-
PDF
- Split View
-
Views
-
Cite
Cite
Oliver Schreiner, Eveline Wandel, Frido Himmelsbach, Peter R. Galle, Elisabeth Märker‐Hermann, Reduced secretion of proinflammatory cytokines of monosodium urate crystal‐stimulated monocytes in chronic renal failure: an explanation for infrequent gout episodes in chronic renal failure patients?, Nephrology Dialysis Transplantation, Volume 15, Issue 5, May 2000, Pages 644–649, https://doi.org/10.1093/ndt/15.5.644
Close - Share Icon Share
Abstract
Background. In gouty arthritis, monosodium urate (MSU) crystals interact with monocytes and neutrophils to produce inflammatory reactions associated with acute synovitis. In patients with end‐stage renal disease (ESRD), gouty arthritis is a rare condition despite often severe hyperuricaemia. We wondered whether differences in the secretion of proinflammatory cytokines by MSU crystal‐stimulated monocytes might be one explanation for the low incidence of gouty arthritis in patients with ESRD compared with healthy controls.
Methods. Thirteen patients with ESRD on intermittent haemodialysis treatment, six patients with chronic renal failure not yet on dialysis, and 15 age‐ and sex‐matched healthy controls were examined. Monocytes, purified from peripheral blood mononuclear cells (PBMC) by immunomagnetic bead separation, were incubated for 18 h in the presence of MSU crystals, Escherichia coli lipopolysaccharide (LPS) or medium alone. The supernatants were studied for the presence of interleukin (IL)‐1β, IL‐6 and tumour necrosis factor‐α (TNF‐α) using cytokine‐specific enzyme‐linked immunosorbent assays.
Results. Monocytes from patients with ESRD produced significantly lower amounts of IL‐1β, IL‐6 and TNF‐α after stimulation with MSU crystals or LPS than did monocytes from healthy subjects. Cytokine production was not significantly different between ESRD patients on haemodialysis and chronic renal failure patients not yet on dialysis. Artificial MSU crystals were stronger stimuli than tophus‐derived ‘natural’ MSU crystals.
Conclusion. We demonstrate that monocyte‐associated immunosuppression in ESRD leads to reduced secretion of proinflammatory cytokines in response to stimuli such as MSU crystals. This may be one of the factors preventing many ESRD patients from the manifestation of acute gout despite often severe hyperuricaemia.
Introduction
Rheumatic disorders are a major complication of chronic renal insufficiency, mainly in patients on long‐term haemodialysis (HD). Numerous metabolic and musculoskeletal complications have been described, including septic arthritis, avascular necrosis, renal osteodystrophy, chronic β2‐microglobulin amyloidosis, aluminium arthropathy and calcium crystal‐related arthropathies [1–4]. However, despite prolonged and severe hyperuricaemia, gouty arthritis is considered to be rare in end‐stage renal disease (ESRD) [5,6]. Recent studies providing information on the incidence of gouty arthritis in long‐term ESRD demonstrated a remarkable improvement in the clinical activity of gout following the initiation of dialysis therapy [7,8]. Furthermore, in the study by Ifudu et al. [7], none of a group of patients on HD with hyperuricaemia developed new‐onset clinical gouty arthritis. These results were confirmed by another smaller prospective study on 25 long‐term HD patients [9], in which only two patients developed monarthritis during a mean follow‐up time of 24 months. However, upto now, we are not aware of any studies on the immunopathophysiological mechanisms leading to the reduced frequency of gout attacks in ESRD.
It is known that in gouty arthritis, monosodium urate (MSU) crystals interact with monocytes, neutrophils and endothelial cells to produce inflammatory reactions associated with acute synovitis [10]. Among the factors that regulate the grade of inflammation in joints that contain MSU crystals, coating of the crystals by different proteins may be one important modulating mechanism [11]. Secondly, MSU crystals and other inorganic crystals have been shown to induce the formation of specific antibodies which in turn can catalyse the more rapid formation of new crystals [12,13]. Thirdly, a balance between various cytokines and other molecules that exert pro‐ and anti‐inflammatory effects has been incriminated in the modulation of MSU crystal‐associated synovitic inflammation. Neutrophils and endothelial cells may be a source of oxygen radicals in crystal‐induced inflammation, since these cells phagocytose MSU crystals and produce superoxide anion [14]. Monocytes exposed to MSU crystals secrete interleukin‐1 (IL‐1), tumour necrosis factor‐α (TNF‐α) and interleukin‐6 (IL‐6) [15,16] which cause both local inflammation and systemic manifestations including fever and elevated C‐reactive protein [17]. In contrast, regulatory synovial cytokines such as transforming growth factor‐β1 (TGF‐β1) may down‐regulate actue and chronic inflammation [18].
Here we analyse crystal–cell interactions in patients with ESRD compared with age‐matched healthy controls and test the hypothesis that suppression of proinflammatory cytokine responses due to an uraemia‐associated monocyte immune deficiency may be one explanation for the relatively low incidence of gout in patients with long‐standing renal insufficiency.
Subjects and methods
Patients
Thirteen patients with ESRD on intermittent HD were studied (five male, mean age 60 years, range 49–70 years, mean duration of HD 4.0 years; eight females, mean age 69 years, range 39–85 years, mean duration of HD 2.8 years). The renal diseases leading to HD were the following: diabetic nephropathy (n=6), chronic pyelonephritis (n=3), Wegener's disease (n=1), arteriolar nephrosclerosis (n=1) and analgesic nephropathy (n=2). None of the patients received steroids, immunosuppressive drugs, allopurinol or any other drug that could lead to alterations in cytokine synthesis. Furthermore, we studied six patients with pre‐terminal chronic renal insufficiency who were not yet on dialysis (three male, mean age 60 years, range 48–71 years; three female, mean age 56 years, range 29–82 years). In these patients, the renal diseases leading to chronic renal failure were diabetic nephropathy (n=3), chronic pyelonephritis (n=2) and analgesic nephropathy (n=1). As healthy control subjects, eight male blood donors (mean age 62 years, range 50–86 years) and seven female blood donors (mean age 73 years, range 40–92 years) were selected.
MSU crystals
Artificial MSU crystals were prepared under pyrogen‐free conditions from a supersaturated solution of sodium urate as described [19]. Natural MSU crystals were obtained from a tophus surgically removed from a patient suffering from gout, purified as described and washed in phosphate‐buffered saline (PBS) [20].
Isolation of peripheral blood monocytes
Mononuclear cells (MNC) were isolated from heparinized blood by standard Ficoll‐Hypaque density centrifugation (Histopaque‐1077, Sigma, Deisenhofen, Germany). In HD patients, blood was drawn directly before HD started in order to avoid monocyte activation by the procedure of HD. In the first series of experiments, various methods for the purification of human monocytes from MNC were evaluated: the E‐rosetting technique to enrich monocytes and B‐lymphocytes and remove T cells, Percoll density gradient centrifugation, counterflow centrifugal elutriation and immunomagnetic separation (IMS). The aim of these evaluation experiments was to achieve a pure population of monocytes with minimum pre‐activation due to plastic adherence or other in vitro manipulations. The monocytes should also be stimulated optimally by mitogens and by MSU crystals.
The E‐rosetting technique to enrich B cells and monocytes
MNC (5×107) were resuspended with 2 ml of a prepared solution of 300 μl of sheep erythrocytes incubated with 100 μl of Test‐Neuraminidase (Centeon, King of Prussia, PA, USA) in 4.6 ml of RPMI‐1640 (Seromed, Berlin, Germany) for 30 min at 37°C, layered on Ficoll 1.090 (Biochrom, Berlin, Germany) and centrifugated for 30 min at 2800 r.p.m. [21]. The interface layer containing B cells and monocytes was collected and washed with PBS/3% fetal calf serum (FCS).
Percoll density gradient centrifugation
MNC (5×107) were suspended in 2 ml of a 100% Percoll solution (Percoll, Sigma, Deisenhofen, Germany) in a 15 ml centrifuge tube and overlayered by Percoll gradients containing 3 ml layers of 60, 50 and 35% of Percoll in PBS. After centrifugation for 20 min at 3000 r.p.m. and 4°C, the monocytes were collected from the interface between the 60 and the 50% layer and were washed with PBS/3% FCS.
Counterflow centrifugal elutriation
A J2‐MC Elutriator system (Beckmann, Munich, Germany) was treated with PBS/1% bovine serum albumin (BSA) to block unspecific binding sites and then washed with elutriation buffer (PBS). The elutriation of monocytes was performed according to a method described by Fidgor et al. [22] and to the manufacturer's instructions at 1600 r.p.m. and 16°C. At an initial flow of 20 ml/min, remaining red blood cells and detritus were removed from the elutriation chamber. Thereafter, 1000 ml of a cell mixture of lymphocytes and a few monocytes was collected at a flow of 29 ml/min. The monocytes were finally collected at a flow of 50 ml/min into 50 ml tubes and kept on ice until they were centrifuged for 10 min at 1200 r.p.m. and 4°C and resuspended in Dulbecco's modified Eagle's medium (DMEM; Gibco, Eggenstein, Germany) containing 10% heat‐inactivated human AB serum.
Immunomagnetic separation of monocytes
IMS was performed using Dynabeads according to the manufacturer's instructions (Deutsche Dynal, Hamburg, Germany). Briefly, Dynabeads M‐450 were washed twice in PBS/3% FCS using a Dynal MPC‐1 magnetic separation device. Peripheral blood MNC (PBHC) prepared by the E‐rosetting technique were suspended in PBS/3% FCS at 2×107 cells/ml in a 15 ml conical tube, and the pre‐washed Dynabeads M‐450 were added together with anti‐CD2 and anti‐CD19 monoclonal antibodies (Immunotech, Hamburg, Germany). After incubation at 4°C for 30 min with gentle tilting and rotation, the tubes were placed in a Dynal MPC for 2 min to remove magnetically the CD2+ and CD19+ cells coated with beads. The unbound, negatively selected cells were transferred to a fresh tube, and a second magnetic separation procedure was performed. The resulting cells were counted, and resuspended in DMEM (Gibco) containing 10% heat‐inactivated human AB serum.
Monocyte stimulation by MSU crystals and lipopolysaccharide
Freshly isolated blood monocytes (0.5×106 cells per well) were cultured with the various stimuli (artificial and natural MSU crystals at different concentrations, purified lipopolysaccharide (LPS) from Escherichia coli, (Sigma, Deisenhofen, Germany) or culture medium alone) in 48‐well flat bottom plates (Greiner, Nürtingen, Germany) for 18 h. An incubation time of 18 h was found to induce peek levels of cytokine secretion by the monocytes. The supernatants were collected, centrifuged to remove crystals and cell debris, and stored at −80°C until they were tested for the presence of cytokines.
ELISAs for IL‐1β, IL‐6 and TNF‐α
Maxi‐Sorb 96‐well plates (Nunc, Roskilde, Denmark) were coated with 2 μg/ml of anti‐IL‐1β (Immunotech, Hamburg, Germany), anti‐IL‐6 (Virotech, Rüsselsheim, Germany) or anti‐TNF‐α (Biozol, Munich, Germany) monoclonal antibodies, respectively. After washing, excess sites were blocked with 100 μl of 5% BSA (Serva, Heidelberg, Germany) in PBS. After subsequent washing with PBS/0.05% Tween, the reaction mixtures (100 μl of culture supernatants) were pipetted into triplicate wells; standards were always included in the same trays. The plates were incubated overnight and washed four times, followed by incubation with 100 μl of biotinylated rabbit anti‐human IgG (2 μg/ml) (Serva) and subsequently with 100 μl of avidin‐conjugated peroxidase (1 μg/ml) (Dako, Hamburg, Germany). Finally, substrate ABTS (Serva) was added. Colour development was measured by determining the optical density at 405 nm with an automated microplate reader.
Statistical analysis
The data were analysed using the Kruskal–Wallis test and the Wilcoxon test according to the advice of the Institute of Medical Statistics and Documentation, University of Mainz. The significance level was P<0.01.
Results
Comparison of different monocyte isolation methods in terms of total cell yield and purity of monocytes
After the PBMC were isolated by Ficoll, the monocytes were separated by E‐rosetting, Percoll density gradient centrifugation, counterflow centrifugal elutriation and IMS as described above, and afterwards analysed by fluorescence‐activated cell sorting (FACS) for purity and yield of monocytes. Three independent experiments (three different blood donors) were performed with each of the above separation methods. Best values for purity and yield of monocytes were measured and calculated for Percoll centrifugation and IMS with comparably good results for monocyte purity (>95%) and monocyte yield (60–75%) (see Table 1). In contrast, elutriation and E‐rosetting showed less favourable results and were therefore considered to be unsuitable for further experiments.
Total yield of peripheral blood mononuclear cells (range of three experiments) and proportion of monocytes, T cells and B cells after different monocyte isolation methods [Percoll density centrifugation, counterflow centrifugal elutriation, E‐rosetting and immunomagnetic separation (IMS)]
| Yield | Monocytes | T‐cells | B‐cells | |
| of cells after | (CD14+) | (CD2+) | (CD19+) | |
| initial MNC | ||||
| separation by | ||||
| Ficoll | ||||
| Ficoll | 100% | 18% | 73% | 9% |
| Percoll | 65–70% | 95% | 4% | 1% |
| Elutriation | 35–45% | 86% | 11% | 3% |
| E‐rosetting | 60–70% | 71% | 3% | 26% |
| IMS | 50–60% | 97% | 1% | 2% |
| Yield | Monocytes | T‐cells | B‐cells | |
| of cells after | (CD14+) | (CD2+) | (CD19+) | |
| initial MNC | ||||
| separation by | ||||
| Ficoll | ||||
| Ficoll | 100% | 18% | 73% | 9% |
| Percoll | 65–70% | 95% | 4% | 1% |
| Elutriation | 35–45% | 86% | 11% | 3% |
| E‐rosetting | 60–70% | 71% | 3% | 26% |
| IMS | 50–60% | 97% | 1% | 2% |
Data for monocytes, T cells and B cells as measured by FACS analysis are given as a percentage of the MNC population initially separated by Ficoll‐Hypaque centrifugation.
Total yield of peripheral blood mononuclear cells (range of three experiments) and proportion of monocytes, T cells and B cells after different monocyte isolation methods [Percoll density centrifugation, counterflow centrifugal elutriation, E‐rosetting and immunomagnetic separation (IMS)]
| Yield | Monocytes | T‐cells | B‐cells | |
| of cells after | (CD14+) | (CD2+) | (CD19+) | |
| initial MNC | ||||
| separation by | ||||
| Ficoll | ||||
| Ficoll | 100% | 18% | 73% | 9% |
| Percoll | 65–70% | 95% | 4% | 1% |
| Elutriation | 35–45% | 86% | 11% | 3% |
| E‐rosetting | 60–70% | 71% | 3% | 26% |
| IMS | 50–60% | 97% | 1% | 2% |
| Yield | Monocytes | T‐cells | B‐cells | |
| of cells after | (CD14+) | (CD2+) | (CD19+) | |
| initial MNC | ||||
| separation by | ||||
| Ficoll | ||||
| Ficoll | 100% | 18% | 73% | 9% |
| Percoll | 65–70% | 95% | 4% | 1% |
| Elutriation | 35–45% | 86% | 11% | 3% |
| E‐rosetting | 60–70% | 71% | 3% | 26% |
| IMS | 50–60% | 97% | 1% | 2% |
Data for monocytes, T cells and B cells as measured by FACS analysis are given as a percentage of the MNC population initially separated by Ficoll‐Hypaque centrifugation.
Cytokine release of monocytes depending on the purification method used
The different separation methods were also compared in order to evaluate the extent of monocyte pre‐activation during these procedures and the optimal activation after mitogen or MSU crystal stimulation by measuring the cytokine release. Monocyte isolation by Percoll led to higher production of IL‐1β, IL‐6 and TNF‐α, (data shown for TNF‐α, see Figure 1) in unstimulated cells (medium) than counterflow centrifugal elutriation, E‐rosetting or IMS. In contrast, Percoll isolation of monocytes induced the highest spontaneous levels of the three cytokines (medium control). Therefore, for further experiments, the IMS method was chosen, since it showed high monocyte purity after isolation (see Table 1) and led to very low spontaneous but high LPS‐ or MSU crystal‐inducible cytokine secretion, thus resulting in an optimal stimulation index.
Spontaneous (medium control), E. coli LPS‐ and artificial monosodium urate crystal‐ (art‐MSU crystals) induced secretion of TNF‐α in peripheral blood‐derived monocytes which had been isolated by different purification methods [Percoll density centrifugation, counterflow centrifugal elutriation, E‐rosetting and immunomagnetic separation (IMS)]. Freshly isolated monocytes (0.5×106 cells per well) were cultured with LPS or art‐MSU crystals or with culture medium alone in 48‐well flat bottom plates for 18 h. The supernatants were collected and the concentration of TNF‐α measured by ELISA.
Monocyte IL‐1β, IL‐6 and TNF‐α secretion induced by MSU crystals and LPS
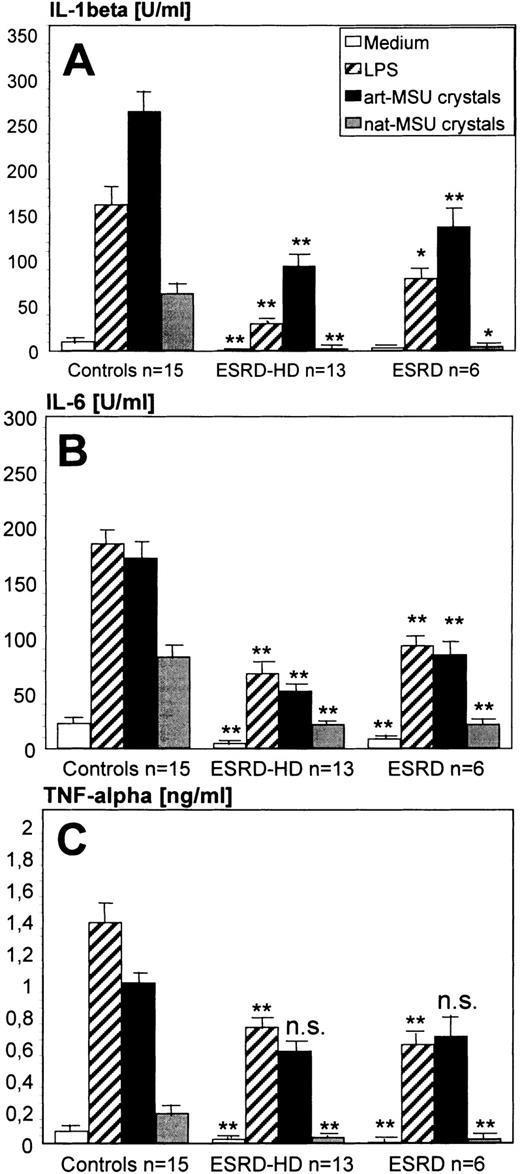
Monocytes separated by immunomagnetic beads spontaneously released only very low amounts of cytokines. When the cells were stimulated with LPS or various amounts of artificial and natural MSU crystals, the cytokine secretion was significantly increased. In these experiments, stimulation with artificial MSU crystals was more effective than stimulation with microcrystals that had been prepared from a tophus (natural MSU crystals) (Figure 2).
For all proinflammatory cytokines (IL‐1β, IL‐6 and TNF‐α) tested, cytokine production by monocytes from patients with ESRD (both HD patients and patients with chronic renal failure not yet on dialysis) was significantly reduced as compared with healthy controls (Figure 2). Although the number of patients with chronic renal failure not yet on dialysis was small, the cytokine production by these patients were statistically not significantly different from the patients who were on intermittent HD.
Proinflammatory cytokine (IL‐1β, IL‐6 and TNF‐α) secretion by peripheral blood monocytes stimulated by medium alone, E. coli LPS, artificial (art‐MSU crystals) or natural (nat‐MSU crystals) monosodium urate crystals. Comparison of patients with ESRD on chronic HD (ESRD‐HD, n=13) and patients with ESRD who were not yet on HD (ESRD, n=6) with healthy normal blood donors (n=15). Monocytes (0.5×106 cells per well) which had been isolated by immunomagnetic separation (IMS) were cultured with LPS, art‐MSU or nat‐MSU crystals or with culture medium alone in 48‐well flat bottom plates for 18 h. The supernatants were collected and the concentration of IL‐1β, IL‐6 and TNF‐α measured by ELISA. Statistical analyses were performed for the differences between the group of ESRD‐HD patients and the group of healthy controls, and the group of ESRD patients and the group of healthy controls, respectively. **P<0.001, *P<0.005, ns=not significant.
Discussion
The clinical background of this study has been the observation that gouty arthritis is rare and less severe in ESRD despite prolonged and sometimes severe hyperuricaemia [7,9]. The events leading to crystallization of urate and subsequent synovitic inflammation in hyperuricaemic patients without renal disease are not yet fully understood. However, there is strong evidence that the monocytic phagocyte system is crucially involved in the pathogenesis of gout [10]. Therefore, this study was designed to compare the capacity of purified monocytes from the peripheral blood of patients with ESRD and from healthy blood donors to produce proinflammatory and regulatory cytokines in response to MSU crystals.
The results found here in the group of healthy subjects are in concordance with previous work on the stimulatory effect of MSU crystals on IL‐1 and IL‐6 release from monocytes [17]. These studies demonstrated that within hours after incubation with MSU crystals, monocytes produced proinflammatory cytokines and expressed cyclooxygenase‐2 [23]. In addition, MSU crystals could activate endothelial cells [24]. The resultant release of inflammatory mediators from the endothelium and from monocytes is believed to be important in specific aspects of the pathogenesis of gout including acute local synovitic inflammation and systemic acute phase responses.
Concerning patients with ESRD, the working hypothesis of our present work has been that suppression of the proinflammatory cytokine response due to an uraemia associated monocyte immune deficiency might be one explanation for the relatively low incidence of gout in long‐standing renal insufficiency. Several previous studies have suggested that ESRD induces a clinical state of immune deficiency, including an increased incidence of bacterial and fungal infections, an impaired efficacy of vaccinations such as hepatitis B [25] and clinical and serological remissions of systemic lupus erythematosus (SLE) as azotaemia develops [26]. In vitro, impaired T cell‐mediated immune responses in ESRD are based mainly on reduced T cell‐activating capabilities of monocytes [27], an involvement of the B7/CD28 pathway [28] as well as enhanced monocyte apoptosis [29].
Our findings of significantly reduced cytokine production by MSU crystal‐stimulated monocytes from patients with ESRD provide direct support for the hypothesis that the ability of monocytes to secrete proinflammatory cytokines is critical in the clinical state of ESRD‐induced immunodeficiency. Moreover, not HD but azotaemia itself seems to be responsible for the defect of monocytes in ESRD, as demonstrated by the fact that there were no significant differences in cytokine production in patients with ESRD on intermittent HD and patients with chronic renal failure not yet on dialysis. This correlates with the general lack of prominent systemic acute phase responses in many patients with long‐standing renal insufficiency. Our findings may in part explain the observation that despite hyperuricaemia in chronic azotaemia and despite the formation of MSU crystals, acute inflammatory gouty arthritis is extremely rare in ESRD and the clinical course is ameliorated in those patients who have had a history of gout in the stage of early renal insufficiency [7].
Measurement of cytokines in patients with ESRD has been performed by several groups and, unfortunately, the results are contradictory. Pereira et al. [30] compared different studies on cytokine secretion in patients on HD and tried to find out the reasons for the inconsistent findings. They made clear that the results strongly depended on the method of cell separation used, the purity and pre‐activation of the cell population as well as the method of cytokine measurement. These aspects were taken into consideration in our present study. We found that monocyte pre‐activation due to the separation procedures was crucial for the amount of proinflammatory cytokines detected by ELISA pre‐ and post‐stimulation with LPS or MSU crystals. Therefore, we would suggest choosing for further studies in this field the IMS method (‘negative isolation’ by antigen–antibody reactions) to separate monocytes from an MNC fraction.
Interestingly, natural MSU crystals were biologically much less active than synthetic counterparts (artificial MSU crystals), which was in contrast to the findings of Stankovic et al. [20]. It should be noted, however, that these human crystals were obtained from tophi in the skin where they are known to evoke little, if any, inflammation aside from phagocytosis by macrophages.
In summary, the findings presented here may represent one aspect of how proinflammatory monocyte reactions in response to MSU crystals and the resulting acute gouty arthritis might be prevented in patients with ESRD. Differences between ESRD patients and healthy controls may also involve differences in MSU‐induced complement activation, neutrophil and endothelial stimulation or T cell mitogenesis. The effects of MSU on neutrophil activation in ESRD patients are now under investigation in our laboratory.
Correspondence and offprint requests to: Dr Elisabeth Märker‐Hermann, MD, First Department of Medicine, University of Mainz, D‐55101 Mainz, Germany.
References
Goldstein S, Winston E, Chung TJ, Chopra S, Pariser K. Chronic arthropathy in long term hemodialysis.
Hermann E, Mayet W‐J, Wandel E et al. Rheumatologische und radiologische Symptome der Dialyse‐assoziierten β2‐Mikroglobulin‐Amyloidose: Retrospektive Langzeituntersuchung an 175 chronischen Hämodialysepatienten.
Ferrari AJ, Rothfuss S, Schumacher HR. Dialysis arthropathy: identification and evaluation of a subset of patients with unexplained inflammatory effussions.
Richet P, Mignon GF, Ardaillou R. Goutte secondaire des néphropathies chroniques.
Ifudu O, Tan CC, Culin AL, Delano BG, Friedman EA. Gouty arthritis in end‐stage renal disease: clinical course and rarity of new cases.
Chou C‐T, Wasserstein A, Schumacher HR Jr, Fernandez P. Musculoskeletal manifestations in hemodialysis patients.
Bertolo MB, Lavras‐Costallat LT. Hiperuricemia e gota: experiencia em hemodialisados.
Terkeltaub RA. Gout and mechanisms of crystal induced inflammation.
Ortiz‐Bravo E, Sieck MS, Schumacher HR. Changes in the proteins coating monosodium urate crystals during active and subsiding inflammation: immunogold studies of synovial fluid from patients with gout and of fluid obtained using the rat subcutaneous air pouch model.
Falasca GF, Ramachandrula A, Kelley KA, O'Connor CR, Reginato AJ. Superoxide anion production and phagocytosis of crystals by cultured endothelial cells.
Di Giovine FS, Malvista SE, Nuki G, Duff GW. Interleukin 1 (IL 1) as a mediator of crystal arthritis: stimulation of T cell and synovial fibroblast mitogenesis by urate crystal‐induced IL 1.
Guerne PA, Zuraw B, Vaughan JH, Carson DA, Lotz M. Synovium as a source of interleukin 6 in vitro: contribution to local and systemic manifestations of arthritis.
Guerne PA, Terkeltaub R, Zuraw B, Lotz M. Inflammatory microcrystals stimulate interleukin‐6 production and secretion by human monocytes and synoviocytes.
Lioté F, Prudhommeaux F, Schiltz C, Champy R, Herbelin A, Ortiz‐Bravo E. Inhibition and prevention of monosodium urate monohydrate crystal‐induced acute inflammation in vivo by transforming growth factor β1.
Seegmiller JE, Howell R, Malawista SE. The inflammatory reaction to sodium urate.
Stakovic A, Front P, Barbara A, Mitrovic DR. Tophus‐derived monosodium urate monohydrate crystals are biologically much more active than synthetic counterpart.
Märker‐Hermann E, Duchmann R. Isolation of T cells and establishment of T cell lines and clones.
Fidgor CG, vanEs WL, Leemans JMM, Bont WS. A centrifugal elutriation system for separating small numbers of cells.
Pouliot M, James MJ, McColl SR et al. Monosodium urate microcrystals induce cyclooxygenase‐2 in human monocytes.
Chapmann PT, Yarwood H, Harrison AA et al. Endothelial activation in monosodium urate monohydrate crystal‐induced inflammation: in vitro and in vivo studies on roles of tumor necrosis factor alpha and interleukin‐1.
Köhler H, Arnold W, Renschin G, Dormeyer HH, Meyer zum Büschenfelde KH. Active hepatitis B vaccination of dialysis patients and medical staff.
Coplon NS, Diskin CJ, Petersen J, Swenson RS. The long‐term course of systemic lupus erythematosis in end‐stage renal disease.
Meuer SC, Hauer M, Kurz P, Meyer zum Büschenfelde K‐H, Köhler H. Selective blockade of the antigen‐receptor‐mediated pathway of T cell activation in patients with impaired immune responses.
Girndt M, Köhler H, Schiedhelm‐Weick E, Schlaak JF, Meyer zum Büschenfelde K‐H, Fleischer B. T‐cell activation defect in hemodialysis patients: evidence for a role of the B7/CD28 pathway.
Heidenreich S, Schmidt M, Bachmann J, Harrack B. Apoptosis of monocytes cultured from long‐term hemodialysis patients.



![Spontaneous (medium control), E. coli LPS‐ and artificial monosodium urate crystal‐ (art‐MSU crystals) induced secretion of TNF‐α in peripheral blood‐derived monocytes which had been isolated by different purification methods [Percoll density centrifugation, counterflow centrifugal elutriation, E‐rosetting and immunomagnetic separation (IMS)]. Freshly isolated monocytes (0.5×106 cells per well) were cultured with LPS or art‐MSU crystals or with culture medium alone in 48‐well flat bottom plates for 18 h. The supernatants were collected and the concentration of TNF‐α measured by ELISA.](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/ndt/15/5/10.1093_ndt_15.5.644/2/m_nd70167.12.1.jpeg?Expires=1717023890&Signature=eVCM2q39ve2xq1TAUcwcAuHbQ5Tla-MCmVnbKbsqCEuLa0Z-TRjBE0sEuOirQR4jAWnq4V2kBPzHKR5gVjJNXEUPEL1VweuS5n6ubjkwFzRp~mrPpQPcwYZfpRkT7SkG56FHWqJVMPxSf3GM0q1c2tb4lmRXeVuozuouj2V2kr-BwHGzbBAypkmith7aeApUl3Qc7gmNqUHaA8jeiXNwmlYqT3dHgGvV5W0o4T~nLFnn0ypsBsbUm92wknR1QjNBnqg2WvgFGd~U2GkTqZEg6SaT5pAUvrO5TWGS-9dAf~76RDJFbJYksEdu7vQLDp-21hDPT1P~CMKLUgQi38CuHA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)


Comments