-
PDF
- Split View
-
Views
-
Cite
Cite
Peter Stenvinkel, Olof Heimbürger, Bengt Lindholm, George A. Kaysen, Jonas Bergström, Are there two types of malnutrition in chronic renal failure? Evidence for relationships between malnutrition, inflammation and atherosclerosis (MIA syndrome), Nephrology Dialysis Transplantation, Volume 15, Issue 7, July 2000, Pages 953–960, https://doi.org/10.1093/ndt/15.7.953
Close - Share Icon Share
Introduction
It is believed that malnutrition is common in patients with chronic renal failure (CRF). They have reduced body weight, depleted energy (fat tissue) stores, loss of somatic protein (low muscle mass) and low levels of serum albumin, transferrin, pre‐albumin and other visceral proteins. Various studies show signs of malnutrition in 23–76% of haemodialysis (HD) and 18–50% of peritoneal dialysis (PD) patients [1–4]. Such variations in the prevalence of malnutrition may be related to factors such as age, case mix, co‐morbid conditions and quality of dialysis therapy. The aetiology of malnutrition in CRF is complex and may include many factors, e.g. poor food intake because of anorexia, nausea and vomiting due to uraemic toxicity, hormonal derangements, acidosis and increased resting energy expenditure.
While malnutrition by definition is caused by poor nutritional intake, laboratory or anthropometric measurements are generally used to define it clinically. Other factors can cause the same changes in body and plasma protein composition, especially inflammatory and infectious complications [5,6] and chronic heart failure (CHF). In addition, factors directly associated with the dialytic procedure, such as bio‐incompatibility, nutrient losses in the dialysate and, during PD, poor appetite due to abdominal discomfort and uptake of glucose may also contribute to what we define as malnourishment in CRF. These may exert their action either by direct nutrient loss or by triggering the inflammatory response. However, since malnutrition also occurs in pre‐dialysis patients [7], it is evident that dialysis‐unrelated factors, e.g. infectious and inflammatory complications as well as co‐morbidity, may also be important contributors to malnutrition in CRF.
Assessment of malnutrition in CRF
Many methods have been used to assess the presence of malnutrition in patients with CRF. A history of weight loss and symptoms such as anorexia, nausea and vomiting may indicate impending or established malnutrition. Anthropometric measurements, such as mid‐arm muscle circumference, skinfold thickness and hand‐grip strength may all be useful tools for estimating malnutrition. Hand‐grip strength, in particular, has been shown to be an inexpensive, reliable and easily performed parameter of nutrition [4,8] that also predicts mortality in CRF patients (unpublished observation). Creatinine kinetics have also been advocated as a method to assess nutritional status. However, recent evidence suggests that it is unreliable in individual CRF patients [8,9]. More sophisticated methods used to evaluate nutritional status include bio‐electrical impedance, dual‐emission X‐ray absorptiometry (DXA), nuclear magnetic resonance, computerized tomography, total body potassium and total body nitrogen, but mostly as research tools. Finally, several biochemical markers [e.g. serum albumin, pre‐albumin, insulin‐like growth factor‐1 (IGF‐1) and transferrin] have been used to evaluate nutritional status. Of these biochemical markers, serum albumin so far has been the most common to assess malnutrition, and hypoalbuminaemia has sometimes, perhaps erroneously (see below), been used to diagnose malnutrition [10].
Protein and energy requirements in chronic renal failure
The protein requirements in maintenance dialysis patients are not well defined. It can be assumed that the variation in protein requirements is much greater among dialysis patients than in healthy subjects, due to additional causes of variation, such as endocrine and biochemical abnormalities, anaemia, drugs, physical inactivity and co‐morbid conditions, e.g. cardiovascular disease, diabetes and infections. In addition, specific effects of the dialytic process may increase the protein requirements, especially in patients treated with HD. The daily protein intake recommended is ∼0.6 g/kg body weight/day in non‐dialysed patients and at least 1.2 g/kg body weight/day in dialysis patients [11]. The energy requirements are dependent on the level of physical activity. In healthy subjects, an energy intake of 35–40 kcal/kg body weight/day is recommended for those not performing heavy physical exercise. There is no strong evidence that the energy requirements of chronic dialysis patients always differ from those of normal subjects [12,13] although increased energy expenditure has been reported in the former group [14]. A sufficient energy intake is needed to prevent protein from being utilized as an energy source via gluconeogenesis, and variations in energy intake can probably explain, at least partly, the inter‐individual variations in nitrogen balance in CRF patients on similar protein intake [15]. However, the inter‐patient variation in nitrogen balance with similar protein intake may also be related in part to the presence and degree of inflammation and/or co‐morbid conditions. Thus, more studies are needed to evaluate dietary protein intake and energy requirements in clinically stable dialysis patients and in dialysis patients with co‐morbid conditions and/or an inflammatory response [11].
Hypoalbuminaemia as a marker of malnutrition and mortality
It is well established that a low serum albumin level is a strong independent predictor of total and cardiovascular mortality in HD [16] and PD [17,18] patients. On the other hand, in non‐renal patients, no association was found between serum albumin and cardiovascular disease [19], suggesting that a low serum albumin level per se does not necessarily contribute to cardiovascular mortality. Moreover, Koch et al. [20] have reported that the nutritional status alone does not predict overall or cardiovascular mortality, and Struijk et al. [21] have shown that serum albumin merely reflects the presence of systemic disease in dialysis patients.
It has been proposed that the primary cause of hypoalbuminaemia in CRF is malnutrition [22]. However, poor food intake does not often result in hypoalbuminaemia if CRF is not present [23] and, although the food intake is markedly lower in patients with anorexia nervosa, serum albumin levels and the catabolic rate of albumin have been shown to be similar to those of control subjects [24]. Furthermore, in a prospective 24 week study in which healthy volunteers were subjected to semi‐starvation (1500 kcal/24 h), serum albumin decreased only moderately (from 42.8 to 38.6 g/l) despite a 23% reduction in body weight (from 69.3 to 53.6 kg) and muscle mass [25]. Serum albumin levels may be low even in apparently well nourished HD patients, and they decrease in relation to the degree of malnutrition [4]. Although they differ markedly in patients with or without inflammation, they do not differ significantly between well‐nourished and malnourished pre‐dialysis patients [8], suggesting that serum albumin is a poor nutritional marker also in dialysis patients [26,27]. Despite these findings and the fact that today several alternative methods are available for assessing nutritional status, serum albumin still seems to be, by far, the most commonly used nutritional marker in CRF patients.
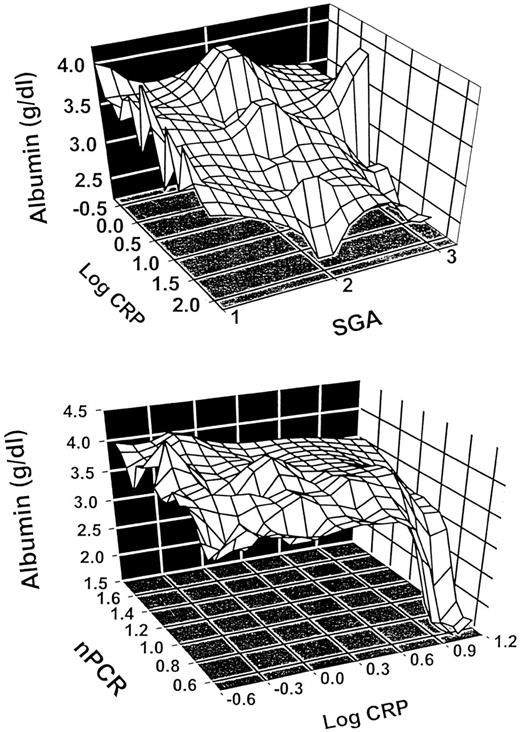
Serum albumin concentration is best seen as being regulated by several factors, especially protein malnutrition, inflammation and external losses. The latter is important in PD, where transperitoneal losses may be as great as 20 g/24 h [28], and in HD, especially following re‐use with bleach [29], although other processes may also increase dialyser permeability to albumin. While dietary protein insufficiency may also cause a modest reduction in serum albumin concentration, inflammation by itself can lead to a very marked reduction [30]. Inflammation can cause hypoalbuminaemia by suppressing albumin synthesis [30] and by causing transfer of albumin from the vascular to the extravascular space. The combination of inflammation and reduced protein intake will lead to a significant reduction in serum albumin concentration [31], as shown in Figure 1.
Relationship between subjective global assessment (SGA), C‐reactive protein (CRP) and serum albumin in 95 pre‐dialysis patients in Stockholm (top) and between normalized protein catabolic rate (nPCR), CRP and serum albumin in 79 haemodialysis patients in Sacramento (bottom). Both CRP and markers of nutrition (SGA and nPCR, respectively) independently controlled serum albumin concentrations in both models.
Pro‐inflammatory cytokines cause malnutrition and cardiovascular disease
In recent years, several reports have suggested that inflammation, alone or in combination with a low protein intake, plays a significant role in causing hypoalbuminaemia in CRF patients [31–34] (Figure 1). This is not unexpected since both serum albumin and C‐reactive protein (CRP) participate reciprocally in the same acute‐phase process. It has been established recently that moderately elevated plasma concentrations of CRP are associated with an increased risk of cardiovascular disease in otherwise healthy subjects. In non‐renal patient populations, elevated levels of CRP are associated with cardiovascular morbidity [35], ischaemic stroke [36] and mortality in the elderly [37]. Likewise, several groups recently have reported that an increased CRP is also a strong risk factor for death [33,38,39], cardiovascular mortality [38] and hospitalization [40] in dialysis patients. Furthermore, recently we have found a strong relationship between malnutrition, elevated CRP levels and atherosclerosis in pre‐dialysis patients [7].
The prevalence of an increased CRP (>8–10 mg/l) has been reported to be high in dialysis [4,38,41] and pre‐dialysis [7] patients. Serum levels of CRP appear to reflect generation of pro‐inflammatory cytokines [interleukin‐1 (IL‐1), IL‐6 and tumour necrosis factor‐α (TNF‐α)] which have also been reported to be increased in CRF patients [42,43]. It is well documented that high levels of pro‐inflammatory cytokines may cause muscle wasting by stimulating protein catabolism via the ubiquitin–proteosome pathway [44] by reducing albumin synthesis and by inhibiting appetite [45]. It has also been observed that IL‐1, TNF‐α and endotoxins may induce net catabolism of muscle protein by stimulating branched‐chain ketoacid dehydrogenase, which leads to greater oxidation of branched‐chain amino acids [46]. In consequence, increased plasma levels of pro‐inflammatory cytokines predict hypoalbuminaemia [34] and mortality [34,43] in dialysis patients. Furthermore, whereas serum albumin and CRP predict mortality in univariate analysis, only CRP is a significant predictor in multivariate analysis [33,38]. Taken together, available evidence suggests that the increased mortality rate observed in CRF may be associated with an acute‐phase response rather than low serum albumin levels caused by other mechanisms.
Inflammation increases resting energy expenditure
Energy deficiency due to low energy intake and increased resting energy expenditure (REE) may be another important cause of wasting in dialysis patients. In general, failure to down‐regulate REE as an adaptation to anorexia is often said to be a major cause of weight loss and wasting. Although REE may be increased in patients with CHF [47], cancer [48,49], rheumatoid arthritis [50] and AIDS [51], either normal [12,13,52] or increased [14] REE have been observed in CRF patients. These discrepant REE findings in dialysis patients are not understood. However, inflammation repeatedly has been shown to be associated with increased REE in other patients with wasting disorders [48–50,53]. On the basis of these observations, some of the reported variations in REE in dialysis patients may reflect differences in the prevalence of inflammation. Thus, the presence of the acute‐phase response permits identification of hypermetabolic patients [48]. Indeed, Schneeweiss et al. [13] have reported increased REE in dialysis patients with infections. Therefore, since inflammation increases REE, we suggest that this may be a factor contributing to protein calorie malnutrition in CRF patients.
Increased oxidative stress in malnourished and inflamed patients
Oxidative stress, which occurs in the presence of excessive free‐radical production or low anti‐oxidant levels, is an important co‐factor for development of endothelial dysfunction and atherogenesis. Recent data indicate that increased oxidative stress occurs in dialysis patients [54]. Malnourished pre‐dialysis patients have biochemical evidence of more oxidative stress than well‐nourished ones [55]. Moreover, malnourished pre‐dialysis patients have lower plasma levels of vitamin E [7], suggesting a low intake of anti‐oxidants when nutritional intake is low. In addition, recent data show that inflammation is associated with an increase in oxidative stress [56] and that advanced oxidation protein products are associated with monocyte activation in CRF patients [57]. On the basis of these findings, one can speculate that an increase in oxidative stress may contribute to the high prevalence of cardiovascular disease in malnourished CRF patients. Human plasma contains a variety of anti‐oxidant biomolecules and it is of interest that serum albumin may act as the principal anti‐oxidant in plasma [58]. Thus, low serum albumin levels associated with malnutrition and/or inflammation may increase atherogenesis by raising oxidative stress. Indeed, Soejima et al. [59] have shown that hypoalbuminaemia accelerates erythrocyte membrane lipid peroxidation in HD patients. One mechanism by which greater oxidative stress may accelerate atherogenesis is by affecting endothelial function. In fact, increased oxidant stress appears to play a pathophysiological role in the deleterious endothelial effects of homocysteine [60].
Inflammation is associated with endothelial dysfunction
Atherosclerosis is related to endothelial dysfunction and reduced bio‐availability of nitric oxide (NO). It is not yet known whether CRF patients have reduced or increased NO production because, although whole‐body NO production is reduced [61], excretion of NO is increased in exhaled air of patients with CRF [62]. However, both PD [63] and HD [64] patients have impaired endothelium‐dependent vasodilation. It should be noted that NO forms an adduct with serum albumin that has endothelium‐derived relaxing properties [65]. Thus, low albumin levels could be associated with impaired endothelial‐dependent vasodilation. Although Kim et al. [66] showed correlations between serum albumin, CRP and serum markers of endothelial function, an infusion of albumin did not normalize endothelial function. Consequently, their findings strongly suggest that the relationship between low serum albumin levels and endothelial dysfunction may be secondary to other factors, such as inflammation. Indeed, Kessler et al. [67] have reported in rabbits that pro‐inflammatory mediators inhibit the formation of endothelium‐dependent hyperpolarizing factor which may contribute to endothelial dysfunction. Moreover, an increased CRP is associated with endothelial dysfunction in patients with diabetes [68]. Altogether, available evidence suggests that inflammation can be associated with endothelial dysfunction, which may accelerate atherosclerosis.
CHF may cause wasting in dialysis patients
It has been well documented that CHF is associated with malnutrition (cardiac cachexia) and increased levels of pro‐inflammatory cytokines. More than 50% of patients with CHF have signs of cardiac cachexia [69]. Evidence of muscle wasting is present even in mild CHF [70], and muscle wasting appears to be related to reduced exercise capacity [71]. The pathogenesis of cardiac cachexia is not understood, and no relationship between nutritional intake and cardiac cachexia has been established. However, elevated serum levels of TNF‐α are related to cardiac cachexia [72]. The serum levels of IL‐6 are also significantly higher even in mild or moderate CHF [73]. It has been suggested that pro‐inflammatory cytokines, generated in response to factors such as reduced tissue perfusion, altered gut permeability and congestion, may play an important role in the loss of lean body mass in these patients. Indeed, cardiac cachexia is a strong independent risk factor for mortality in patients with CHF [74]. Since the prevalence of CHF is high among dialysis patients [75], CHF may contribute to wasting, elevated levels of pro‐inflammatory cytokines and increased mortality even in dialysis patients.
Relationship between malnutrition, inflammation and cardiovascular disease
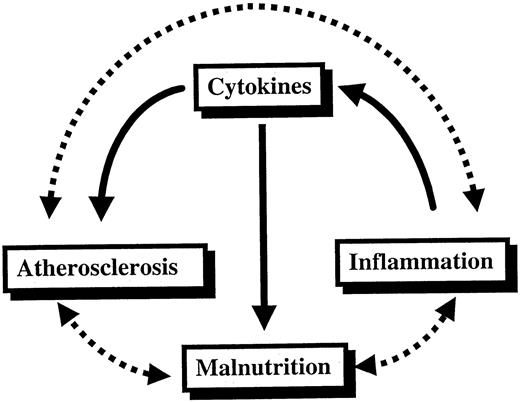
It may seem puzzling that whereas hypoalbuminaemia and inflammation have been shown to be important predictors of mortality in dialysis patients, complications from malnutrition as such are not common causes of mortality in dialysis patients [76]. In fact, malnutrition accounts for <5% of deaths in renal patients [77] while atherosclerotic cardiovascular disease is by far the most common cause of mortality in the dialysis population [78]. How can this finding be explained? A recent study suggests that strong interactions exist between cardiovascular disease and inflammatory as well as nutritional parameters in CRF patients [7]. We have therefore suggested the existence of a syndrome consisting of malnutrition, inflammation and atherosclerosis (MIA syndrome) in some patients with CRF [79]. Indeed, inflammation is more common in malnourished HD patients [4], and Ikizler et al. [40] have shown that the nutritional status and inflammatory response are independent predictors of hospitalization in HD patients. Moreover, malnutrition [80] and inflammation [38] are associated with a higher cardiovascular mortality rate in HD patients. Taken together, available evidence suggests that nutritional and inflammatory markers are closely linked to cardiovascular disease in CRF. It therefore seems likely that elevated levels of pro‐inflammatory cytokines could be the link between the high prevalence of inflammation, malnutrition and cardiovascular disease in patients with CRF (Figure 2).
The vicious circle of malnutrition, inflammation and atherosclerotic cardiovascular disease (MIA syndrome) in patients with chronic renal failure. A central role in this scenario is played by the pro‐inflammatory cytokines generated in response to factors such as chronic heart failure and infectious/inflammatory co‐morbid diseases.
Are there two types of malnutrition in dialysis patients?
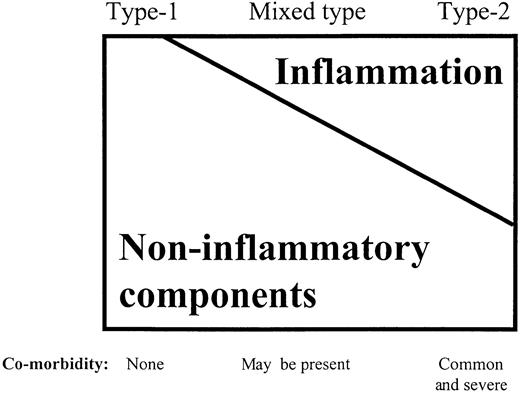
On the basis of findings discussed above, it seems reasonable to conclude that the acute‐phase response and malnutrition are closely linked and that both these conditions may contribute to the excessive atherosclerotic cardiovascular mortality observed in dialysis patients. Therefore, like Baltzan and Shoker [81], we believe that at least two types of malnutrition may occur in dialysis patients (Table 1). The first type (type 1) is associated with the uraemic syndrome per se or factors associated with uraemia (such as physical inactivity, underdialysis, dietary restrictions and psychosocial factors). It is characterized by a modest reduction in serum albumin levels, because of lower protein and energy intake due to uraemic toxicity. The first signs of protein and energy malnutrition begin early in the course of progressive renal failure [82] and the development of renal failure is associated with a spontaneous decrease in dietary protein intake [83]. Significant co‐morbidity, such as CHF, and elevated levels of pro‐inflammatory cytokines are usually not present in this type of malnutrition. The main feature is a low protein energy intake due to uraemic anorexia, with a corresponding decrease in protein catabolism. REE may be normal in this type of malnutrition. More marked hypoalbuminaemia, higher REE, markedly increased oxidative stress and increased protein catabolism, on the other hand, would characterize the other type of malnutrition (type 2). Significant co‐morbid conditions, such as CHF, frequently are found in this type of malnutrition, and there is usually an inflammatory response, as evidenced by higher levels of CRP and pro‐inflammatory cytokines. However, the spectrum of malnutrition in CRF may involve a continuous overlap between both types of malnutrition (Figure 3), and most dialysis patients probably have a mixed type of malnutrition. It is of interest that if one uses either normalized protein catabolic rate in HD patients, or subjective global assessment in pre‐dialysis patients as markers of nutrition, reduction in serum albumin concentration below values of 3.5 g/dl is only encountered when CRP levels are increased (Figure 1). Thus, hypoalbuminaemia in these patient populations may well reflect this combination between malnutrition and inflammation. It seems likely that ‘cytokine‐driven’ type 2 malnutrition occurs not only in CRF patients but also in those with wasting disorders, e.g. CHF, chronic inflammatory diseases (e.g. rheumatoid arthritis and AIDS), advanced cancer and chronic respiratory insufficiency.
Proposed relative contribution of non‐inflammatory components (such as low intake of protein and energy due to uraemic anorexia, underdialysis, physical inactivity, etc.) and the inflammatory components of malnutrition in patients with type 1 and type 2 malnutrition, respectively.
Proposed features of type 1 and type 2 malnutrition
| Type 1 | Type 2 | |
| Serum albumin | Normal/low | Low |
| Co‐morbidity | Uncommon | Common |
| Presence of inflammation | No | Yes |
| Food intake | Low | Low/normal |
| Resting energy expenditure | Normal | Elevated |
| Oxidative stress | Increased | Markedly increased |
| Protein catabolism | Decreased | Increased |
| Reversed by dialysis and | ||
| nutritional support | Yes | No |
| Type 1 | Type 2 | |
| Serum albumin | Normal/low | Low |
| Co‐morbidity | Uncommon | Common |
| Presence of inflammation | No | Yes |
| Food intake | Low | Low/normal |
| Resting energy expenditure | Normal | Elevated |
| Oxidative stress | Increased | Markedly increased |
| Protein catabolism | Decreased | Increased |
| Reversed by dialysis and | ||
| nutritional support | Yes | No |
Proposed features of type 1 and type 2 malnutrition
| Type 1 | Type 2 | |
| Serum albumin | Normal/low | Low |
| Co‐morbidity | Uncommon | Common |
| Presence of inflammation | No | Yes |
| Food intake | Low | Low/normal |
| Resting energy expenditure | Normal | Elevated |
| Oxidative stress | Increased | Markedly increased |
| Protein catabolism | Decreased | Increased |
| Reversed by dialysis and | ||
| nutritional support | Yes | No |
| Type 1 | Type 2 | |
| Serum albumin | Normal/low | Low |
| Co‐morbidity | Uncommon | Common |
| Presence of inflammation | No | Yes |
| Food intake | Low | Low/normal |
| Resting energy expenditure | Normal | Elevated |
| Oxidative stress | Increased | Markedly increased |
| Protein catabolism | Decreased | Increased |
| Reversed by dialysis and | ||
| nutritional support | Yes | No |
Nutritional support in uraemic patients with malnutrition
To date, >20 studies have investigated the effects of intradialytic nutritional support in patients with CRF [84]. Overall, the findings have been difficult to interpret usually because of the lack of control groups. A recent review indicated that the data supporting the use of intradialytic parenteral nutrition are weak [84]. One reason for this may be that the patient populations were mixed, i.e. both patients with type 1 and type 2 malnutrition were included. In our opinion, whereas adequate nutritional support and dialysis treatment is probably sufficient to treat type 1 malnutrition, this will hardly improve the nutritional status in type 2 malnutrition. Indeed, pro‐inflammatory effects of the dialytic procedure per se (such as bio‐incompatibility of the dialyser membrane, the exposure to endotoxins in the dialysis water and blood access infections) may aggravate this ‘cytokine‐driven’ type of malnutrition. Therefore, it is unlikely that type 2 malnutrition can be treated adequately unless concomitant co‐morbid disease(s) and/or source(s) of chronic inflammation are eliminated. Indeed, some recent findings suggest that the effect of giving nutritional supplements may be blunted if co‐morbid conditions and/or inflammation are present. At first, recent data show that co‐morbid conditions seem to hinder the potential benefits on the nutritional state in PD patients of increasing dialysis dose [85]. Moreover, Ericsson et al. [86] have found recently that low‐grade inflammatory activity abolishes the anabolic effect of growth hormone in HD patients. Finally, nutritional interventions to increase serum albumin have been reported to improve outcome, especially in patients with low co‐morbidity [87].
New treatment strategies are needed
Despite the considerable improvement in dialysis technology, the mortality rate from cardiovascular disease is still unacceptably high in dialysis patients [78] and new treatments obviously are needed for the next century. Indeed, several new strategies may be of interest and should be tested in prospective interventional trials in patients having malnutrition associated with MIA syndrome. In view of the strong associations between CHF, muscle wasting and elevated levels of pro‐inflammatory cytokines, we believe that it is very important to optimize cardiac performance in dialysis patients. In this respect, treatment with angiotensin‐converting enzyme inhibitors (ACEIs) may be of particular interest. This class of drugs has been shown not only to improve cardiac function and reduce the mortality rate in patients with cardiovascular disease [88,89], but also to be associated with a better nutritional status and lower levels of TNF‐α in CRF patients [90]. Anker et al. [91] recently found that treatment with ACEIs reduced the risk of developing weight loss in patients with CHF. It is thus possible that ACEIs, in addition to their favourable haemodynamic effects, may also improve the response to metabolic and immunological abnormalities in CRF. In view of the documented association between increased oxidative stress and endothelial dysfunction [60], we speculate that anti‐oxidant supplementation may improve endothelial function in malnourished dialysis patients. Indeed, vitamin C improves endothelial dysfunction of the pericardial coronary arteries in hypertensive patients [92].
Since there is now mounting evidence suggesting that certain persistent bacterial and viral infections may play a role in the pathogenesis of cardiovascular disease [93], prospective studies are needed to determine whether anti‐bacterial or anti‐viral treatment may improve the cardiovascular and nutritional status of dialysis patients. Finally, anti‐cytokine therapy (e.g. anti‐TNF‐α antibodies, soluble TNF‐α receptors and IL‐1 receptor antagonists) in other patient groups with wasting disorders [94] has been found to be associated with a rapid improvement not only in clinical findings but also in inflammatory parameters. It should also be noted that thalidomide (which selectively inhibits the production of TNF‐α, primarily by accelerating the degradation of TNF‐α mRNA transcripts) reverses the wasting syndrome associated with HIV [95] and tuberculosis [96]. Prospective studies are therefore needed to investigate whether anti‐cytokine therapies are safe and may have a beneficial effect on cardiovascular and nutritional status and mortality rate in CRF patients with signs of wasting and inflammation.
Conclusions
Chronic inflammation, as evidenced by increased levels of pro‐inflammatory cytokines and CRP, is common in CRF patients and may cause malnutrition and progressive atherosclerotic cardiovascular disease by several pathogenetic mechanisms. We therefore propose that at least two types of malnutrition exist in CRF: one without (type 1) and one with (type 2) a concomitant inflammatory response and significant co‐morbidity. It is likely that the treatment strategies for these types of malnutrition should be very different. We suggest that in future prospective studies, in which the effects of various interventions on nutritional and/or cardiovascular status are evaluated in CRF patients, an appropriate assessment of inflammatory status and distinction between the two types of malnutrition should be made.
Correspondence and offprint requests to: Peter Stenvinkel, MD, Department of Renal Medicine, K56, Huddinge University Hospital, S‐141 86 Huddinge, Sweden.
This study was supported by the Trone‐Holsts Foundation (P.S.) and the Swedish Medical Research Foundation (P.S.).
References
Marckmann P. Nutritional status of patients on hemodialysis and peritoneal dialysis.
Bergström J, Lindholm B. Nutrition and adequacy of dialysis. How do hemodialysis and CAPD compare?
Cianciaruso B, Brunori G, Kopple JD et al. Cross‐sectional comparison of malnutrition in continuous ambulatory dialysis and hemodialysis patients.
Qureshi AR, Alvestrand A, Danielsson A, Divino‐Filho JC, Gutierrez A, Lindholm B et al. Factors influencing malnutrition in hemodialysis patients. A cross‐sectional study.
Simons JP, Scols AM, Buurman WA, Wouters EF. Weight loss and low body cell mass in males with lung cancer: relationship with systemic inflammation, acute‐phase response, resting energy expenditure, and catabolic and anabolic hormones.
Munro R, Capell H. Prevalence of low body mass in rheumatoid arthritis: associations with the acute phase response.
Stenvinkel P, Heimbürger O, Paultre F et al. Strong associations between malnutrition, inflammation and atherosclerosis in chronic renal failure.
Heimbürger O, Qureshi AR, Blaner WS, Berglund L, Stenvinkel P. Hand‐grip muscle strength, lean body mass and plasma proteins as markers of nutritional status in patients with chronic renal failure close to start of dialysis therapy.
Johanson A, Samuelsson O, Haraldson B, Bosaeus J, Attman P‐O. Body composition in patients treated with peritoneal dialysis.
Kopple JD. Dietary protein and energy requirements in ESRD patients.
Monteon FJ, Laidlaw SA, Shaib JK, Kopple JD. Energy expenditure in patients with chronic renal failure.
Schneeweiss B, Graninger W, Stockenhuher F et al. Energy metabolism in acute and chronic renal failure.
Ikizler TA, Wingard RL, Sun M, Harvell J, Parker RA, Hakim RM. Increased energy expenditure in hemodialysis patients.
Bergström J, Fürst P, Alvestrand A, Lindholm B. Protein and energy intake, nitrogen balance and nitrogen losses in patients treated with continuous ambulatory peritoneal dialysis.
Lowrie EG, Lew NL. Death risk in hemodialysis patients: the predictive value of commonly measured variables and an evaluation of death rate differences between facilities.
Avram MM, Fein PA, Bonomini L et al. Predictors of survival in continuous ambulatory peritoneal dialysis patients: a five year prospective study.
Avram MM, Goldwasser P, Erroa M, Fein PA. Predictors of survival in continuous ambulatory peritoneal dialysis patients: the importance of prealbumin and other nutritional and metabolic parameters.
Folsom AR, Ma J, Eckfeldt JH, Nieto FJ, Metcalfe PA, Barnes RW. Low serum albumin. Association with diabetes mellitus and other cardiovascular risk factors but not with prevalent cardiovascular disease or carotid artery intima media–media thickness.
Koch M, Kutkuhn B, Grabensee B, Ritz E. Apolipoprotein A, fibrinogen, age, and a history of stroke are predictors of death in dialyzed diabetic patients: a prospective study in 412 subjects.
Struijk DG, Krediet RT, Koomen GCM, Boeschoten EW, Arisz L. The effect of serum albumin at the start of continuous ambulatory peritoneal dialysis treatment on patient survival.
Acchiardo SR, Moore LW, Latour PA. Malnutrition as a main factor in morbidity and mortality of hemodialysis patients.
Smith G, Robinson PH, Fleck A. Serum albumin distribution in early treated anorexia nervosa.
Keys A, Brozek J, Henschel A, Mickelsen O, Taylor HL. The biology of human starvation. The University of Minnesota Press, Minneapolis, 1950
Heimbürger O, Bergström J, Lindholm B. Is serum albumin an indication of nutritional status in CAPD patients?
Kaysen GA, Schonfeld PY. Albumin homeostasis in patients undergoing continuous ambulatory peritoneal dialysis.
Kaplan AA, Halley SE, Lapkin RA, Graeber CW. Dialysate protein losses with bleach processed polysulphone dialyzers.
Moshage HJ, Janssen JAM, Franssen JH, Hafkenscheid JCM, Yap SH. Study of the molecular mechanisms of decreased liver synthesis of albumin in inflammation.
Kaysen GA, Stevenson FT, Depner TA. Determinants of albumin concentration in hemodialysis patients.
Yeun JY, Kaysen GA. Acute phase proteins and peritoneal dialysate albumin loss are the main determinants of serum albumin and peritoneal dialysis patients.
Bergström J, Heimbürger O, Lindholm B, Qureshi AR. Elevated serum C‐reactive protein is a strong predictor of increased mortality and low serum albumin in hemodialysis (HD) patients.
Bologa RM, Levine DM, Parker TS et al. Interleukin‐6 predicts hypoalbuminemia, hypocholesterolemia, and mortality in hemodialysis patients.
Ridker PM, Cushman M, Stampfer MJ, Russell PT, Hennekens CH. Inflammation, aspirin and the risk of cardiovascular disease in apparently healthy men.
Muir KW, Weir CJ, Alwan W, Squire IB, Lees KR. C‐reactive protein and outcome after ischemic stroke.
Harris TB, Ferrucci L, Tracy RP et al. Association of elevated interleukin‐6 and C‐reactive protein levels with mortality in the elderly.
Zimmermann J, Herrlinger S, Pruy A, Metzger T, Wanner C. Inflammation enhances cardiovascular risk and mortality in hemodialysis patients.
Iseki K, Tozawa M, Yoshi S, Fukiyama K. Serum C‐reactive (CRP) and risk of death in chronic dialysis patients.
Ikizler TA, Wingard RL, Harvell J, Shyr Y, Hakim RM. Association of morbidity with markers of nutrition and inflammation in chronic hemodialysis patients: a prospective study.
Owen WF, Lowrie EG. C‐reactive protein as an outcome predictor for maintenance hemodialysis patients.
Pereira BJG, Shapiro L, King AJ, Falagas ME, Strom JA, Dinarello CA. Plasma levels of IL‐1β, TNF‐α and their specific inhibitors in undialyzed chronic renal failure, CAPD and hemodialysis patients.
Kimmel PL, Phillips TM, Simmens SJ et al. Immunologic function and survival in hemodialysis patients.
Bistrian BR, Schwartz J, Istfan NW. Cytokines, muscle proteolysis, and the catabolic response to infection and inflammation.
Nawabi MD, Block KP, Chakrabarti MC, Buse MG. Administration of endotoxin, tumour necrosis factor, or interleukin‐1 to rats activates skeletal muscle branched‐chain alpha‐keto acid dehydrogenase.
Schneeweiss B, Druml W, Graninger W et al. Energy metabolism in patients with severe heart failure.
Falconer JS, Fearon KCH, Plesler CE, Ross JA, Carter DC. Cytokines, the resting acute‐phase response, and resting energy expenditure in cachectic patients with pancreatic cancer.
Staal‐van den Brekel AJ, Dentener MA, Schols AM, Buurman WA, Wouters EF. Increased resting energy expenditure and weight loss are related to a systemic inflammatory response in lung cancer patients.
Roubenhoff R, Roubenhoff RA, Cannon JG et al. Rheumatoid cachexia: cytokine‐driven hypermetabolism accompanying reduced body cell mass in chronic inflammation.
Heijligenberg R, Romijn JA, Westerterp KR, Jonkers CF, Prins JM, Sauerwein HP. Total energy expenditure in human immunodeficiency virus‐infected men and healthy controls.
Harty J, Conway L, Keegan M et al. Energy metabolism during CAPD; a controlled study.
Campillo B, Bories PN, Devanlay M et al. Aging, energy expenditure and nutritional status: evidence for denutrition‐related hypermetabolism.
Maggi E, Bellazzi R, Falaschi F et al. Enhanced LDL oxidation in uremic patients: an additional mechanism for accelerated atherosclerosis.
Stenvinkel P, Holmberg I, Heimbürger O, Diczfalusy U. A study of plasmalogen as an index of oxidative stress in patients with chronic renal failure. Evidence of increased oxidative stress in malnourished patients.
Sguyen‐Choa T, Massy ZA, DeBandt JP et al. Factors influencing oxidative stress in hemodialysis (HD) patients.
Witko‐Sarsat V, Friedlander M, Khoa TN et al. Advanced oxidation protein products as novel mediators of inflammation and monocyte activation in chronic renal failure.
Cha MK, Kim IH. Glutahthione‐linked thiol peroxidase activity of human serum albumin: a possible antioxidant role of serum albumin in blood plasma.
Soejima A, Matsuzawa N, Miyake N et al. Hypoalbuminemia accelerates erythrocyte membrane lipid peroxidation in chronic hemodialysis patients.
Kanani PM, Sinkey CA, Browning RL, Allaman M, Knapp HR, Haynes WG. Role of oxidant stress in endothelial dysfunction produced by experimental hyperhomocysteinemia in humans.
Werver R, Boer P, Hijmering M et al. Nitric oxide production is reduced in patients with chronic renal failure.
Matsumoto A, Hirata Y, Kakoki M et al. Increased excretion of nitric oxide in exhaled air of patients with chronic renal failure.
van Guldener C, Janssen MJ, Lambert J, Steyn M, Donker AJ, Steuhower CC. Endothelium‐dependent vasodilation is impaired in peritoneal dialysis patients.
Hand MF, Haynes WG, Webb DJ. Hemodialysis and l‐arginine, but not d‐arginine, correct renal‐failure associated endothelial dysfunction.
Keaney JF, Simon DI, Stamler JS et al. NO forms an adduct with serum albumin that has endothelium‐derived relaxing factor‐like properties.
Kim SB, Chi HS, Park JS, Hong CD, Yang WS. Effect of increasing serum albumin on plasma d‐dimer, von Willebrand factor, and platelet aggregation in CAPD patients.
Kessler P, Popp R, Busse R, Schini‐Kerth VB. Proinflammatory mediators chronically downregulate the formation of the endothelium‐derived hyperpolarizing factor in arteries via a nitric oxide/cyclic GMP‐dependent mechanism.
Schalkwijk CG, Poland DC, van Dijk W et al. Plasma concentrations of C‐reactive protein is increased in type 1 diabetic patients without clinical macroangiopathy and correlates with markers of endothelial dysfunction: evidence for chronic inflammation.
Carr JG, Stevensson LW, Walden JA, Heber D. Prevalence and hemodynamic correlates of malnutrition in severe congestive heart failure secondary to ischemic or idiopathic dilated cardiomyopathy.
Mancini DM, Walter G, Reicek N et al. Contribution of skeletal muscle atrophy to exercise intolerance and altered muscle metabolism in heart failure.
Volterrani M, Clark AL, Ludman PF et al. Determinants of exercise capacity in chronic heart failure.
Levine B, Kalman J, Mayer L, Fillit HM, Packer M. Elevated circulating levels of tumor necrosis factor in severe chronic heart failure.
Munger MA, Johnsson B, Amber IJ, Callahan KS, Bilbert EM. Circulating concentrations of proinflammatory cytokines in mild or moderate heart failure secondary to ischemic or idiopathic dilated cardiomyopathy.
Anker SD, Ponikowski P, Varney S et al. Wasting as independent risk factor for mortality in chronic heart failure.
Parfrey PS, Foley RN, Harnett JD, Kent GM, Murray DC, Barre PE. Outcome and risk factors for left ventricular disorders in chronic uraemia.
Bergström J, Lindholm B. Malnutrition, cardiac disease and mortality—an integrated point of view.
EDTA/ERA Registry Report. Demography of dialysis and transplantation in Europe 1984.
Foley RN, Parfrey PS, Sarnak MJ. Clinical epidemiology of cardiovascular disease in chronic renal failure.
Stenvinkel P, Heimbürger O, Wang T, Elinder C‐G, Bergström J, Lindholm B. A syndrome of malnutrition, inflammation and atherosclerosis (MIA) is associated with elevated serum hyaluronan and increased mortality in chronic renal failure (CRF).
Keane WF, Collins AJ. Influence of co‐morbidity on mortality and morbidity of hemodialysis patients.
Kopple JD, Chumlea WC, Gassman JJ, et al. Relationship between GFR and nutritional status—results from the MDRD study.
Ikizler TA, Greene JH, Wingard RL, Parker RA, Hakim RM. Spontaneous dietary intake during progression of chronic renal failure.
Foulks CJ. An evidence‐based evaluation of intradialytic parenteral nutrition.
Davies SJ, Phillips L, Griffiths AM, Naish PF, Russell GI. An analysis of the effects of increasing delivered dialysis treatment to malnourished peritoneal dialysis patients.
Ericsson F, Divino JD, Lindgren B. Low grade inflammatory activity abolishes the anabolic effect of growth hormone (GH) on metabolism in hemodialysis patients.
Beddhu S, Zeidel S, Stark S, Saul M, Bruns F. Comorbidity influences the impact of albumin (Alb) on dialysis outcomes.
The SOLVD Investigators. Effect of enalapril on mortality and the development of heart failure in asymptomatic patients with reduced left ventricular ejection fractions.
CONSENSUS Trial Study Group. Effects of enalapril on mortality in severe congestive heart failure. Results of the north Scandinavian enalapril survival study.
Stenvinkel P, Andersson P, Wang T et al. Do ACE‐inhibitors suppress tumor necrosis factor‐α production in advanced chronic renal failure?
Anker SD, Negassa A, Coats AJ, Poole‐Wilson PA, Yusuf S. Weight loss in chronic heart failure (CHF) and the impact of treatment with ACE inhibitors. Results from the SOLVD treatment trial.
Solzbach U, Hornig B, Jeserich M, Just H. Vitamin C improves endothelial dysfunction of epicardial coronary arteries in hypertensive patients.
Danesh J, Collins R, Peto R. Chronic infections and coronary heart disease: is there a link?
Elliot MJ, Maini RN, Feldmann M et al. Randomised double‐blind comparison of chimeric monoclonal antibody to tumour necrosis factor alpha (cA2) versus placebo in rheumatoid arthritis.
Reyes‐Terán G, Sierra‐Madero JG, Martínez del Cerro V et al. Effects of thalidomide on HIV‐associated wasting syndrome: a randomized, double blind, placebo‐controlled clinical study.





Comments