-
PDF
- Split View
-
Views
-
Cite
Cite
Martha Lappas, A20, an essential component of the ubiquitin-editing protein complex, is a negative regulator of inflammation in human myometrium and foetal membranes, Molecular Human Reproduction, Volume 23, Issue 9, September 2017, Pages 628–645, https://doi.org/10.1093/molehr/gax041
Close - Share Icon Share
Abstract
Does A20 regulate mediators involved in the terminal processes of human labour in primary myometrial and amnion cells?
A20 is a nuclear factor-kappa B (NF-κB) responsive gene that acts as a negative regulator of NF-κB-induced expression of pro-labour mediators.
Inflammation is commonly implicated in spontaneous preterm birth and the processes involved in rupture of foetal membranes and uterine contractions. In myometrium and foetal membranes, the pro-inflammatory transcription factor NF-κB regulates the transcription of pro-labour mediators in response to inflammatory stimuli. In non-gestational tissues, A20 is widely recognised as an anti-inflammatory protein that inhibits inflammation-induced NF-κB signalling.
Primary human amnion and myometrial cells were used to determine the effect of pro-inflammatory mediators on A20 expression and the effect of A20 siRNA on the expression and secretion of pro-labour mediators. The expression of A20 was assessed in myometrium and foetal membranes from non-labouring and labouring women at preterm and or term (n = 8 or nine samples per group).
The effects of pro-inflammatory mediators and of A20 siRNA in cell cultures were determined by quantitative RT-PCR (qRT-PCR), western blots, immunoassays, gelatin zymography and luciferase assays. A20 expression in tissue samples was assessed by qRT-PCR. Statistical significance was ascribed to a P value < 0.05.
In primary cells isolated from myometrium and or amnion, the pro-inflammatory cytokines IL1B and TNF, the bacterial products flagellin and fsl-1, and the viral double stranded RNA analogue poly(I:C) significantly increased A20 mRNA expression via NF-κB. A20 siRNA studies in primary myometrial and amnion cells demonstrated an augmentation of inflammation-induced expression and or secretion of pro-inflammatory cytokines (IL1A, IL6), chemokines (CXCL1, CXCL8, CCL2), adhesion molecules (ICAM1, VCAM1), contraction-associated proteins (PTGS2, PTGFR, PGF2α) and the extracellular matrix degrading enzyme MMP9, as well as NF-κB activation. Inhibition of NF-κB activity significant attenuated inflammation-induced expression of pro-labour mediators in A20 siRNA transfected cells. Finally, A20 mRNA expression was decreased in myometrium and foetal membranes with labour, and in foetal membranes with chorioamnionitis.
Not applicable.
The conclusions of this study are solely reliant on the data from in vitro experiments using cells isolated from myometrium and amnion.
The results of this study raise the possibility that targeting A20 may be a therapeutic approach to reduce inflammation associated with spontaneous preterm birth.
Associate Professor Martha Lappas is supported by a Career Development Fellowship from the National Health and Medical Research Council (NHMRC; grant no. 1047025). Funding for this study was provided by the NHMRC (grant no. 1058786), Norman Beischer Medical Research Foundation and the Mercy Research Foundation. There are no competing interests.
Introduction
Preterm birth is a worldwide health issue which accounts for over 1 million annual neonatal deaths (Liu et al., 2012). Spontaneous preterm birth, either due to idiopathic preterm labour or, in the absence of labour, due to pre-labour rupture of membranes (PROMs), accounts for up to 70% of all preterm births (Goldenberg et al., 2008). Inflammation, which can be triggered by infection or pro-inflammatory insults, is a common cause of early preterm birth (Romero et al., 1994). This results in the stimulation of the maternal immune system (Yuan et al., 2009) and ensuing pro-inflammatory cytokine and chemokine production. Chemokines promote the recruitment of leucocytes into the uterus and foetal membranes (Osman et al., 2003) leading to local release of pro-inflammatory cytokines. These cytokines can: (i) further induce cytokine and chemokine production; (ii) upregulate the expression of cell adhesion molecules (CAMs) (Ledingham et al., 2001) which control leucocyte trafficking into these tissues; (iii) activate cyclooxygenase-2 (PTGS2) (Belt et al., 1999), promoting production of the uterotonic prostaglandin production (O'Brien, 1995) via the prostaglandin F receptor (PTGFR) (Fortier et al., 2008); and (iv) and increase the expression of the extracellular matrix (ECM) remodelling enzyme matrix metalloproteinase 9 (MMP9) (Roh et al., 2000) which is involved in tissue remodelling and foetal membrane rupture.
The canonical (or classical) nuclear factor κB (NF-κB) pathway is a critical controller of the terminal processes of human labour and delivery (Lindstrom and Bennett, 2005; Lappas and Rice, 2007, 2009). There is higher expression of the RELA subunit of NF-κB in labouring human foetal membranes (Vora et al., 2008), cervix, (Stjernholm-Vladic et al., 2004) and myometrium (Choi et al., 2007). Furthermore, higher NF-κB DNA binding activity has also been detected in labouring human foetal membranes (Allport et al., 2001). In these tissues, NF-κB is activated by pro-inflammatory cytokines such as TNF and IL1B, and microbial or viral components that activate toll-like receptors (TLRs) (Lindstrom and Bennett, 2005; Lappas and Rice, 2007, 2009; Lappas, 2015). Activation of NF-κB involves the phosphorylation of the NFKBIA protein by the IκB kinase (IKK) complex; NFKBIA is ubiquitinated and subsequently degraded by proteasomes. This releases NF-κB which is then free to translocate to the nucleus where it can bind to the κB region of specific genes to alter their transcription. Functional studies have revealed that NF-κB is required for the genesis of pro-inflammatory cytokines chemokines, adhesion molecules, PTGS2 and MMP9 (Lappas et al., 2005; Lindstrom and Bennett, 2005; Lappas and Rice, 2007). Thus, controlling NF-κB signalling may be beneficial in treating spontaneous preterm birth.
Numerous studies have shown that there are several autoregulatory feedback loops that can terminate NF-κB signalling (Renner and Schmitz, 2009). One such protein is A20 (also known as TNF-induced protein 3, TNFAIP3), an essential component of a ubiquitin-editing protein complex. A20 has been found to be required for terminating activation of NF-κB induced by various pro-inflammatory insults including IL1B, TNF, or pathogens via TLRs (Dixit et al., 1990; Cooper et al., 1996; Boone et al., 2004; Onose et al., 2006; Luo et al., 2015). The phenotype of A20 knockout mice have been vital in demonstrating the potent anti-inflammatory role of A20. Mice deficient in A20 show enhanced NF-κB responses and die early because of severe multi-organ inflammation (Lee et al., 2000). Total or partial loss of A20 in mice results in spontaneous neuroinflammation as evidenced by significantly higher levels of TNF, IL6 and CCL2 (mRNA and protein) in cerebral cortex and hippocampus (Guedes et al., 2014). Similarly, excessive NF-κB activation and or inflammatory responses is observed in mice with myeloid cell-specific deletion of A20 (A20myel-KO) (Matmati et al., 2011), mice that are deficient for A20 in both intestinal epithelial cells and myeloid cells (Vereecke et al., 2014) and in mice lacking liver parenchymal cell A20 (Catrysse et al., 2016). In vitro, basal, IL1B-, TNF- or LPS-induced cytokine production is much higher in cells lacking A20 (Guedes et al., 2014; Luo et al., 2015). Conversely, overexpression of A20 in pancreatic islets of Langerhans confers resistance to cytokine-mediated activation of NF-κB (Grey et al., 2003); A20 overexpression in liver is protective in hepatectomy (Longo et al., 2005) and acute toxic hepatitis (Arvelo et al., 2002) through anti-inflammatory functions; and adenoviral delivery of A20 attenuates allergic airway inflammation (Kang et al., 2009). Finally, in humans, A20 polymorphisms have been associated with a plethora of different immune pathologies including inflammatory bowel disease (Bates et al., 2009; Adrianto et al., 2011; Catrysse et al., 2014).
Genome-wide expression profiling of a myometrial cell line PHM1-31 following exposure to IL1B identified a ~30-fold increase in A20 mRNA expression (Chevillard et al., 2007). Apart from this one study, not much is known about the role of A20 in human labour. Thus, this study aimed to investigate the involvement of A20 in the termination of NF-κB signalling in response to pro-inflammatory mediators. The specific aims of this study were to: (i) investigate the effect of pro-inflammatory mediators on A20 expression in myometrium, foetal membranes and cells isolated from the human myometrium and amnion; (ii) elucidate whether A20 siRNA knockdown regulates pro-labour mediators in primary cells isolated from human myometrium and amnion and the role of NF-κB in this process; and (iii) characterise the expression of A20 in human myometrium and foetal membranes obtained from labouring and non-labouring women at preterm and term. To mimic the processes of inflammation-induced preterm labour, the following stimuli were used in cell culture: pro-inflammatory cytokines IL1B and TNF, the bacterial product and TLR2 ligand fibroblast-stimulating lipopeptide (fsl-1), and the viral dsRNA analogue and TLR3 ligand polyinosinic polycytidilic acid (poly(I:C)).
Materials and Methods
Ethics statement
Approval for this study was obtained from the Research Ethics Committee of Mercy Hospital for Women. Written, informed consent was obtained from all participating women.
Tissue collection for expression studies
For the expression studies, myometrium and foetal membranes were obtained from women at the time of delivery at term or preterm as detailed below. All tissues were brought to the research laboratory, processed within 15 min of delivery and samples were immediately snap frozen in liquid nitrogen and stored at −80°C. Women with any underlying medical conditions such as diabetes, asthma, polycystic ovary syndrome, pre-eclampsia and macrovascular complications were excluded. Additionally, women with multiple pregnancies, obese women and fetuses with chromosomal abnormalities were excluded.
Myometrial biopsies, obtained from the upper margin of the lower uterine segment incision during Caesarean section, were collected from (i) women undergoing elective Caesarean section in the absence of labour (n = 8 patients; mean gestational age 39.4 ± 0.3 weeks) and (ii) women who were delivered during active labour (n = 8 patients; mean gestational age 39.8 ± 0.2 weeks). Labour was defined as the presence of regular uterine contractions (every 3–4 min) resulting in cervical effacement and dilation. Indications for Caesarean section in the absence of labour were breech presentation and/or previous Caesarean section. Indications for Caesarean section in the labouring samples were for placenta praevia, foetal distress and delayed or failure to progress. In the labouring group, none of the patients received any medications to augment or induce labour, and the average length of labour was 10 h ± 6 h 40 min.
Foetal membranes were obtained from women (i) at term, no labour undergoing elective Caesarean section (n = 9 patients; mean gestational age 39.3 ± 0.3 weeks) and (ii) at term after spontaneous labour, spontaneous membrane rupture and normal vaginal delivery (n = 9 patients; mean gestational age 40.4 ± 0.2 weeks). Indications for Caesarean section were breech presentation and/or previous Caesarean section. None of the patients received any medications to augment or induce labour, and the average ± SEM length of labour was 8 h 31 min ± 2 h 7 min. The relevant clinical characteristics of the patients used for this study have previously been described (Lim et al., 2017).
Foetal membranes were also obtained from women at preterm birth for two studies: preterm labour and preterm chorioamnionitis. For these two studies, different patients were used, with the clinical details of the patients detailed elsewhere (Lim et al., 2017). For the preterm labour study, foetal membranes (amnion and choriodecidua) were obtained from women (i) at Caesarean section in the absence of labour with intact membranes (n = 9 patients; mean gestational age 33.3 ± 0.8 weeks) and (ii) after spontaneous labour and normal vaginal delivery (n = 9 patients; mean gestational age 33.6 ± 0.7 weeks). For the chorioamnionitis study, only amnion was collected as the choriodecidual tissue was degraded and could not be collected. Amnion was collected from women at (i) Caesarean section in the absence of labour (n = 8 patients; mean gestational age 33.6 ± 0.7 weeks) and (ii) Caesarean section in the absence of labour with histologically confirmed acute chorioamnionitis (n = 8 patients; mean gestational age 28.5 ± 1.5 weeks). Indications for preterm delivery (in the absence of labour) were placenta praevia, placental abruption, antepartum haemorrhage or Rhesus isoimmunisation. All placentas collected from preterm gestations were subject to histopathological examination and foetal membranes were swabbed for microbiological culture investigations. Chorioamnionitis was diagnosed pathologically according to standard criteria which included histological evidence of macrophages and neutrophils permeating the chorionic cell layer and often infiltrating the amniotic cell layer (Tita and Andrews, 2010).
Primary amnion and myometrial cell culture
For these studies, fresh amnion and myometrium was obtained from women who delivered healthy, singleton infants at term (37–40 weeks gestation) undergoing elective Caesarean section in the absence of labour. Patient exclusion criteria for these studies were the same as those described above. Amnion cells (epithelial and mesenchymal) were prepared as previously described (Lim et al., 2013b). Briefly, amnion strips were washed in PBS and digested, twice, with 0.125% collagenase A and 0.25% trypsin in serum-free DMEM for 35 min at 37°C. The cell suspension was filtered through a cell strainer and the eluate was neutralised with 1% FCS. The cell suspensions were centrifuged at 500 × g for 10 min and the cells cultured in DMEM/F-12, 10% FCS and 1% penicillin-streptomycin. The media was replaced after 4 h then every 24–48 h thereafter. Myometrial cells were isolated and cultured as previously described (Lappas, 2015). Briefly, myometrium was minced and digested for 1 h in Dulbecco's Modified Eagle's Medium/Nutrient Mixture F-12 Ham (DMEM/F-12) with 3 mg/ml type one collagenase (Worthington Biochemical, Freehold, USA) and 80 μg/ml DNase 1 (Roche Diagnostics, Castle Hill, Australia) at 37°C. Cells were centrifuged at 400 × g for 10 min and grown in DMEM/F-12 enriched with 10% heat-inactivated foetal calf serum (containing 100 U/ml penicillin G and 100 mg/ml streptomycin).
To determine the effect of pro-inflammatory mediators on A20 mRNA expression, cells at approximately 80% confluence were incubated in the absence or presence of 1 ng/ml IL1B (PeproTech; Rocky Hill, NJ, USA), 10 ng/ml TNF (PeproTech; Rocky Hill, NJ, USA), 250 ng/ml fsl-1 (InVivoGen; San Diego, CA, USA), 5 μg/ml poly(I:C) (Sigma-Aldrich; St. Louis, MO, USA), or 1 μg/ml flagellin (purified flagellin from B. subtilis; InVivoGen; San Diego, CA, USA) for up to 20 h. To determine the effect of the NF-κB inhibitor BAY 11-7082 on A20 expression, experiments were performed as detailed above; however, cells were pre-treated with 10 μM BAY 11-7082 for 30 min, then 1 ng/ml IL1B, 10 ng/ml TNF, 250 ng/ml fsl-1, 5 μg/ml poly(I:C) or 1 μg ml flagellin for an additional 20 h. The concentration of BAY 11-7082 is based on previous studies in myometrial cells (Lim et al., 2013a). Cells were collected and stored at −80°C until assayed for A20 mRNA expression by qRT-PCR as detailed below. Experiments were performed in amnion and myometrial cells obtained from five patients.
Loss-of-function studies, using siRNA, were also performed to determine if A20 regulates pro-labour mediators. Transfection of primary amnion and myometrial cells with siRNA was performed as previously described (Lim et al., 2015a). Briefly, cells at approximately 50% confluence were transfected using Lipofectamine 3000 according to manufacturer's guidelines (Life Technologies; Mulgrave, Victoria, Australia). A20 siRNA (siA20) and negative control siRNA (siCONT) were obtained from Ambion (Thermo Fisher Scientific; Scoresby, Vic, Australia). Cells were transfected with 50 nM siA20 or 50 nM siA20 in DMEM/F-12 for 48 h. The medium was then replaced with DMEM/F-12 (containing 0.5% BSA) with or without 1 ng/ml IL1B, 10 ng/ml TNF, 250 ng/ml fsl-1 or 5 μg/ml poly(I:C) and the cells were incubated at 37°C for an additional 20 h. For a subset of experiments, cells were treated with 250 ng/ml fsl-1 in the absence or presence of 10 μM BAY 11-7082 or 400 μM naringenin for 20 h. After final incubation, cells and media were collected separately and stored at −80°C. Cell viability was assessed by the 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide proliferation assay as previously described (Lim et al., 2014b). The response to IL1B, TNF, fsl-1 and poly(I:C) between patients varied greatly, as previously reported (Lim et al., 2015a). Thus, data are presented as fold change in expression relative to the expression level in the IL1B-, TNF-, fsl-1- or poly(I:C)-stimulated siCONT transfected cells, which was set at 1. Data could not be normalised to siCONT transfected cells alone as some of the readings were 0. Experiments were performed in cells obtained from five patients.
RNA extraction and qRT-PCR
RNA extractions and qRT-PCR was performed as previously described (Lappas, 2015). RNA concentration and purity were measured using a NanoDrop ND1000 (Thermo Fisher Scientific; Scoresby, Vic, Australia). RNA was converted to cDNA using the high-capacity cDNA reverse transcription kit (Thermo Fisher Scientific; Scoresby, Vic, Australia) according to the manufacturer's instructions. The RT-PCR was performed using the CFX384 Real-Time PCR detection system (Bio-Rad Laboratories; Gladesville, NSW, Australia) using 100 nM of pre-designed and validated QuantiTect primers (primer sequences not available) (Qiagen; Chadstone Centre, Vic, Australia). Average gene Ct values were normalised to the average YWHAZ and succinate dehydrogenase (SDHA) Ct values of the same cDNA sample. Fold differences were determined using the comparative Ct method.
Western blotting
Western blotting was performed as previously described (Lappas, 2015). Blots were incubated in a 1/1000 dilution of rabbit monoclonal anti-A20 #5630 (Cell Signalling Technology; Danvers, MA, USA) or 1 μg/ml rabbit polyclonal anti-NFKBIA (Santa Cruz Biotechnology, Santa Cruz, CA, USA) prepared in blocking buffer (5% skim milk in TBS (50 mM Tris–HCl, pH 7.5, 150 mM NaCl) with 0.05% Tween-20) for 16 h at 4°C. Semi-quantitative analysis of the relative density of the bands in Western blots was performed using Quantity One 4.2.1 image analysis software (Bio-Rad Laboratories, Hercules, CA, USA).
Enzyme immunoassays
Assessment of cytokine and chemokine release of IL6 and CXCL8 was performed using the CytoSet™ sandwich ELISA according to the manufacturer's instructions (Life Technologies; Mulgrave, Vic, Australia). The release of CXCL1, CCL2, sICAM1 and sVCAM1 was performed by sandwich ELISA from R&D Systems (Minneapolis, MN, USA) according to the manufacturer's instructions. The release of PGF2α into the incubation medium was assayed using a commercially available competitive enzyme immunoassay kit according to the manufacturer's specifications (Kookaburra Kits from Sapphire Bioscience, NSW, Australia). The interassay and intraassay coefficients of variation for all assays were less than 10%.
Gelatin zymography
Incubation media was also collected and assessment of MMP9 was performed by gelatin zymography as previously described (Lim et al., 2013c). Proteolytic activity was visualised as clear zones of lysis on a blue background of undigested gelatin. Gels were scanned using a ChemiDoc XRS system (Bio-Rad Laboratories; Gladesville, NSW, Australia) and inverted, and densitometry was performed using using Quantity One image analysis software (Bio-Rad Laboratories; Gladesville, NSW, Australia).
Luciferase assay
A luciferase assay was also used to determine the effect of siA20 on NF-κB RELA subunit as previously described (Lim et al., 2016b). Primary myometrial cells were transfected with 300 ng/ml RELA reporter construct (Qiagen; Chadstone Centre, Vic, Australia) using FuGENE HD transfection reagent (Promega; Alexandria NSW, Australia). After 6 h, cells were transfected with 50 nM of siA20 or siCONT (as detailed above) for 48 h. The medium was then replaced with DMEM/F-12 (containing 0.5% BSA), with or without 1 ng/ml IL1B, 250 ng/ml fsl-1 or 5 μg/ml poly(I:C), and the cells were incubated at 37°C for an additional 20 h. After the final incubation, cells were harvested in lysis buffer, and luminescence activity was measured using a Luciferase Reporter Assay Kit (Life Research; Scoresby, Vic, Australia) and Renilla Luciferase Flash Assay kit (Thermo Fisher Scientific; Scoresby, Vic, Australia) as instructed. The ratio of the firefly luciferase level to the Renilla luciferase level was determined and the results are expressed as a ratio of normalised luciferase activity. The experiments were performed from myometrium obtained from five patients.
Statistical analysis
All statistical analyses were undertaken using GraphPad Prism (GraphPad Software, La Jolla, CA, USA). For two sample comparisons, either a paired or unpaired Student's t-test was used to assess statistical significance between normally distributed data; otherwise, the nonparametric Mann–Whitney U (unpaired) or the Wilcoxon (matched pairs) tests were used. For all other comparisons, the homogeneity of data was assessed by the Bartlett's test, and when significant, the data were logarithmically transformed before further analysis using a one-way ANOVA (with LSD post-hoc testing to discriminate among the means). Statistical significance was ascribed to a P value <0.05. Data were expressed as mean ± SEM.
Results
Effect of pro-inflammatory mediators on A20 expression in primary myometrial and amnion cells
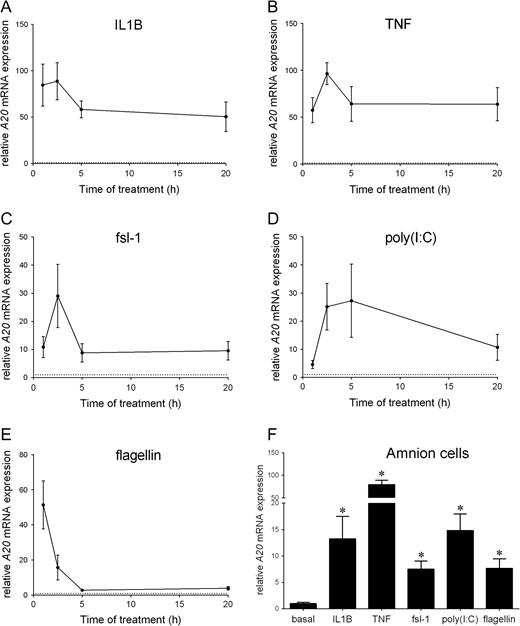
The first goal of this study was to define the outcome of pro-inflammatory mediators on the expression of A20. In primary myometrial cells, a time-course study was performed over 20 h and the data were presented in Fig. 1A–E. For IL1B (Fig. 1A) and TNF (Fig. 1B), A20 mRNA expression was rapidly induced and remained high over the 20 h period. Within 1 h of treatment, there was a ~70-fold increase in A20 mRNA expression. A20 mRNA expression was further increased at 2.5 h and then decreased by 20 h. A20 mRNA levels surged >30-fold at 2.5 h of treatment with fls-1 (Fig. 1C) or poly(I:C) (Fig. 1D) treatment. A20 mRNA expression subsequently decreased, yet remained above the baseline for the 20 h treatment period (~10-fold increase compared to basal levels). For flagellin (Fig. 1E), A20 mRNA expression was rapidly induced within 1 h (50-fold increase) and then decreased over time to ~5-fold by 5 and 20 h.
Effect of pro-inflammatory mediators on A20 expression in primary myometrial and amnion cells. (A–E) Human myometrial cells were incubated in the absence or presence of (A) 1 ng/ml IL1B, (B) 10 ng/ml TNF, (C) 250 ng/ml fsl-1, (D) 5 μg/ml poly(I:C) or (E) 1 μg/ml flagellin for 1, 2.5, five or 20 h (n = 6 patients per treatment). For all data, A20 mRNA expression was analysed by qRT-PCR and the fold change was calculated relative to basal. The dotted line denotes basal A20 levels at each time point. Data are displayed as mean ± SEM. (F) Human amnion cells were incubated in the absence or presence of 1 ng/ml IL-1β, 10 ng/ml TNF, 250 ng/ml fsl-1, 5 μg/ml poly(I:C) or 1 μg/ml flagellin for 2.5 h (n = 6 patients per treatment). For all data, A20 mRNA expression was analysed by qRT-PCR and the fold change was calculated relative to basal. Data are displayed as mean ± SEM. *P < 0.05 vs. basal (paired sample comparison).
The effect of pro-inflammatory mediators on A20 was also assessed in amnion cells after 2.5 h of treatment. As shown in Fig. 1F, there was >7.5-fold increase in A20 mRNA expression by all mediators, with TNF inducing the greatest increase (~100-fold).
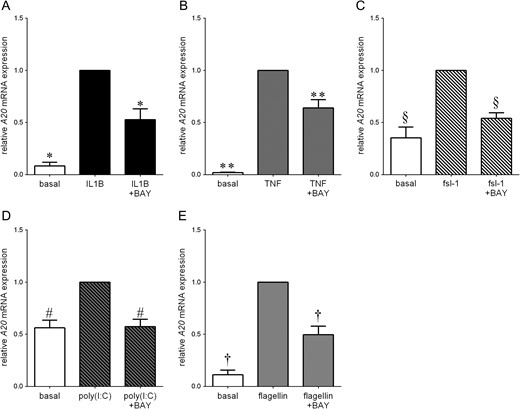
The next aim was to determine whether these pro-inflammatory mediators regulate A20 expression through activation of NF-κB. To test this, the pharmacological inhibitor of NF-κB, BAY 11-7082, was used at concentrations previously shown to inhibit NF-κB transcriptional activity in primary myometrial cells (Lim et al., 2013a). As shown in Fig. 2, BAY 11-7082 significantly attenuated IL1B (Fig. 2A), TNF (Fig. 2B), fsl-1 (Fig. 2C), poly(I:C) (Fig. 2D) and flagellin (Fig. 2E) induced A20 mRNA expression.
NF-κB regulates A20 expression in primary myometrial cells. Human primary myometrial cells were treated with (A) 1 ng/ml IL1B, (B) 10 ng/ml TNF, (C) 250 ng/ml fsl-1, (D) 5 μg/ml poly(I:C) or (E) 1 μg/ml flagellin with or without BAY 11-7082 for 2.5 h (n = 5 patients). For all data, A20 mRNA expression was analysed by qRT-PCR and the fold change was calculated relative to basal. Data are displayed as mean ± SEM. *P < 0.05 vs. IL1B (one-way ANOVA); **P < 0.05 vs. TNF (one-way ANOVA); §P < 0.05 vs. fls-1 (one-way ANOVA); #P < 0.05 vs. poly(I:C) (one-way ANOVA); †P < 0.05 vs. flagellin (one-way ANOVA).
Effect of siA20 on pro-inflammatory cytokines and chemokines in primary myometrial and amnion cells
The efficacy of siRNA knockdown was firstly assessed and the data presented in Supplementary Figure 1. The effect of siA20 was an 85% decrease in A20 protein expression in myometrial cells and a 70% decrease in A20 protein expression in amnion cells. Cell viability was not affected by siA20.
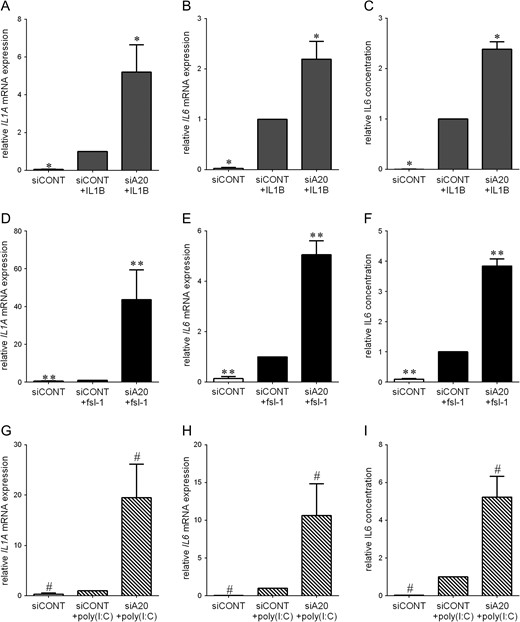
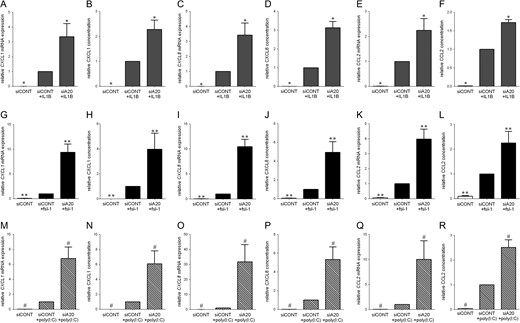
Consequently, cells were treated with IL1B, TNF, fsl-1 or poly(I:C) as models of inflammation associated with preterm labour. The effect of siA20 in pro-inflammatory cytokine (IL1A, IL6) and chemokine (CXCL1, CXCL8, CCL2) mRNA expression and secretion in myometrial cells is presented in Figs 3 and 4, respectively; the data for amnion cells is presented in Table I. As expected in siCONT transfected cells, IL1B, TNF, fsl-1 or poly(I:C) treatment of cells significantly increased IL-1α, IL6, CXCL1, CXCL8 and CCL2 mRNA expression and IL6, CXCL1, CXCL8 and CCL2 release. For all treatments, the effect of transfection with siA20 was a significant augmentation of pro-inflammatory cytokine (IL1A, IL6) and chemokine (CXCL1, CXCL8, CCL2) mRNA expression and secretion in both myometrial and amnion cells.
Effect of siA20 on pro-inflammatory cytokines and chemokines in human primary amnion cells.
| . | IL6 mRNA expression . | IL6 release . | CXCL1 mRNA expression . | CXCL1 release . | CXCL8 mRNA expression . | CXCL8 release . | CCL2 mRNA expression . |
|---|---|---|---|---|---|---|---|
| siCONT | 0.07 ± 0.04* | 0.00 ± 0.00* | 0.02 ± 0.01* | 0.00 ± 0.00* | 0.38 ± 0.22* | 0.00 ± 0.00* | 0.00 ± 0.00* |
| siCONT + IL1B | 1.00 ± 0.00 | 1.00 ± 0.00 | 1.00 ± 0.00 | 1.00 ± 0.00 | 1.00 ± 0.00 | 1.00 ± 0.00 | 1.00 ± 0.00 |
| siA20 + IL1B | 3.74 ± 0.79* | 2.77 ± 0.42* | 5.40 ± 1.42* | 2.19 ± 0.25* | 14.24 ± 6.95* | 1.90 ± 0.13* | 7.03 ± 1.12* |
| siCONT | 0.02 ± 0.01** | 0.00 ± 0.00** | 0.08 ± 0.04** | 0.00 ± 0.00** | 0.04 ± 0.02** | 0.00 ± 0.00** | 0.00 ± 0.00** |
| siCONT + TNF | 1.00 ± 0.00 | 1.00 ± 0.00 | 1.00 ± 0.00 | 1.00 ± 0.00 | 1.00 ± 0.00 | 1.00 ± 0.00 | 1.00 ± 0.00 |
| siA20 + TNF | 2.16 ± 0.01** | 1.31 ± 0.15 | 3.02 ± 0.85** | 2.70 ± 0.36** | 7.84 ± 2.33** | 2.19 ± 0.14** | 5.04 ± 0.91** |
| siCONT | 0.06 ± 0.03# | 0.00 ± 0.00# | 0.11 ± 0.04# | 0.00 ± 0.00# | 0.05 ± 0.02# | 0.00 ± 0.00# | 0.01 ± 0.01# |
| siCONT + fsl-1 | 1.00 ± 0.00 | 1.00 ± 0.00 | 1.00 ± 0.00 | 1.00 ± 0.00 | 1.00 ± 0.00 | 1.00 ± 0.00 | 1.00 ± 0.00 |
| siA20 + fsl-1 | 2.26 ± 0.22# | 2.17 ± 0.33# | 11.91 ± 5.26# | 4.60 ± 0.71# | 10.58 ± 1.49# | 3.56 ± 0.94# | 9.73 ± 2.03# |
| siCONT | 0.21 ± 0.07§ | 0.00 ± 0.00§ | 0.15 ± 0.09§ | 0.00 ± 0.00§ | 1.85 ± 1.51§ | 0.00 ± 0.00§ | 0.00 ± 0.00§ |
| siCONT + poly(I:C) | 1.00 ± 0.00 | 1.00 ± 0.00 | 1.00 ± 0.00 | 1.00 ± 0.00 | 1.00 ± 0.00 | 1.00 ± 0.00 | 1.00 ± 0.00 |
| siA20 + poly(I:C) | 3.34 ± 0.72§ | 2.13 ± 0.43§ | 4.82 ± 1.94§ | 3.93 ± 0.82§ | 6.33 ± 2.22§ | 2.35 ± 0.59§ | 15.52 ± 3.97§ |
| . | IL6 mRNA expression . | IL6 release . | CXCL1 mRNA expression . | CXCL1 release . | CXCL8 mRNA expression . | CXCL8 release . | CCL2 mRNA expression . |
|---|---|---|---|---|---|---|---|
| siCONT | 0.07 ± 0.04* | 0.00 ± 0.00* | 0.02 ± 0.01* | 0.00 ± 0.00* | 0.38 ± 0.22* | 0.00 ± 0.00* | 0.00 ± 0.00* |
| siCONT + IL1B | 1.00 ± 0.00 | 1.00 ± 0.00 | 1.00 ± 0.00 | 1.00 ± 0.00 | 1.00 ± 0.00 | 1.00 ± 0.00 | 1.00 ± 0.00 |
| siA20 + IL1B | 3.74 ± 0.79* | 2.77 ± 0.42* | 5.40 ± 1.42* | 2.19 ± 0.25* | 14.24 ± 6.95* | 1.90 ± 0.13* | 7.03 ± 1.12* |
| siCONT | 0.02 ± 0.01** | 0.00 ± 0.00** | 0.08 ± 0.04** | 0.00 ± 0.00** | 0.04 ± 0.02** | 0.00 ± 0.00** | 0.00 ± 0.00** |
| siCONT + TNF | 1.00 ± 0.00 | 1.00 ± 0.00 | 1.00 ± 0.00 | 1.00 ± 0.00 | 1.00 ± 0.00 | 1.00 ± 0.00 | 1.00 ± 0.00 |
| siA20 + TNF | 2.16 ± 0.01** | 1.31 ± 0.15 | 3.02 ± 0.85** | 2.70 ± 0.36** | 7.84 ± 2.33** | 2.19 ± 0.14** | 5.04 ± 0.91** |
| siCONT | 0.06 ± 0.03# | 0.00 ± 0.00# | 0.11 ± 0.04# | 0.00 ± 0.00# | 0.05 ± 0.02# | 0.00 ± 0.00# | 0.01 ± 0.01# |
| siCONT + fsl-1 | 1.00 ± 0.00 | 1.00 ± 0.00 | 1.00 ± 0.00 | 1.00 ± 0.00 | 1.00 ± 0.00 | 1.00 ± 0.00 | 1.00 ± 0.00 |
| siA20 + fsl-1 | 2.26 ± 0.22# | 2.17 ± 0.33# | 11.91 ± 5.26# | 4.60 ± 0.71# | 10.58 ± 1.49# | 3.56 ± 0.94# | 9.73 ± 2.03# |
| siCONT | 0.21 ± 0.07§ | 0.00 ± 0.00§ | 0.15 ± 0.09§ | 0.00 ± 0.00§ | 1.85 ± 1.51§ | 0.00 ± 0.00§ | 0.00 ± 0.00§ |
| siCONT + poly(I:C) | 1.00 ± 0.00 | 1.00 ± 0.00 | 1.00 ± 0.00 | 1.00 ± 0.00 | 1.00 ± 0.00 | 1.00 ± 0.00 | 1.00 ± 0.00 |
| siA20 + poly(I:C) | 3.34 ± 0.72§ | 2.13 ± 0.43§ | 4.82 ± 1.94§ | 3.93 ± 0.82§ | 6.33 ± 2.22§ | 2.35 ± 0.59§ | 15.52 ± 3.97§ |
For all data, the fold change was calculated relative to IL1B-, TNF-, fsl-1- or poly(I:C)-stimulated siCONT transfected cells. All data are displayed as mean ± SEM. *P < 0.05 vs. IL1B-stimulated siCONT transfected cells (one-way ANOVA); **P < 0.05 vs. TNF-stimulated siCONT transfected cells (one-way ANOVA); #P < 0.05 vs. fsl-1-stimulated siCONT transfected cells (one-way ANOVA); §P < 0.05 vs. poly(I:C)-stimulated siCONT transfected cells (one-way ANOVA).
Effect of siA20 on pro-inflammatory cytokines and chemokines in human primary amnion cells.
| . | IL6 mRNA expression . | IL6 release . | CXCL1 mRNA expression . | CXCL1 release . | CXCL8 mRNA expression . | CXCL8 release . | CCL2 mRNA expression . |
|---|---|---|---|---|---|---|---|
| siCONT | 0.07 ± 0.04* | 0.00 ± 0.00* | 0.02 ± 0.01* | 0.00 ± 0.00* | 0.38 ± 0.22* | 0.00 ± 0.00* | 0.00 ± 0.00* |
| siCONT + IL1B | 1.00 ± 0.00 | 1.00 ± 0.00 | 1.00 ± 0.00 | 1.00 ± 0.00 | 1.00 ± 0.00 | 1.00 ± 0.00 | 1.00 ± 0.00 |
| siA20 + IL1B | 3.74 ± 0.79* | 2.77 ± 0.42* | 5.40 ± 1.42* | 2.19 ± 0.25* | 14.24 ± 6.95* | 1.90 ± 0.13* | 7.03 ± 1.12* |
| siCONT | 0.02 ± 0.01** | 0.00 ± 0.00** | 0.08 ± 0.04** | 0.00 ± 0.00** | 0.04 ± 0.02** | 0.00 ± 0.00** | 0.00 ± 0.00** |
| siCONT + TNF | 1.00 ± 0.00 | 1.00 ± 0.00 | 1.00 ± 0.00 | 1.00 ± 0.00 | 1.00 ± 0.00 | 1.00 ± 0.00 | 1.00 ± 0.00 |
| siA20 + TNF | 2.16 ± 0.01** | 1.31 ± 0.15 | 3.02 ± 0.85** | 2.70 ± 0.36** | 7.84 ± 2.33** | 2.19 ± 0.14** | 5.04 ± 0.91** |
| siCONT | 0.06 ± 0.03# | 0.00 ± 0.00# | 0.11 ± 0.04# | 0.00 ± 0.00# | 0.05 ± 0.02# | 0.00 ± 0.00# | 0.01 ± 0.01# |
| siCONT + fsl-1 | 1.00 ± 0.00 | 1.00 ± 0.00 | 1.00 ± 0.00 | 1.00 ± 0.00 | 1.00 ± 0.00 | 1.00 ± 0.00 | 1.00 ± 0.00 |
| siA20 + fsl-1 | 2.26 ± 0.22# | 2.17 ± 0.33# | 11.91 ± 5.26# | 4.60 ± 0.71# | 10.58 ± 1.49# | 3.56 ± 0.94# | 9.73 ± 2.03# |
| siCONT | 0.21 ± 0.07§ | 0.00 ± 0.00§ | 0.15 ± 0.09§ | 0.00 ± 0.00§ | 1.85 ± 1.51§ | 0.00 ± 0.00§ | 0.00 ± 0.00§ |
| siCONT + poly(I:C) | 1.00 ± 0.00 | 1.00 ± 0.00 | 1.00 ± 0.00 | 1.00 ± 0.00 | 1.00 ± 0.00 | 1.00 ± 0.00 | 1.00 ± 0.00 |
| siA20 + poly(I:C) | 3.34 ± 0.72§ | 2.13 ± 0.43§ | 4.82 ± 1.94§ | 3.93 ± 0.82§ | 6.33 ± 2.22§ | 2.35 ± 0.59§ | 15.52 ± 3.97§ |
| . | IL6 mRNA expression . | IL6 release . | CXCL1 mRNA expression . | CXCL1 release . | CXCL8 mRNA expression . | CXCL8 release . | CCL2 mRNA expression . |
|---|---|---|---|---|---|---|---|
| siCONT | 0.07 ± 0.04* | 0.00 ± 0.00* | 0.02 ± 0.01* | 0.00 ± 0.00* | 0.38 ± 0.22* | 0.00 ± 0.00* | 0.00 ± 0.00* |
| siCONT + IL1B | 1.00 ± 0.00 | 1.00 ± 0.00 | 1.00 ± 0.00 | 1.00 ± 0.00 | 1.00 ± 0.00 | 1.00 ± 0.00 | 1.00 ± 0.00 |
| siA20 + IL1B | 3.74 ± 0.79* | 2.77 ± 0.42* | 5.40 ± 1.42* | 2.19 ± 0.25* | 14.24 ± 6.95* | 1.90 ± 0.13* | 7.03 ± 1.12* |
| siCONT | 0.02 ± 0.01** | 0.00 ± 0.00** | 0.08 ± 0.04** | 0.00 ± 0.00** | 0.04 ± 0.02** | 0.00 ± 0.00** | 0.00 ± 0.00** |
| siCONT + TNF | 1.00 ± 0.00 | 1.00 ± 0.00 | 1.00 ± 0.00 | 1.00 ± 0.00 | 1.00 ± 0.00 | 1.00 ± 0.00 | 1.00 ± 0.00 |
| siA20 + TNF | 2.16 ± 0.01** | 1.31 ± 0.15 | 3.02 ± 0.85** | 2.70 ± 0.36** | 7.84 ± 2.33** | 2.19 ± 0.14** | 5.04 ± 0.91** |
| siCONT | 0.06 ± 0.03# | 0.00 ± 0.00# | 0.11 ± 0.04# | 0.00 ± 0.00# | 0.05 ± 0.02# | 0.00 ± 0.00# | 0.01 ± 0.01# |
| siCONT + fsl-1 | 1.00 ± 0.00 | 1.00 ± 0.00 | 1.00 ± 0.00 | 1.00 ± 0.00 | 1.00 ± 0.00 | 1.00 ± 0.00 | 1.00 ± 0.00 |
| siA20 + fsl-1 | 2.26 ± 0.22# | 2.17 ± 0.33# | 11.91 ± 5.26# | 4.60 ± 0.71# | 10.58 ± 1.49# | 3.56 ± 0.94# | 9.73 ± 2.03# |
| siCONT | 0.21 ± 0.07§ | 0.00 ± 0.00§ | 0.15 ± 0.09§ | 0.00 ± 0.00§ | 1.85 ± 1.51§ | 0.00 ± 0.00§ | 0.00 ± 0.00§ |
| siCONT + poly(I:C) | 1.00 ± 0.00 | 1.00 ± 0.00 | 1.00 ± 0.00 | 1.00 ± 0.00 | 1.00 ± 0.00 | 1.00 ± 0.00 | 1.00 ± 0.00 |
| siA20 + poly(I:C) | 3.34 ± 0.72§ | 2.13 ± 0.43§ | 4.82 ± 1.94§ | 3.93 ± 0.82§ | 6.33 ± 2.22§ | 2.35 ± 0.59§ | 15.52 ± 3.97§ |
For all data, the fold change was calculated relative to IL1B-, TNF-, fsl-1- or poly(I:C)-stimulated siCONT transfected cells. All data are displayed as mean ± SEM. *P < 0.05 vs. IL1B-stimulated siCONT transfected cells (one-way ANOVA); **P < 0.05 vs. TNF-stimulated siCONT transfected cells (one-way ANOVA); #P < 0.05 vs. fsl-1-stimulated siCONT transfected cells (one-way ANOVA); §P < 0.05 vs. poly(I:C)-stimulated siCONT transfected cells (one-way ANOVA).
Effect of siA20 on pro-inflammatory cytokines in primary myometrial cells. Human primary myometrial cells were transfected with 50 nM siCONT or 50 nM siA20 for 48 h and then treated with (A–C) 1 ng/ml IL1B, (D–F) 250 ng/ml fsl-1, or (G–I) 5 μg/ml poly(I:C) for an additional 20 h (n = 5 patients). (A,B,D,E,G,H) IL1A and IL6 mRNA expression was analysed by qRT-PCR. (C,F,I) The concentration of IL6 in the incubation medium was assayed by ELISA. For all data, the fold change was calculated relative to IL1B-, fsl-1- or poly(I:C)-stimulated siCONT transfected cells, and displayed as mean ± SEM. *P < 0.05 vs. IL1B-stimulated siCONT transfected cells (one-way ANOVA); **P < 0.05 vs. fsl-1-stimulated siCONT transfected cells (one-way ANOVA); #P < 0.05 vs. poly(I:C)-stimulated siCONT transfected cells (one-way ANOVA).
Effect of siA20 on chemokines in primary myometrial cells. Human primary myometrial cells were transfected with 50 nM siCONT or 50 nM siA20 for 48 h and then treated with (A–F) 1 ng/ml IL1B, (G–L) 250 ng/ml fsl-1 or (M–R) 5 μg/ml poly(I:C) for an additional 20 h (n = 5 patients). (A,C,E,G,I,K,M,O,Q) CXCL1, CXCL8 and CCL2 mRNA expression was analysed by qRT-PCR. (B,D,F,H,J,L,N,P,R) The concentration of CXCL1, CXCL8 and CCL2 in the incubation medium was assayed by ELISA. For all data, the fold change was calculated relative to IL1B-, fsl-1- or poly(I:C)-stimulated siCONT transfected cells, and displayed as mean ± SEM. *P < 0.05 vs. IL1B-stimulated siCONT transfected cells (one-way ANOVA); **P < 0.05 vs. fsl-1-stimulated siCONT transfected cells (one-way ANOVA); #P < 0.05 vs. poly(I:C)-stimulated siCONT transfected cells (one-way ANOVA).
Effect of siA20 on adhesion molecules in primary myometrial cells
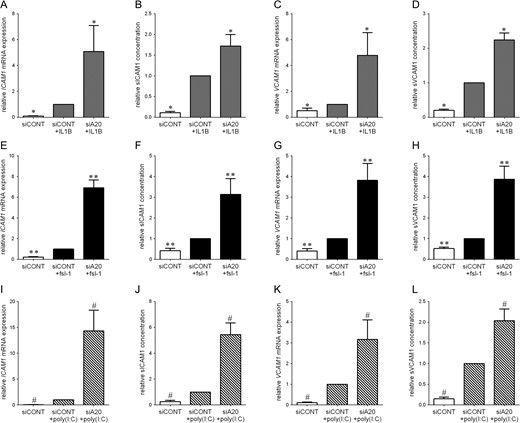
The effect of siA20 on the mRNA expression and secretion of the adhesion molecules ICAM1 and VCAM1 in myometrial cells is presented in Fig. 5. Treatment of siCONT transfected cells with IL1B, fsl-1 or poly(I:C) significantly increased ICAM1 and VCAM1 mRNA expression and sICAM1 and sVCAM1 secretion. This increase was significantly amplified in cells transfected with siA20.
Effect of siA20 on adhesion molecules in primary myometrial cells. Human primary myometrial cells were transfected with 50 nM siCONT or 50 nM siA20 for 48 h and then treated with (A–C) 1 ng/ml IL1B, (D–F) 250 ng/ml fsl-1 or (G–I) 5 μg/ml poly(I:C) for an additional 20 h (n = 5 patients). (A,C,E,G,I,K) ICAM1 and VCAM1 mRNA expression was analysed by qRT-PCR. (B,D,F,H,J,L) The concentration of sICAM1 and sVCAM1 in the incubation medium was assayed by ELISA. For all data, the fold change was calculated relative to IL1B-, fsl-1- or poly(I:C)-stimulated siCONT transfected cells, and displayed as mean ± SEM. *P < 0.05 vs. IL1B-stimulated siCONT transfected cells (one-way ANOVA); **P < 0.05 vs. fsl-1-stimulated siCONT transfected cells (one-way ANOVA); #P < 0.05 vs. poly(I:C)-stimulated siCONT transfected cells (one-way ANOVA).
Effect of siA20 on the prostaglandin pathway in primary myometrial and amnion cells
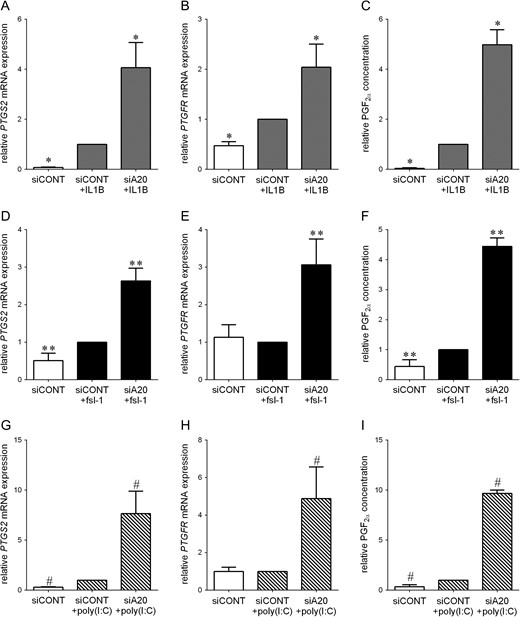
As is expected, in siCONT transfected cells, PTGS2 and PTGFR mRNA expression and PGF2α release was significantly higher after treatment with IL1B (Fig. 6A–C). Fsl-1 and poly(I:C) also significantly increased PTGS2 mRNA expression (Fig. 6D and G) and PGF2α release (Fig. 6F and I) but had no effect on PTGFR mRNA expression (Fig. 6E and H) in siCONT transfected cells. Regardless, for all treatments, the effect of transfection with siA20 was a significant intensification in PTGS2 and PTGFR mRNA expression and PGF2α release.
Effect of siA20 on PTGS2-prostaglandin pathway in primary myometrial cells. Human primary myometrial cells were transfected with 50 nM siCONT or 50 nM siA20 for 48 h and then treated with (A–C) 1 ng/ml IL1B, (D–F) 250 ng/ml fsl-1 or (G–I) 5 μg/ml poly(I:C) for an additional 20 h (n = 5 patients). (A,B,D,E,G,H) PTGS2 and PTGFR mRNA expression was analysed by qRT-PCR. (C,F,I) The concentration of PGF2α in the incubation medium was assayed by ELISA. For all data, the fold change was calculated relative to IL1B-, fsl-1- or poly(I:C)-stimulated siCONT transfected cells, and displayed as mean ± SEM. *P < 0.05 vs. IL1B-stimulated siCONT transfected cells (one-way ANOVA); **P < 0.05 vs. fsl-1-stimulated siCONT transfected cells (one-way ANOVA); #P < 0.05 vs. poly(I:C)-stimulated siCONT transfected cells (one-way ANOVA).
Effect of siA20 on MMP9 expression in primary myometrial and amnion cells
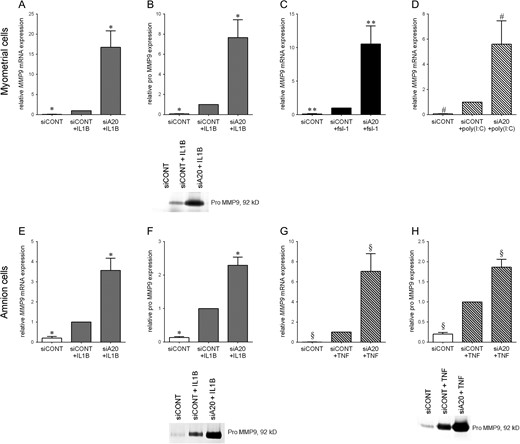
In siCONT transfected myometrial and amnion cells, IL1B increased MMP9 mRNA (Fig. 7A and E) expression and the secretion of pro MMP9 (Fig. 7B and F). Likewise, in iCONT transfected amnion cells, TNF increased MMP9 mRNA (Fig. 7G) expression and the secretion of pro MMP9 (Fig. 7H). Finally, in siCONT transfected myometrial cells, fls-1 (Fig. 7C) and poly(I:C) (Fig. 7D) significantly increased MMP9 mRNA expression (pro MMP9 levels were undetectable for fsl-1 and poly(I:C)). For all treatments, the mRNA expression of MMP9 and the secretion of pro MMP9 was further significantly intensified in cells transfected with siA20.
Effect of siA20 on MMP9 in primary myometrial and amnion cells. Human primary (A–D) myometrial or (E–H) amnion cells were transfected with 50 nM siCONT or 50 nM siA20 for 48 h and then treated with (A,B,E,F) 1 ng/ml IL1B, (C) 250 ng/ml fsl-1, (D) 5 μg/ml poly(I:C) or (G,H) 10 ng/ml TNF for an additional 20 h (n = 5 patients). (A,C-E,G) MMP9 mRNA expression was analysed by qRT-PCR. (B,F,H) The concentration of pro-MMP9 in the incubation medium was assayed by zymography. Representative zymography images from one patient are also shown. For all data, the fold change was calculated relative to IL1B-, fsl-1- poly(I:C)-or TNF-stimulated siCONT transfected cells, and displayed as mean ± SEM. *P < 0.05 vs. IL1B-stimulated siCONT transfected cells (one-way ANOVA); **P < 0.05 vs. fsl-1-stimulated siCONT transfected cells (one-way ANOVA); #P < 0.05 vs. poly(I:C)-stimulated siCONT transfected cells (one-way ANOVA); §P < 0.05 vs. TNF-stimulated siCONT transfected cells (one-way ANOVA).
Effect of siA20 on NF-κB activation in primary myometrial and amnion cells
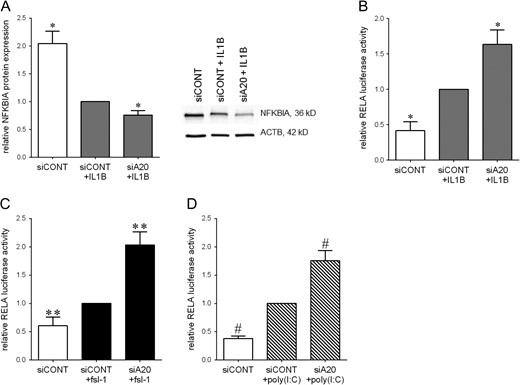
The effect of siA20 knockdown on NF-κB activation was also explored. In myometrial cells, IL1B significantly decreased NFKBIA protein levels (Fig. 8A) and significantly increased RELA transcriptional activity (Fig. 8B). There was no effect of fsl-1 or poly(I:C) on NFKBIA protein levels (data not shown); however, fsl-1 (Fig. 8C) and poly(I:C) (Fig. 8D) significantly increased NF-κB RELA transcriptional activity. In cells transfected with siA20, there was a further significant decrease in NFKBIA protein expression (Fig. 8A) and a significant increase in RELA transcriptional activity (Fig. 8B–D).
Effect of siA20 on NF-κB activation in primary myometrial cells. (A) Human myometrial cells were transfected with 50 nM siCONT or 50 nM siA20 for 48 h, then treated with 1 ng/ml IL1B for an additional 5 min. NFKBIA protein expression was assessed by Western blotting, normalised to β-actin protein expression. Representative Western blot from one patient is also shown. (B–D) Human myometrial cells were transfected with 0.75 ng RELA reporter construct. After 6 h, cells were transfected with 50 nM siCONT or 50 nM siA20 for 48 h, then treated with (B) 1 ng/ml IL1B, (C) 250 ng/ml fsl-1, (D) 5 μg/ml poly(I:C) for an additional 20 h (n = 5 patients). RELA promoter activity is expressed as a ratio of luciferase activity of IL1B-, fsl-1- poly(I:C)-stimulated siCONT transfected cells. All data displayed as mean ± SEM. *P < 0.05 vs. IL1B-stimulated siCONT transfected cells (one-way ANOVA); **P < 0.05 vs. fsl-1-stimulated siCONT transfected cells (one-way ANOVA); #P < 0.05 vs. poly(I:C)-stimulated siCONT transfected cells (one-way ANOVA).
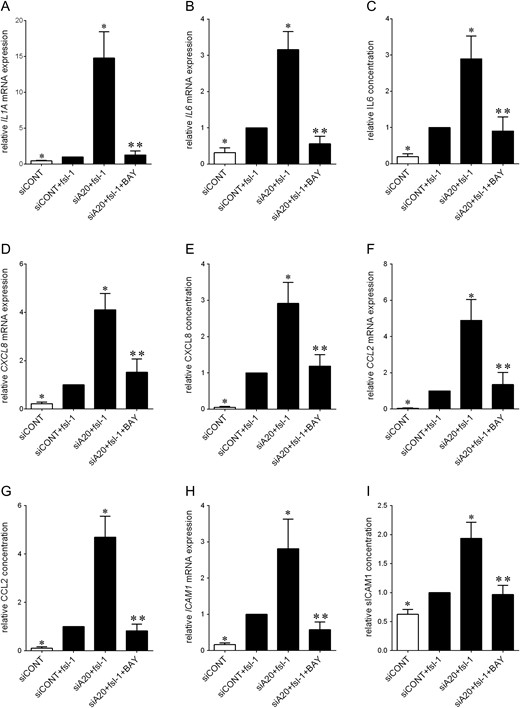
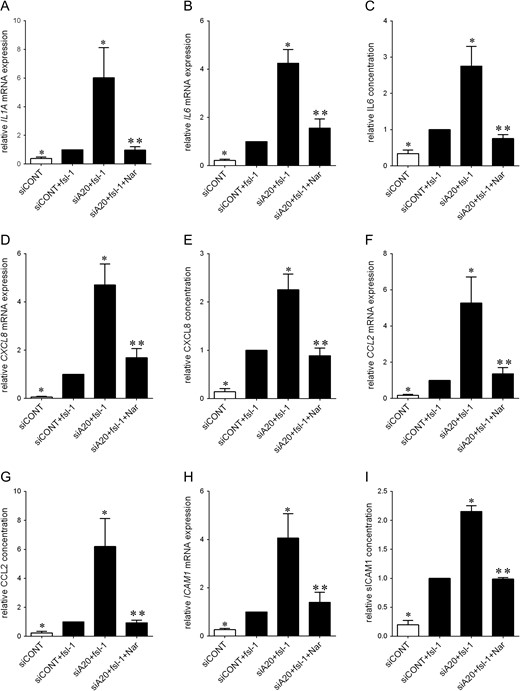
Given these findings, it was of importance to explore whether A20 regulates pro-inflammatory and pro-labour mediators through activation of the transcription factor NF-κB. This was assessed by utilising two inhibitors of NF-κB - the pharmacological inhibitor BAY 11-7082 and the polyphenol naringenin - on fsl-1-induced inflammation was assessed. As depicted in Fig. 9, co-treatment of siA20 transfected cells with BAY 11-7082 (Fig. 9) significantly decreased IL1A, IL6, CXCL8, CCL2 and ICAM1 mRNA expression and the secretion of IL6, CXCL8, CCL2 and ICAM1 back to levels similar to fsl-1-transfected siCONT cells. Similar results were obtained for cells treated with naringenin (Fig. 10).
Effect of BAY 11-7082 on fsl-1-induced expression of pro-labour mediators in primary myometrial cells. Human primary myometrial cells were transfected with or without 50 nM siCONT or 50 nM siA20 for 48 h, and then treated with 250 ng/ml fsl-1 in the absence or presence of 10 μM BAY 11-7082 for an additional 20 h (n = 5 patients). (A,B,D,F,H) IL1A, IL6, CXCL8, CCL2 and ICAM1 mRNA expression was analysed by qRT-PCR and the fold change was calculated relative to fsl-1-stimulated siCONT transfected cells. (C,E,G,I) IL6, CXCL8, CCL2 and sICAM1 concentration in the incubation medium was assayed by ELISA. The fold change was calculated relative to fsl-1-stimulated siCONT transfected cells. All data are displayed as mean ± SEM. *P < 0.05 vs. fsl-1-stimulated siCONT transfected cells (one-way ANOVA); **P < 0.05 vs. fsl-1-stimulated siA20 transfected cells (one-way ANOVA).
Effect of naringenin on fsl-1-induced expression of pro-labour in primary myometrial cells. Human primary myometrial cells were transfected with or without 50 nM siCONT or 50 nM siA20 for 48 h, and then treated with 250 ng/ml fsl-1 in the absence or presence of 400 μM naringenin for an additional 20 h (n = 5 patients). (A,B,D,F,H) IL1A, IL6, CXCL8, CCL2 and ICAM1 mRNA expression was analysed by qRT-PCR and the fold change was calculated relative to fsl-1-stimulated siCONT transfected cells. (C,E,G,I) IL6, CXCL8, CCL2 and sICAM1 concentration in the incubation medium was assayed by ELISA. The fold change was calculated relative to fsl-1-stimulated siCONT transfected cells. All data are displayed as mean ± SEM. *P < 0.05 vs. fsl-1-stimulated siCONT transfected cells (one-way ANOVA); **P < 0.05 vs. fsl-1-stimulated siA20 transfected cells (one-way ANOVA).
Expression of A20 in human myometrium and foetal membranes from non-labouring and labouring women
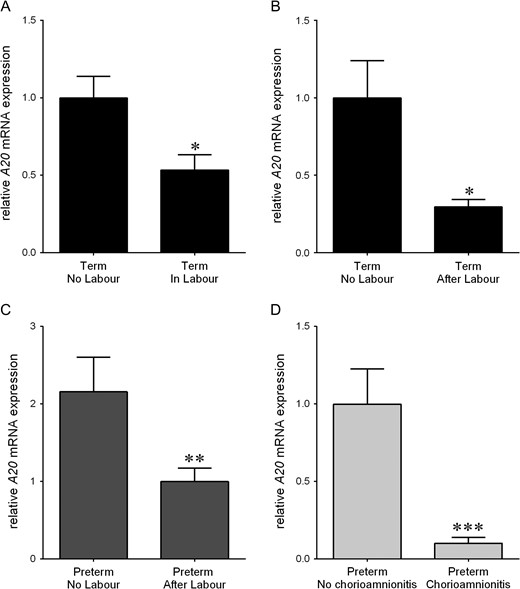
Having shown that silencing of A20 increases pro-labour mediators, the next aim was to characterise A20 expression in myometrium and foetal membranes obtained from term and preterm deliveries that have undergone labour and thus are likely to be associated with increased inflammation. A20 mRNA expression was significantly reduced in myometrium (Fig. 11A), and foetal membranes after spontaneous labour at term (Fig. 11B) and preterm (Fig. 11C) when compared to gestationally-matched non-labouring tissues. Furthermore, as shown in Fig. 11D, when compared to the preterm group without histologic chorioamnionitis, the expression of A20 mRNA was significantly lower in the preterm group with histologic chorioamnionitis.
A20 expression in human myometrium and foetal membranes. (A) Human myometrium was obtained from non-labouring and labouring women at term Caesarean section (n = 8 patients per group). (B) Foetal membranes were obtained from women not in labour at term Caesarean section and women after term spontaneous labour onset and delivery (n = 9 patients per group). (C) Foetal membranes were obtained from women not in labour at preterm Caesarean section and women after preterm spontaneous labour onset and delivery (n = 9 patients per group). (D) Amnion was obtained from women at preterm Caesarean section with or without histological chorioamnionitis (n = 8 patients per group). A20 mRNA abundance was analysed by qRT-PCR. All data are displayed as mean ± SEM. *P < 0.05 versus term no labour (Student's t-test); **P < 0.05 vs. preterm no labour (Student's t-test); ***P < 0.05 vs. preterm no chorioamnionitis (Student's t-test).
Discussion
The chief discoveries of this study are that there is a robust induction of A20 by various pro-inflammatory stimuli in human myometrium, foetal membranes and primary cells isolated from human myometrium and amnion. Functional studies revealed that siRNA knockdown of A20 protein augmented the expression and production of pro-labour mediators in the presence of pro-inflammatory mediators. The effects of A20 are through NF-κB as (i) siA20 increased RELA transcriptional activity and (ii) inhibition of NF-κB, using the pharmacological NF-κB inhibitor BAY 11-7082 and the polyphenol naringenin, significantly suppressed siA20-induced expression and secretion of key pro-labour mediators.
A20 expression and activity are subject to tight regulation (Verstrepen et al., 2010). Under basal conditions, constitutive expression of A20 is quite low. There are, however, two NF-κB binding sites in the A20 promoter that upon activation, increase the re-initiation rates of transcription resulting in a total increase in A20 mRNA levels (Krikos et al., 1992). Increased A20 mRNA expression has been reported to various stimuli including pro-inflammatory cytokines IL1B or TNF, and bacterial products including LPS (Dixit et al., 1990; Cooper et al., 1996; Boone et al., 2004; Onose et al., 2006; Luo et al., 2015). In agreement with these studies, A20 mRNA expression was increased in foetal membranes, myometrium, and primary human amnion and myometrial cells treated with the pro-inflammatory cytokines IL1B or TNF, the bacterial products fsl-1 or flagellin, or the viral dsRNA analogue poly(I:C). Time-course studies in myometrial cells revealed that A20 is rapidly induced (within 2.5 h of treatment). The rapid kinetics of A20 expression suggests that it may be a part of the normal physiological response to protect cells against inflammatory insults or injury. Furthermore, in keeping with the existence of two NF-κB binding sites in the A20 promoter, A20 expression was regulated by the NF-κB inhibitor BAY 11-7082.
In vivo and in vitro studies have described a potent anti-inflammatory role for A20. In vivo, A20 deficient mice severe multi-organ inflammation leads to a premature death (Lee et al., 2000). The defensive role of A20 has also been observed in other diseases where inflammation plays a central role like asthma and rheumatoid arthritis (Kang et al., 2009; Hah et al., 2010). In vitro, inhibition or overexpression of A20 has demonstrated a role in basal, IL1B-, TNF- or LPS-induced cytokine or chemokine production (Grey et al., 2003; Guedes et al., 2014; Luo et al., 2015)
Given the central role for A20 in inflammation, the expression of A20 was manipulated to determine if it may play a role in controlling the expression of a number of pro-labour mediators in human myometrial and amnion cells. For these studies, the pro-inflammatory cytokines IL1B or TNF, the bacterial product fsl-1 and the dsRNA analogue poly(I:C) were used to induce cytokines, chemokines, adhesion molecules, MMP9, PTGS2 and prostaglandins (Gillaux et al., 2011; Hoang et al., 2014; Lim et al., 2014a) associated with preterm birth. Notably, pro-inflammatory cytokines (Yoshimura and Hirsch, 2005; Sadowsky et al., 2006) and bacterial and viral products (Mussalli et al., 1999; Cardenas et al., 2010) can also induce preterm labour and delivery in experimental animal models. This study, for the first time, reports that A20 deficiency leads to a more robust inflammatory response in response to pro-inflammatory cytokines (IL1B, TNF), the bacterial product fsl-1 and the dsRNA analogue poly(I:C). Specifically, when compared to siCONT transfected cells, the expression and secretion of pro-inflammatory cytokines (IL-1α and IL6), chemokines (CXCL1, CXCL8 and CCL2), adhesion molecules (ICAM1 and VCAM1), MMP9, PTGS2 and PTGFR mRNA and PGF2α were significantly increased in siA20 transfected cells.
We and others have shown that IL1B, TNF, fsl-1 and poly(I:C) can induce pro-labour mediators by activating NF-κB (Lappas et al., 2006; Gillaux et al., 2011; Hoang et al., 2014; Lim et al., 2014a, 2015b). In response to these pro-inflammatory insults, NFKBIA (which keeps NF-κB inactive in the nucleus) is rapidly degraded to allow NF-κB to translocate to the nucleus to induce the transcription of genes involved in labour processes, such as pro-inflammatory cytokines, chemokines, MMP9 and PTGS2 (Lappas et al., 2005, 2006; Keelan et al., 2009; De Silva et al., 2010; Gillaux et al., 2011; Hoang et al., 2014; Lim et al., 2014a, 2015b). In non-gestational tissues, there is much proof to show that A20 reduces the inflammatory action by interfering with NF-κB signalling via multiple mechanisms (Catrysse et al., 2014). The modulation of ubiquitin-dependent signalling cascades has been shown to be vital to many of the functions of A20, as it can act as a deubiquitinating enzyme (DUB) and an E3 ubiquitin ligase. For example, A20 can restrict NF-κB activation by sequential deubiquitination and ubiquitin-mediated degradation of adaptor proteins essential for TNF- or TLR-induced NF-κB activation (Boone et al., 2004; Wertz et al., 2004; Shembade et al., 2010).
In support of these studies, NF-κB activation was significantly augmented by siA20 knockdown. This was demonstrated by an augmentation of NFKBIA degradation and or increased RELA transcriptional activity (as assessed by luciferase assay). Collectively, these results show that NF-κB-dependent upregulation of pro-labour mediators may play a part in the A20-responsive inflammatory response. To test this hypothesis, the effect of BAY 11-7082, a pharmacological inhibitor of NF-κB, on fsl-1-induced inflammation in siA20 transfected myometrial cells was examined. The findings demonstrated that in cells transfected with siA20, treatment with BAY 11-7082 significantly suppressed fsl-1-induced expression and secretion of pro-labour mediators. Thus, in human myometrium, A20 acts as a negative regulator of TLR signalling by interfering with NF-κB activation. Although not directly assessed in this study, it is proposed that A20 regulates IL1B-, TNF- and TLR3-induced pro-labour mediators via a similar mechanism given that (i) IL1B-, TNF- and poly(I:C)-induced NF-κB transcriptional activity was significantly augmented by siA20 knockdown and (ii) NF-κB regulates pro-labour mediators induced by IL1B, TNF or poly(I:C) (Lindstrom and Bennett, 2005; Lappas and Rice, 2007, 2009; Lappas, 2015).
Given its significant role in the modification of NF-κB signalling, it can be anticipated that deficiencies in the expression and or function of A20 expression may lead to increased inflammation. In support of this, abnormal A20 expression has been described in numerous disease states, including several human malignancies, inflammatory bowel disease and rheumatoid arthritis (Bates et al., 2009; Vereecke et al., 2009; Adrianto et al., 2011; Catrysse et al., 2014). In this context, the expression of A20 was examined in myometrium and foetal membranes obtained from women after spontaneous labour onset at preterm and or term and it was found that A20 expression was significantly lower after labour. This is unsurprising given that human labour is highly linked with inflammation. Histological studies have shown an invasion of leucocytes into myometrium and foetal membranes with labour (Osman et al., 2003), while gene array studies have identified inflammation as a key component of labouring myometrium and foetal membranes (Bollapragada et al., 2009; Weiner et al., 2010). Further, and in keeping with the observations of increased inflammation in pregnancies affected by infection (Goldenberg et al., 2000), decreased A20 expression was observed in samples taken from women at preterm with histological chorioamnionitis.
Collectively, the findings raise the possibility that targeting A20 could be a therapeutic approach to decrease inflammation associated with preterm birth. Although the therapeutic potential of targeting the A20 has been widely recognised for various inflammatory diseases (Catrysse et al., 2014), there is no therapeutic agent currently available. Polyphenols, however, may represent such an approach as they have been shown to regulate inflammation through A20 (Malcomson et al., 2016; Reihill et al., 2016). We have previously shown that polyphenols, including naringenin, a flavonoid found in grapefruit, regulates pro-labour mediators via NF-κB (Lim et al., 2013c). Thus, it was of interest to determine the effect of naringenin on pro-labour mediators in siA20 transfected myometrial cells. Using fsl-1 as a model of inflammation, our results demonstrate that naringenin significantly suppressed fsl-1-induced expression and secretion of pro-labour mediators. These findings further support the possibility of polyphenols as possible therapeutics to prevent inflammation associated with preterm birth.
To design effective therapeutic approaches to prevent preterm birth, it is imperative that we increase our knowledge of the mechanisms regulating the terminal processes of human labour. This has been a main objective of the studies performed in this laboratory, and many others, over the last 15 years. Using similar approaches to those described in this manuscript, our previous studies have revealed that a number of signalling proteins can positively or negatively regulate the expression of pro-labour mediators in human myometrium and foetal membranes in the context of inflammation. In addition to NF-κB, these include, Kruppel-like factor 5 (KLF5) (Lappas, 2015), sirtuins (SIRTs) (Lappas et al., 2011; Lim et al., 2013b, 2016a; Poljak et al., 2014) and forkhead box O (FOXO) proteins (Lim et al., 2013a). Whether A20 also regulates pro-labour mediators via interacting with these signalling proteins is not known and an avenue for further research.
The novel findings of this study highlight an important role for A20 in controlling mediators involved the terminal processes of human labour in myometrium and foetal membranes. Specifically, pro-inflammatory mediators induced an immediate and robust increase in A20 expression in primary myometrial and amnion cells while functional studies revealed that A20 is a negative regulator of NF-κB-induced expression of pro-labour. Altogether, these findings suggests that negative feedback regulation by A20 restricts the production of pro-inflammatory mediators in response to inflammatory insults. Consistently, A20 expression in myometrium and or foetal membranes was decreased with spontaneous term or preterm labour and preterm chorioamnionitis, conditions associated with enhanced inflammation. A greater understanding of A20, both its mechanism of action and its regulation, may be beneficial in the development of novel therapeutics to prevent inflammation associated with preterm birth.
Supplementary data
Supplementary data are available at Molecular Human Reproduction online.
Acknowledgements
The following are gratefully acknowledged: Dr Ratana Lim and Gillian Barker for their excellent technical assistance; the clinical research midwives, Genevieve Christophers, Gabrielle Pell and Rachel Murdoch, for sample collection; and the Obstetrics and Midwifery staff of the Mercy Hospital for Women for their co-operation.
Authors’ roles
M.L. conceived and designed the study, performed some of the experiments, analysed the data and wrote the manuscript.
Funding
Associate Professor Martha Lappas is supported by a Career Development Fellowship from the National Health and Medical Research Council (NHMRC; grant no. 1047025). Funding for this study was provided by the NHMRC (grant no. 1058786), Norman Beischer Medical Research Foundation and the Mercy Research Foundation.
Conflicts of interest
None declared.
References
- cytokine
- polymerase chain reaction
- tumor necrosis factors
- cyclooxygenase-2
- signal transduction
- cell culture techniques
- inflammation
- interleukin-1
- cell adhesion molecules
- chemokines
- amnion
- bodily secretions
- chorioamnionitis
- fetal membranes
- flagellin
- gelatin
- gelatinase b
- intercellular adhesion molecule 1
- labor
- luciferases
- monocyte chemoattractant protein-1
- rna, messenger
- ubiquitin
- interleukin-6
- myometrium
- premature birth
- rna, small interfering
- chemokine (c-x-c motif) ligand 1