-
PDF
- Split View
-
Views
-
Cite
Cite
A. C. I. T. L. Tan, J. T. Brouwer, P. Glue, R. van Leusen, R. H. Kauffmann, S. W. Schalm, R. A. de Vries, B. Vroom, Safety of interferon and ribavirin therapy in haemodialysis patients with chronic hepatitis C: results of a pilot study, Nephrology Dialysis Transplantation, Volume 16, Issue 1, January 2001, Pages 193–195, https://doi.org/10.1093/ndt/16.1.193
Close - Share Icon Share
Sir,
Haemodialysis patients have an increased risk of chronic hepatitis C (CHC), and this is related to duration of haemodialysis and to the number of blood transfusions received. After renal transplantation, immunosuppression to sustain the renal graft can increase serum HCV‐RNA titres. Furthermore, CHC infection after renal transplantation has been shown to decrease both graft and patient survival. However, interferon treatment of CHC may precipitate renal graft rejection, so that treatment of CHC must occur before renal transplantation is considered. At present, the standard of therapy for CHC in patients with normal kidney function is interferon and ribavirin. In dialysis patients, only interferon has been given in small studies [1]. Single‐dose ribavirin clearance is substantially diminished in patients with renal dysfunction [2], and ribavirin dosing guidelines have not been established for this patient group. This is the first description of clinical experience with combination interferon and ribavirin therapy in this patient group.
Patients.
Five patients (three females, two males; age range 22–65 years) with CHC, who were on chronic haemodialysis, participated in the study. Approval from the local Hospital Ethical Committee was obtained. Prior to and during the study, all patients received erythropoietin, and blood transfusions were permitted if clinically required. Treatment consisted of open‐label subcutaneous interferon‐α2b 3×106 IU three times weekly (TIW) plus oral ribavirin. Based on a previous study describing single‐dose pharmacokinetics in patients with kidney dysfunction [2] we anticipated a daily dose of 400–600 mg of ribavirin (standard dose in patients with normal renal function is 1000–1200 mg/day). The initial ribavirin dose was 200 mg/day, which could be increased by 200 mg every 6 weeks depending on the haemoglobin (Hb) response (less than 10% reduction from baseline). Trough plasma ribavirin concentrations were obtained every 4–6 weeks and measured by a validated high performance liquid chromatography/mass spectrometric (HPLC/MS) assay.
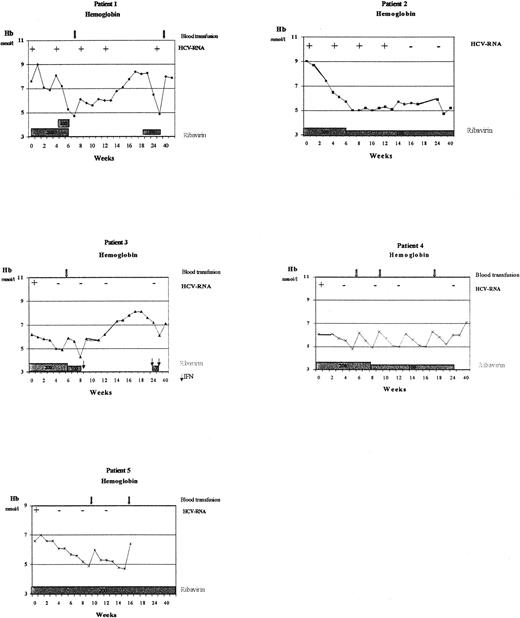
Mean haemoglobin levels and treatment histories for the five patients are shown in Figure 1. In all patients, Hb levels decreased. The erythropoietin dose was increased and blood transfusions administered (arrows, Figure 1) to try and increase Hb levels. Despite this, reticulocyte responses remained low (1–2%). Ribavirin dosing had to be stopped in two patients after 6–7 weeks, due to anaemia, which reversed after stopping ribavirin. After 2–3 months, ribavirin was restarted with a lower dose of 200 mg TIW. Despite this low dose, Hb decreased again and ribavirin was stopped permanently. One patient also suffered from a serious adverse event (fevers, chills), which resolved after permanently stopping interferon. In two other patients, Hb stabilized at a ribavirin dose of 200 mg TIW. The fifth patient remained at a dose of 200 mg daily. Ribavirin plasma concentrations at a daily dose of 200 mg/day were 2517 ng/ml, and at 200 mg 3 times weekly were 1594 ng/ml. In comparison, trough plasma ribavirin concentrations in CHC patients with normal renal function dosed with 1200 mg/day for 4 weeks were ∼2300 ng/ml [3]. Interim analysis revealed that in all patients, quantitative HCV‐RNA levels (as measured by polymerase chain reaction) decreased markedly, resulting in a loss of HCV‐RNA in four of five patients during treatment.
Individual haemoglobin values, relation to ribavirin dose, and qualitative HCV‐RNA results of the five patients treated.
Comment.
In this pilot study, combination therapy with α‐interferon and ribavirin suppressed HCV‐RNA in the majority of haemodialysis patients with CHC, consistent with results in CHC patients with normal renal function. This suggests that a high percentage of sustained response is possible in this difficult‐to‐treat patient group. Interferon clearance is substantially reduced in haemodialysis patients [4] meaning that serum interferon concentrations are considerably higher in these patients compared with those with normal renal function. This may affect its tolerability, as has been previously reported. Determining an appropriate ribavirin dose is more important, as shown in the present study by anaemia‐related dose reductions and discontinuations. The present study showed that trough plasma ribavirin concentrations at 200 mg/day were comparable to those of historical controls with normal renal function [3], while receiving only 1/6 of the controls' daily ribavirin dose. It is likely that even at comparable plasma concentrations, haemodialysis patients experience more severe anaemia than control subjects. In part this may be due to inadequate marrow responsiveness (as shown by the modest reticulocytosis), and it is also possible that ribavirin further reduces erythrocyte half‐life [5] which may be already reduced by anaemia and haemodialysis. Until appropriate dose‐selection studies are completed, interferon and ribavirin combination therapy should not be used in CHC patients on haemodialysis.
Contributing physicians and centres in The Netherlands
Dr K. Jie, Groene Hart Hospital, Gouda
Dr P. L. Rensma, St. Elisabeth Hospital, Tilburg
Dr S. v. Veen, Leyenburg Hospital, 's‐Gravenhage
Dr F. H. Bosch, Rijnstate Hospital, Arnhem
Dr R. M. Valentijn, Rode Kruis Hospital, 's‐Gravenhage
We thank Dr L. J. van Doorn for the measure- ment of HCV‐RNA levels.
References
Fabrizi F, Locatelli F. Combination of interferon alpha and ribavirin in the treatment of hepatitis C: implications for the clinical nephrologist.
Khakoo S, Glue P, Grellier L et al. Ribavirin and interferon alpha‐2b in chronic hepatitis C: assessment of possible pharmacokinetic and pharmacodynamic interactions.
Uchihara M, Izumi N, Sakai Y et al. Interferon therapy for chronic hepatitis C in hemodialysis patients: increased serum levels of interferon.





Comments