-
PDF
- Split View
-
Views
-
Cite
Cite
M. P. Stoffel, M. Pollok, J. Fries, C. A. Baldamus, Radiation nephropathy after radiotherapy in metastatic medullary thyroid carcinoma, Nephrology Dialysis Transplantation, Volume 16, Issue 5, May 2001, Pages 1082–1083, https://doi.org/10.1093/ndt/16.5.1082
Close - Share Icon Share
Sir,
Moll et al. [1] recently published a case report on the development of thrombotic microangiopathy after irradiation in ovarian malignancy. We would like to report our case of radiation nephropathy developing after ‘internal’ radiotherapy in a female patient with metastatic medullary carcinoma. Radiation nephritis is an inflammatory and degenerative disease of the kidneys after exposure to radioactive substances or body irradiation. Technical development, increasing knowledge of the adverse effects of radiation, and implementation of preventive measures have reduced its incidence during the last decades. However, recent therapeutic advances in oncology (such as administration of radiometal‐labelled peptide conjugates or combined high dose chemotherapy and radiation therapy in stem cell transplantation) increase nephrotoxicity. As demonstrated in our patient, the treatment with radioactive substances specifically bound to tumour‐selective cell receptors (i.e. somatostatin receptors in metastatic medullary thyroid carcinoma) caused thrombotic microangiopathy that led to end‐stage radiation nephropathy after 8 months.
Case.
A 63‐year‐old Caucasian female patient was admitted to our hospital in May 1999 for progressive weakness and newly diagnosed arterial hypertension. Blood tests revealed normochromic, normocytic anaemia (haemoglobin 8.0 g/dl (12–16)), renal insufficiency (creatinine 4.4 mg/dl (0.5–0.9), urea 129 mg/dl (<50)), hyperphosphataemia (phosphate 1.53 mmol/l (0.84–1.45)), hypercalcaemia (calcium 2.98 mmol/l (2.2–2.65)) and inflammation (ferritin 707 μg/l (30–150), ESR 88 mm/h (<21 mm/h)). Other routine blood tests were normal. Except for appendectomy in 1952, prior history was uneventful until 1991 when follicular thyroid carcinoma was diagnosed. The thyroid and the parathyroid glands were removed completely, followed by ablative radioiodine therapy. In August 1994 a ‘paraganglioma’ on the right cervical side was removed. With the appearance of a neuroendocrine hepatic tumour in September 1997, histological re‐examination of the removed thyroid gland yielded the diagnosis of medullary carcinoma instead of follicular carcinoma. Re‐evaluation of the cervical paraganglioma was consistent with early metastasis of a medullary carcinoma. At that time metastatic spread into liver, lung and hilar lymph nodes was found. Without any therapeutic option we considered a new, experimental radioactive somatostatin‐receptor based therapy (90Y‐DOTATOC‐SMS) of which six cycles were performed between January and July 1998. Kidney function was normal at that time. The patient was followed routinely on an outpatient basis every 3 months. With completion of radiotherapy, partial remission of metastases was noted.
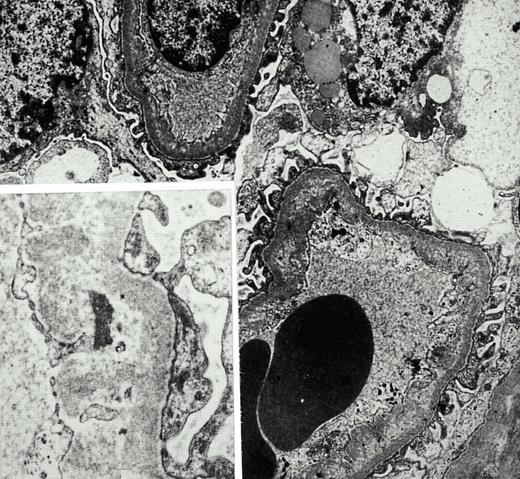
On admission in May 1999, paleness of skin and mucosae were noted. Blood pressure was 170/80 mmHg. Twenty‐four hour blood pressure profile demonstrated severe hypertension (day‐time levels of 175/79 mmHg). Catecholamine‐secreting tumours as well as renal artery stenosis or primary hyperaldosteronism were excluded. Creatinine clearance was 14 ml/min, parathyroid hormone was normal and urine analysis showed mild selective glomerular and tubular proteinuria (albumin 280 mg/l, alpha 1‐microglobulin 88 mg/l) with minimal erythrocyturia (10 erythrocytes/HPF without casts). Ultrasound revealed normal kidneys. Retrospectively, creatinine began to rise in January 1999 without clinical correlates. A renal biopsy in May 1999 revealed light microscopic changes of segmental mesangiolysis in six out of 15 glomeruli with disruption and vacuolar expansion of the effected mesangial area. In addition, electron‐microscopic analysis showed stretches of subendothelial space widened by flocculent, lucent material incorporated into the basement membrane and matrix material newly synthesized by overlying endothelial cells (Figure 1). One glomerulus showed global sclerosis. Immunohistology did not reveal any pathognomonic glomerular deposition of immunoglobulins or complement factors. A marked tubular atrophy and compensatory interstitial fibrosis was present. In contrast to the prominent glomerular changes there was minimal arterial damage consisting of intimal thickening and segmental hyalinosis. Intra‐arterial thrombosis or necrosis were not observed. Six months later serum creatinine had risen to 7.3 mg/dl and a vascular access was created. Haemodialysis was started in February 2000.
Discussion.
This case demonstrates the rare development of radiation nephropathy caused by ‘internal’ radioactive therapy of a neuroendocrine tumour. The histologic hallmark of radiation nephropathy is radiation‐induced thrombotic microangiopathy. In a desperate situation with progressive metastatic disease the new concept of receptor‐mediated selective tumour therapy was applied. Efficacy of this therapy has been documented in neuroendocrine tumours [2], though controlled studies are not yet available. In earlier days radiation nephritis was a common disease after radiotherapy of diverse malignancies [3]. Principally, an acute form that develops within months after irradiation can be differentiated from a chronic form occuring sometimes more than a year after the exposure. In this patient the β‐emitter DOTATOC (a radioactively labelled analogue of octreotide, which in itself is a somatostain analogue (14 amino acids, molecular weight 11‐12 kD)) was used, because medullary thyroid carcinoma expressed somatostatin receptors. The radioactive agent DOTATOC is coupled to somatostatin and binds selectively to tumour cells. Kidney disease is a recognized therapeutic complication, because DOTATOC is filtrated through the glomeruli and probably reabsorbed by tubular cells. Although pathophysiologic conditions leading to the development of radiation nephritis are incompletely understood, the common denominator for the development of thrombotic microangiopathy in small kidney vessels is probably a sublethal endothelial cell injury [4,5]. This leads to inhibition of cell‐replication and to the formation of reactive oxygen intermediates inducing vascular damage [4,5]. In our case histologic changes were mostly prominent in the glomeruli, hinting at a relatively early stage of radiation nephropathy.
Tumour‐selective receptor‐mediated radiation therapy is a fascinating new concept in oncology which will probably gain more interest. Therefore it seems prudent to highlight potential harmful effects early. Use of nephroprotective cationic amino acids and inhibition of angiotensin‐converting enzymes are promising interventions to prevent radiation nephropathy [6,7].
Electron‐microscopic scan of a renal biopsy with glomerular mesangiolysis and peripheral capillary endothelial lesions.
References
Moll S, Pommer W, Mihatsch MJ, Nickeleit V. The lady who had a remote history of ovarian malignancy and developed thrombotic microangiopathy.
Otte A, Mueller‐Brand J, Nitzsche EU, Herrmann R, Maecke HR. Yttrium‐90‐labeled somatostatin‐analogue for cancer treatment.
Hopewell JW, Campling D, Calvo W et al. Vascular irradiation damage: its cellular basis and likely consequences.
Cohen EP, Bonsib SA, Whitehouse E, Hopewell JW, Robbins ME. Mediators and mechanisms of radiation nephropathy.
Behr TM, Sharkey RM, Sgouros G et al. Overcoming the nephrotoxicity of radiometal‐labeled immunoconjugates: improved cancer therapy administered to a nude mouse model in relation to the internal radiation dosimetry.





Comments