red reverse on several media, positive urease test, negative hair-perforation test, absence of growth at 34°C, absence of macroconidia, formation of one-celled clavate microconidia, and spiral hyphae. The species grows well on sterilized human hairs placed on agar medium without any additional nutrients and forms gymnothecium-like structures covered by peridial hyphae. The combination of unique micro- and macromorphological features and physiological and sequence data from four unlinked loci (ITS, benA, RPB2, and act1 gene) justified the proposal of a new species T. onychocola sp. nov.
The European Molecular Biology Laboratory (EMBL) database accession numbers for the internal transcribed spacer rDNA, benA, RPB2, and act1 of the ex-type strain of Trichophyton onychocola sp. nov. are HF937405, HF937401, HF937393, and HF937400, respectively.
The MycoBank (http://www.mycobank.org) accession number for Trichophyton onychocola sp. nov. is MB804199.
Introduction
Dermatophytes are members of the order Onygenales and are one of the most common human and animal pathogens. In the last two decades, substantial changes have been made in the taxonomy of dermatophytes based on data derived using molecular methods [1,2]. Numerous names of well-recognized species have been synonymized, some of them then reappraised and their separate species status again justified. The species concept in anthropophilic and zoophilic dermatophytes is especially problematic as they represent phylogenetically young and genetically similar species with incomplete reproductive barriers [3–6]. Genealogical concordance, phylogenetic species recognition, and biological species concept (based on mating experiments), which are popular in many fungal groups, have limited application as separate identification procedures in these taxa [4,5,7]. In contrast, the DNA sequence divergencies in geophilic dermatophytes are much more pronounced than in anthropophilic and zoophilic species, and species boundaries can be also established using mating experiments [3,8–10]. Only two dermatophytic species, Trichophyton eboreum (teleomorph Arthroderma olidum) [8,9] and Microsporum mirabile (teleomorph A. mirabile) [10], were described in the last two decades despite the increasing application of molecular taxonomic techniques. Other taxa described in this period [11,12] were later synonymized with previously described species [2,13].
During a two-year molecular–epidemiological study that focused on dermatophytes, with the exception of T. rubrum, in the Czech Republic, a species with unusual morphological features was isolated from a case of suspected onychomycosis. This species showed a unique fingerprinting pattern and internal transcribed spacer [ITS] rDNA sequence [Hubka V, et al. One year overview of non–T. rubrum dermatophytes isolated in Czech Republic and identified by molecular methods. 18th Congress of the International Society for Human and Animal Mycology, Berlin, Germany, 2012, P541]. Additional morphological, physiological, and molecular data collected in this study confirmed isolation of an undescribed species that is described here based on a polyphasic approach.
Case report
A 33-year-old man presented in November 2011 with suspected onychomycosis of his right great toenail. Yellow discoloration, hyperkeratosis, and onycholysis were present on the medial margin of the plate of the entire nail; other toenails as well as fingernails were without abnormalities. There was a history of probable onychomycosis (not verified by mycological examination) of the same nail five years previously (in 2006) that had been treated with 1% ciclopirox olamine solution. However, the nail abnormalities reappeared 12 months after the initial remission associated with the indicated treatment. A working diagnosis of recurrent distal lateral subungual onychomycosis was made, and the patient underwent mycological examination to confirm the diagnosis.
At the time of nail sampling, the patient was treated with perindopril for hypertension and was dispensarized due to suspected glomerulonephritis. The patient did not have diabetes, peripheral arterial disease, or psoriasis and had not undergone any immunosuppressive treatment. He did not take any other medication on a regular basis, and also his professional history was insignificant.
The mycological examination was performed in November 2011; the direct microscopic nail examination was negative for the presence of fungi (20% KOH). Remaining portions of the clinical samples were placed on two slants of Sabouraud glucose agar (SGA) without chloramphenicol (Bio-Rad, Marnes-la-Coquette, France) and one slant of SGA with chloramphenicol containing 400 mg/l of cycloheximide (Fluka, Buchs, Switzerland). A pure culture of a fungus was observed that was first identified as T. rubrum based on intensive red reverse pigmentation on SGA slants. Later, the colonies became granular, and abundant spiral hyphae were observed. These hyphae precipitated, in turn, modifying the identification to probable T. mentagrophytes (T. interdigitale according to the recent taxonomy). The isolate was sent to the Department of Botany, Charles University, Prague, where further morphological, physiological, and molecular analyses were conducted. Neither treatment nor further examination were possible because the patient did not return for therapy.
Materials and methods
Cultivation, morphology, and preservation
The isolates were grown on a set of media that included malt extract agar (MEA; Oxoid, Basingstoke, UK), SGA [14], potato dextrose agar (PDA; Roth, Karlsruhe, Germany), potato carrot agar (PCA) [14], and oatmeal agar (OA; Oxoid). A second set of media was supplemented with sterilized hair from blond children and placed on the medium surface, which consisted of OA, Takashio medium (1 g peptone, 2 g glucose, 1 g MgSO4 · 7 H2O, 1 g KH2PO4, and 20 g distilled water), and agar medium without any other additions (20 g agar and 1000 ml distilled water). The plates were incubated at 25°C in the dark and checked weekly for 3 months for growth and morphologic features. Colony color determinations were made using the ISCC-NBS Centroid Color Charts (Foster, J. C., 2004; http://tx4.us/nbs/nbs-1.htm). Growth at 27, 30, 34, and 37°C was evaluated on MEA after incubation for 7 days. An in vitro hair perforation test and a urease test were performed in accordance with the report by Ajello and Georg [15] and Philpot [16], respectively. The ex-type strain of T. onychocola was deposited into the Culture Collection of Fungi (CCF), Department of Botany, Charles University in Prague (CCF 4259T) and into the Centraalbureau voor Schimmelcultures (CBS), Utrecht, Netherlands (CBS 132920T).
Molecular studies
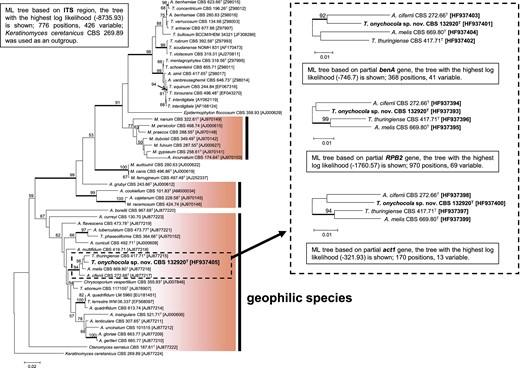
DNA was isolated as described by Hubka et al. [17]. Polymerase chain reaction (PCR) conditions used for amplification of the ITS region, partial benA gene (encoding beta-tubulin), and partial RPB2 (encoding RNA polymerase beta) were as described by Nováková et al. [18]. The act1 locus (encoding actin) was amplified using primers ACT-512F (5′-ATGTGCAAGGCCGGTTTCGC) and ACT-783R (5′-TACGAGTCCTTCTGGCCCAT); the annealing temperature was 60°C. PCR product purification and sequencing were performed at Macrogen Europe (Netherlands, Amsterdam) using the terminal primers. The sequences were inspected and alignments were performed as described by Hubka and Kolařík [19]. Maximum-likelihood (ML) analysis was performed using the same parameters as those described by Nováková et al. [18]. Additional alignment characteristics are listed in the caption of Fig. 1. Sequences were deposited into the EMBL database under accession numbers listed in Fig. 1 (bold print).
ML trees showing relationships of Trichophyton onychocola sp. nov. to other dermatophytes. Nodes supported at 90% bootstrap or higher are double thick; ex-type isolates are designated by a superscript T. Geophilic species are designated by a darker background. The dashed line defines taxa analyzed in three single-locus trees (benA, RPB2, and act1). This Figure is reproduced in color in the online version of Medical Mycology.
Results
Molecular analysis
The ITS rDNA region of isolate CCF 4259 (=CBS 132920) showed 96%–97% similarity when using BLAST similarity search to the ex-type isolate of A. melis CBS 669.80T (766/787 bp; AJ877216), T. thuringiense CBS 417.71T (762/786 bp; AJ877215), and A. ciferrii CBS 272.66T (554/578 bp; AJ877217). In phylogenetic analysis, these taxa created a well-supported clade (Fig. 1) that was located in between other geophilic Trichophyton species. Three additional loci (benA, RPB2, and act1) were sequenced in the ex-type isolates of mentioned species. In all three cases, the isolate CCF 4259 was found to have only a 93%–96% similarity to these taxa, suggesting discovery of an undescribed species.
Taxonomy
Trichophyton onychocola Cmokova, Hubka, Skorepova & M. Kolařík sp. nov., MB804199, Figs 2 and 3.
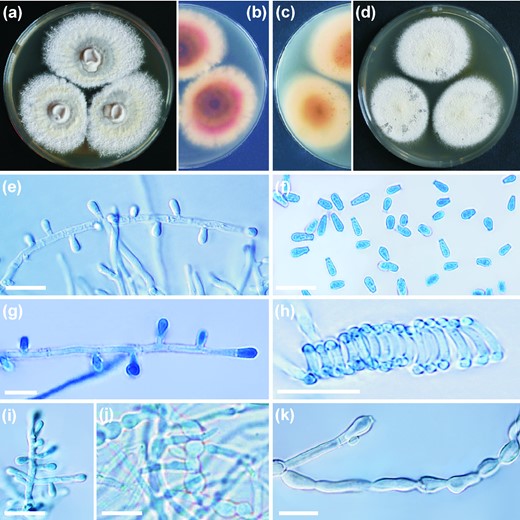
Trichophyton onychocola CCF 4259T colonies after three weeks of cultivation at 25°C on SGA (a) and reverse (b); on MEA (c) and reverse (d); microconidia on short pedicels or sessile (e, g) on the hyphae (conidiophore); clavate microconidia (f); spiral hypha (h); slightly branched conidiophore (i); chlamydospore-like cells and swollen hyphae (j,k). Scale bars, 10 μm. This Figure is reproduced in color in the online version of Medical Mycology.
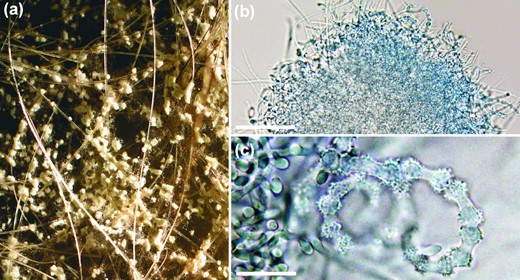
Gymnothecium-like structures present on OA with sterilized human hairs and observed under stereo microscope (a) and light microscope (b); scale bar, 50 μm. Peridial hyphae consisting of ossiform peridial cells (c); scale bar, 10 μm. This Figure is reproduced in color in the online version of Medical Mycology.
Diagnosis: The species has yellowish colonies with red reverse on SGA, PCA, and PDA; is unable to grow at 34°C; on nutrient-poor media or on media supplemented with human hairs produces sterile ascomata-like structures with peridial hyphae consisting of dumbbell-shaped peridial cells; produces abundant pyriform or clavate microconidia, spiral hyphae, chlamydospores; does not produce macroconidia.
Type: Czech Republic, Prague, General University Hospital in Prague, the great toenail of 33-year-old man, 14 November 2011, Dr Magdalena Skorepova, SK 3941/11 (PRM 860611, a dried herbarium specimen – holotype; PRM 860612 – isotype; CCF 4259T = CBS 132920T – cultures ex-holotype).
Description: Colonies on SGA attaining 31–36 mm diameter in 21 d at 25°C, colony color yellowish white (#F0EAD6) to light greenish yellow (#EAE679), floccose, central part of colony raised, crateriform, reverse light orange (#FAB57F). Colonies on MEA attaining 35–40 mm diameter in 21 d at 25°C, colony color yellowish white (#F0EAD6) to pale yellow (#F3E5AB), coarsely granular, flat, reverse light yellow (#F8DE7E) in the marginal part, deep yellow (#AF8D13) in the center. Colonies on PCA attaining diameter of 42–55 mm after 21 d at 25°C, colony color similar to that on MEA, finely granular, plane, reverse deep yellowish pink (#E66761). Colonies on PDA attaining 39–48 mm diameter in 21 d at 25°C, colony color and texture similar to PCA, reverse vivid yellowish pink (#FFB7A5). No growth observed at 37°C on all media tested above. Hair perforation test negative, urease test positive.
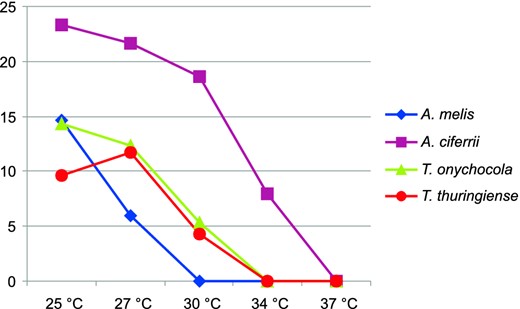
Micromorphology observed on SGA. Vegetative mycelium consists of hyaline, smooth, septate hyphae, 1.3–2.8 μm in diameter. Racquet hyphae sometimes present, mostly associated with ascomata-like structures, spiral hyphae abundantly present. Microconidia abundantly produced, borne singly on short lateral branches, terminally or sessile on the hyphae, pyriform to clavate, smooth-walled, nonseptate, 2.6–5.2 × 1.4–2.6 μm. Ascoma-like structures develop after 5–6 weeks of cultivation on nutrient-poor media with addition of sterilized hair from children, 200–650 μm, solitary or in clusters, globose to subglobose, white at first, becoming pale yellow. Peridial hyphae hyaline, septate, uncinately branched, sometimes terminated by spiral appendages with curled ends or by blunt prominences, peridial dumbbell-shaped cells, symmetrical, echinulate, 4.9–16.4 μm in length and 2.4–5.1 μm in width at the enlarged ends and 1.7–3.1 μm at constrictions. Thick-walled chlamydospores frequently present after 6–7 weeks of cultivation on MEA, in limited numbers on other media, mostly arranged in chains, variable in shape, asymmetrical, spherical, or barrel-shaped, thick walled, up to 6 μm wide. The optimal temperature for growth was between 18°C and 25°C, maximum growth temperature was 30°C, the species did not grow at 34°C (Fig. 4).
Average growth (mm) of four ex-type isolates representing Trichophyton onychocola and three most closely related species on MEA at various temperatures and evaluated after 7 days. This Figure is reproduced in color in the online version of Medical Mycology.
Etymology: “inhabiting nails”: combining onyx, onychos = nail (from the Greek) and colo, -ere, -ui, cultus = inhabit (from the Latin).
Discussion
Atypical causal agents are isolated in dermatomycology with increasing frequency. Several new infectious agents known only from dermatomycological specimens were described recently, belonging, as do dermatophytes, to the order Onygenales. Onychocola canadensis [20–22] and Auxarthron ostraviense [23] are examples of such fungi. Geophilic T. eboreum, which represents the most recently described Trichophyton species, was associated with cases of tinea pedis in a patient who was positive for the human immunodeficiency virus [8] and in erythematous skin lesions of an otherwise healthy patient [24]. However, the etiological significance of the isolated fungus in both cases was not confirmed with sufficient confidence. The geophilic nature of T. eboreum was first predicted by sequence data analysis (the species was close to geophilic Trichophyton species) [8] and later confirmed by repeated isolation of the species from badger and rabbit burrows [9].
Similarly, based on phylogenetic analysis, it is probable that the species described here is also a geophilic Trichophyton species since all such fungi are phylogenetically related and concentrated in one clade (Fig. 1). However, gymnothecium-like structures observed in T. onychocola (Fig. 3), which lack ascospores and may represent structures analogous to the cleistothecium-like structures covered by dumbbell-shaped hyphae, were described by Brasch and Gräser [8] in T. eboreum. As shown by Campbell et al. [9], T. eboreum is, in fact, a heterothallic species, and two opposite mating-type isolates are needed for ascospore production. This observation may suggest that T. onychocola is also a heterothallic species. Mating experiments with the ex-type isolates representing related species were provided on various media without success (data not shown). No isolate that is phylogenetically similar to the ex-type of T. onychocola is currently available for mating experiments, and this species is described here without teleomorph.
Although the isolation of common onychomycosis agents (e.g., T. rubrum and T. interdigitale) from cases is generally considered to be sufficient to establish these fungi as etiologic agents, this is not true for geophilic dermatophytes and less common agents of onychomycosis for which postive direct microscopy and repeated isolation should be conducted to verify their etiological significance. Our T. onychocola isolate probably does not represent the true causal agent of suspected onychomycosis in the presented case. Its pathogenic role was not confirmed by repeated isolation in temporally separated samples, and direct microscopic examination was negative at the time of sampling. Close relatives of this species are members of the T. terrestre complex and other so-called dermatophytoids, which are usually considered to be nonpathogenic. It is likely that T. onychocola represents a fungus that accompanies the true causal agent of infection or saprophyte attached to the nail in the area of a previous onychomycosis. Other noninfectious nail changes, which are commonly associated with an increased level of saprophyte and dermatophytoid isolation, cannot be excluded. However, the history of distal lateral subungual onychomycosis in our patient is probably the result of the lesion with typical morphology, which occurred on the same nail five years earlier and healed after antimycotic therapy with a 1% solution of ciclopirox olamine. Trichophyton onychocola was the only microbial agent isolated after five years when recurrence of the nail infection appeared. However, a more detailed investigation of the case was not possible because the patient did not return for follow-up inspection and therapy.
The good growth of T. onychocola on human hairs, placed on the agar medium without any other additional nutrients, could indicate that this species is capable of using human keratin as its only nutrient source. However, keratinized substrates are also composed of proteins and fatty acids, which could serve as nutrient sources for the fungi. Moreover, perforation structures were not observed in this species, and additional test should be performed in order to confirm keratinolytic abilities of T. onychocola [25,26].
The species most closely related to T. onychocola are homothallic A. ciferrii [27], heterothallic A. melis [28], and anamorphic T. thuringiense [29]. Two of them, A. melis and T. thuringiense, were similarly to T. onychocola originally described as from the central Europe region, and both species were isolated from soil and associated with mammals [28,29]. In contrast, A. ciferri was first described in soil and opossum hairs in the United States [27]; it was subsequently isolated in central Europe [30,31]. This may suggest that all of these species coexist in relatively similar ecological and geographical conditions. Neither ascomata nor sterile ascomata-like bodies (as observed in T. onychocola; Fig. 3) are produced on human hairs by T. thuringiense. In addition, the colonies of T. thuringiese are fasciculate or cottony on all media, and the species produce large chlamydospores that reach up to 15 μm in diameter [29]. Arthroderma ciferii differs from T. onychocola by having wider peridial hyphae at the swelling ends and a length of constricted zone that is shorter (“ossiform” peridial cells in T. onychocola; Fig. 3; “burger-shaped” in A. ciferii). Two- to six-celled macroconidia are abundantly produced by T. thuringiense, and two- to four-celled macroconida are less frequently produced by A. ciferrii and A. melis. In contrast, no macroconidia were observed in T. onychocola. Hair-perforating organs develop in A. melis when grown on human hairs in contrast to T. onychocola. The latter also has distinctive pale yellow colonies with red reverse on SGA (Fig. 2), PCA, and PDA in contrast to colonies of related species, which are white or brown with pink tint. Arthroderma ciferrii was the most rapidly growing species and also the only one able to grow at 34°C (Fig. 4). The growth profile of T. onychocola was most similar to that of T. thuringiense, with a maximum growth temperature at 30°C. Arthroderma melis grew only at 27°C.
This research was supported by the Ministry of Education, Youth and Sports (CZ.1.07/2.3.00/20.0055 and CZ.1.07/2.3.00/30.0003). Molecular genetic analyses were supported by the project GAUK 607812.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and the writing of the paper.