-
PDF
- Split View
-
Views
-
Cite
Cite
George R. Bailie, George Eisele, Lei Liu, Erik Roys, Margaret Kiser, Frederick Finkelstein, Robert Wolfe, Friedrich Port, Sally Burrows-Hudson, Rajiv Saran, Patterns of medication use in the RRI-CKD study: focus on medications with cardiovascular effects, Nephrology Dialysis Transplantation, Volume 20, Issue 6, June 2005, Pages 1110–1115, https://doi.org/10.1093/ndt/gfh771
Close - Share Icon Share
Abstract
Background. Patients with chronic kidney disease (CKD) stages 2–5 are known to suffer numerous complications and co-morbidities associated with kidney disease. The medication prescription patterns in this population are not well understood. We report on prescription data collected as part of a multicentre longitudinal study in patients with CKD, with a focus on medications with cardiovascular or cardioprotective effects.
Methods. Patients were recruited from four academic nephrology centres in the USA, with patient recruitment from June 2000 to March 2002. Medication data were captured at the time of first enrolment into the study. Individual medications were classified into medication groups, and those with predominant cardioprotective effects or for prevention of progression of kidney disease (e.g. medications for treatment of anaemia, lipid-lowering agents, antihypertensives, statins, etc.) were recorded for analysis. Descriptive statistics were used for medication prescription according to baseline demographics and co-morbidities. Predictors of epoetin and iron use were determined by logistic regression adjusting for age, race, sex, diabetes, glomerular filtration rate (GFR), haemoglobin and serum albumin.
Results. Medication data were available for 619 patients with stages 2–5 CKD. Patients were 60.6±16.0 years of age, and were prescribed 8±4 (range 1–28) medications. Overall, the proportion of patients prescribed different classes of medications included epoetin (20%), intravenous iron (13%), HMG-CoA reductase inhibitors (16%), angiotensin-converting enzyme (ACE) inhibitors (44%), angiotensin receptor blockers (13%), β-blockers (46%), calcium channel blockers (52%) and aspirin (37%). There was a low use of epoetin (45%) and iron (20%) in patients with anaemia. Only 24% of patients with coronary artery disease were prescribed statins, and ACE inhibitors and angiotensin receptor blockers were used in only 58 and 23% of diabetic patients with proteinuria. Positive predictors of epoetin and iron therapy included white race and diabetes. Higher GFR and higher serum albumin were associated with lower odds of being prescribed epoetin. White race and diabetics were more likely to be prescribed iron.
Conclusions. This study provides an overview of prescription practices in a cohort of CKD patients. Substantial underutilization of certain classes of cardioprotective medications is apparent, and systematic educational efforts in this direction may well prove worthwhile to impact outcomes.
Introduction
Patients with stage 5 chronic kidney disease (CKD) on dialysis are known to take a large number and variety of medications, with potential for development of significant medication-related problems [1,2]. Despite the possibility of 5–10 million individuals in the USA with stages 2–5 CKD, relatively little is known about the global medication prescription patterns used in individuals with declining kidney function. There is no clear picture of the overall medication profile or burden in this population. These patients have multiple co-morbidities and complications, and the inference can be made that they have a need for a large number of prescription medications, including those that might alter the rate of progression of decline in kidney function, and those used to treat hypertension, lipid disorders, diabetes, anaemia and osteodystrophy [3]. The extent of potential medication-related problems, such as over- and underprescription, drug interaction types and frequencies, and adverse reactions, is largely unknown [2,4,5]. Recent data do suggest that specific populations of patients with kidney disease are prone to suboptimal prescription of medications, but the extent of this phenomenon is unclear [4,5].
The purpose of this study is to describe the prescription patterns of selected medication classes [those with predominant cardioprotective effects or for prevention of progression of kidney disease (e.g. medications for treatment of anaemia, lipid-lowering agents, antihypertensives, statins, etc.)] and to determine predictors of types of medication used in pre-dialysis CKD patients. Data were obtained from a cohort study of CKD patients being conducted at four academic nephrology practices [the Renal Research Institute Chronic Kidney Disease (RRI-CKD) study].
Subjects and methods
The RRI-CKD study is a multicentre prospective cohort study of patients with moderate to advanced CKD (stages 3–5). Patients were recruited from four academic nephrology practices [University of Michigan (UM), Albany Medical Center (AMC), New Haven (NH) and University of North Carolina (UNC)]. Patients with a glomerular filtration rate (GFR) ≤50 ml/min/1.73 m2, determined using the modified MDRD formula [6] at two separate measurements at least 1 month apart, were invited to participate in the study. The institutional review boards at each of the four institutions approved the study independently, and patients provided written informed consent prior to data collection.
Details of the study design have been published previously and are summarized here [7].
The study was conducted in two phases. Phase 1 was designed as an observational study with patient enrolment from June 2000 until March 2002, collecting medical and quality of life data. Phase 2 (January 2003 onwards) involves recall of phase 1 patients to undergo non-invasive cardiovascular testing as well as collection of blood and urine specimens. The data described in this report are from phase 1 only. Medication, demographic and co-morbidity data were abstracted by the study coordinator at each site, using a standard data collection form, from patients’ records during the enrolment clinic visit. Individual medications were classified into major groups of drugs used to prevent progression of CKD or for cardioprotective effects (e.g. antihypertensives, statins, anaemia medications, lipid-lowering agents, aspirin, etc.), using a systematic method for classification previously used in the Dialysis Outcomes and Practice Patterns Study [8].
Statistical analysis
Descriptive statistics were utilized initially. Medication use was described by baseline GFR, anaemia (yes/no; defined as baseline haemoglobin <11 g/dl or haematocrit <33% or taking epoetin within 6 months of enrolment), coronary artery disease (yes/no), diabetes (yes/no) and iron status (iron deficient or not, if iron indices were available, or if the patient was receiving iron). Mean GFR between epoetin- and/iron-treated and untreated groups for anaemic patients was compared using the t-test. Predictors of epoetin and iron use were determined by logistic regression adjusting for age, race, sex, diabetes, GFR, haemoglobin, serum albumin and participating site. Proteinuria was defined (yes/no) based on the results of a urinary dipstick (>2+) or urinalysis (>100 mg/dl protein), either at baseline or at any subsequent visit. Statistical analyses were conducted using SAS software (version 9.1), and statistical significance was defined as P<0.05.
Results
A total of 634 patients were enrolled during phase 1 of the RRI-CKD study. Of these, medication data were available for 619 (94.5%) patients (UM 249, AMC 90, NH 133, UNC 147). The baseline demographic data are shown in Table 1. The mean age was 60.6±16.0 years, with no significant difference in age between patients by CKD stage. The mean GFR was 23.6±9.6 ml/min/1.73 m2. The most common aetiologies of CKD were hypertension alone (25%), diabetes alone (17%) and glomerular diseases (8%). Patients were predominantly white (75%), male (56%) and obese [body mass index (BMI) = 29.0±6.9] with mean haemoglobin 11.8±1.6 g/dl, and mean serum albumin 3.8±0.5 g/l. Patients had many of the co-morbid conditions frequently seen in the CKD population [diabetes (37%), hypertension (90%) and coronary artery disease (28%)].
Baseline demographics for the 619 patients with available medication data
| Characteristic . | . |
|---|---|
| Age (mean±SD, years) | |
| Total (n = 619) | 60.6±16.0 |
| Stage 2 and 3 (n = 150) | 61.3±15.8 |
| Stage 4 (n = 350) | 60.8±16.6 |
| Stage 5 (n = 116) | 59.1±14.1 |
| Causes of CKDa | |
| Diabetes aloneb | 102 (16%) |
| Hypertension alone | 154 (25%) |
| Glomerulonephritis | 50 (8%) |
| Interstitial kidney disease | 17 (3%) |
| Polycystic kidney disease | 28 (5%) |
| Other | 224 (36%) |
| Ethnicity and gender | |
| Female | 271 (44%) |
| White | 463 (75%) |
| Black | 130 (21%) |
| Other | 26 (4%) |
| Co-morbid conditions | |
| Diabetes | 37% |
| Hypertension | 90% |
| Coronary artery disease | 28% |
| Body mass index (mean±SD) | 29.0±6.9 |
| Haemoglobin (mean±SD, g/dl) | 11.8±1.6 |
| Albumin (mean±SD, g/l) | 3.8±0.5 |
| GFR (mean±SD, ml/min/1.73 m2) | 23.6±9.6 |
| Characteristic . | . |
|---|---|
| Age (mean±SD, years) | |
| Total (n = 619) | 60.6±16.0 |
| Stage 2 and 3 (n = 150) | 61.3±15.8 |
| Stage 4 (n = 350) | 60.8±16.6 |
| Stage 5 (n = 116) | 59.1±14.1 |
| Causes of CKDa | |
| Diabetes aloneb | 102 (16%) |
| Hypertension alone | 154 (25%) |
| Glomerulonephritis | 50 (8%) |
| Interstitial kidney disease | 17 (3%) |
| Polycystic kidney disease | 28 (5%) |
| Other | 224 (36%) |
| Ethnicity and gender | |
| Female | 271 (44%) |
| White | 463 (75%) |
| Black | 130 (21%) |
| Other | 26 (4%) |
| Co-morbid conditions | |
| Diabetes | 37% |
| Hypertension | 90% |
| Coronary artery disease | 28% |
| Body mass index (mean±SD) | 29.0±6.9 |
| Haemoglobin (mean±SD, g/dl) | 11.8±1.6 |
| Albumin (mean±SD, g/l) | 3.8±0.5 |
| GFR (mean±SD, ml/min/1.73 m2) | 23.6±9.6 |
aMultiple causes are possible so the total number exceeds 619. The percentage is obtained by dividing the separate number by 619.
bAlso includes patients with diabetes as co-morbidity with proteinuria.
Baseline demographics for the 619 patients with available medication data
| Characteristic . | . |
|---|---|
| Age (mean±SD, years) | |
| Total (n = 619) | 60.6±16.0 |
| Stage 2 and 3 (n = 150) | 61.3±15.8 |
| Stage 4 (n = 350) | 60.8±16.6 |
| Stage 5 (n = 116) | 59.1±14.1 |
| Causes of CKDa | |
| Diabetes aloneb | 102 (16%) |
| Hypertension alone | 154 (25%) |
| Glomerulonephritis | 50 (8%) |
| Interstitial kidney disease | 17 (3%) |
| Polycystic kidney disease | 28 (5%) |
| Other | 224 (36%) |
| Ethnicity and gender | |
| Female | 271 (44%) |
| White | 463 (75%) |
| Black | 130 (21%) |
| Other | 26 (4%) |
| Co-morbid conditions | |
| Diabetes | 37% |
| Hypertension | 90% |
| Coronary artery disease | 28% |
| Body mass index (mean±SD) | 29.0±6.9 |
| Haemoglobin (mean±SD, g/dl) | 11.8±1.6 |
| Albumin (mean±SD, g/l) | 3.8±0.5 |
| GFR (mean±SD, ml/min/1.73 m2) | 23.6±9.6 |
| Characteristic . | . |
|---|---|
| Age (mean±SD, years) | |
| Total (n = 619) | 60.6±16.0 |
| Stage 2 and 3 (n = 150) | 61.3±15.8 |
| Stage 4 (n = 350) | 60.8±16.6 |
| Stage 5 (n = 116) | 59.1±14.1 |
| Causes of CKDa | |
| Diabetes aloneb | 102 (16%) |
| Hypertension alone | 154 (25%) |
| Glomerulonephritis | 50 (8%) |
| Interstitial kidney disease | 17 (3%) |
| Polycystic kidney disease | 28 (5%) |
| Other | 224 (36%) |
| Ethnicity and gender | |
| Female | 271 (44%) |
| White | 463 (75%) |
| Black | 130 (21%) |
| Other | 26 (4%) |
| Co-morbid conditions | |
| Diabetes | 37% |
| Hypertension | 90% |
| Coronary artery disease | 28% |
| Body mass index (mean±SD) | 29.0±6.9 |
| Haemoglobin (mean±SD, g/dl) | 11.8±1.6 |
| Albumin (mean±SD, g/l) | 3.8±0.5 |
| GFR (mean±SD, ml/min/1.73 m2) | 23.6±9.6 |
aMultiple causes are possible so the total number exceeds 619. The percentage is obtained by dividing the separate number by 619.
bAlso includes patients with diabetes as co-morbidity with proteinuria.
The mean number of medications prescribed per patient was 8±4 (range 1–28). Table 2 shows the percentage of patients prescribed selected groups of medications by co-morbid condition. In patients with known coronary artery disease, 65% of patients were on aspirin, 65% on β-blockers and 55% on calcium channel blockers (Table 2). Less than half the patients were on angiotensin-converting enzyme (ACE) inhibitors (44%) and 13% were receiving angiotensin receptor blockers, while 16% were on HMG-CoA reductase inhibitors. In patients with hypertension, 50% were prescribed β-blockers, 55% calcium channel blockers, 45% ACE inhibitors and 14% angiotensin receptor blockers. There were small differences in medications prescribed for patients with proteinuria, based on whether or not they were diabetic. For example, ACE inhibitors were prescribed for 58 and 52% of proteinuric patients with and without diabetes, angiotensin receptor blockers for 23 and 13%, β-blockers for 51 and 44%, calcium channel blockers for 64 and 55%, and aspirin for 44 and 27%, respectively.
Percentage of patients on selected medications by condition
| Condition . | No. . | EPO % . | Iron % . | ACEI % . | ARB % . | BB % . | CCB % . | Aspirin % . | HMG % . |
|---|---|---|---|---|---|---|---|---|---|
| All patients | 619 | 20 | 13 | 44 | 13 | 46 | 52 | 37 | 16 |
| Diabetic | 231 | 21 | 17 | 48 | 15 | 48 | 53 | 47 | 19 |
| Hypertension | 555 | 19 | 13 | 45 | 14 | 50 | 55 | 39 | 17 |
| Anaemia | 270 | 45 | 20 | 45 | 14 | 46 | 53 | 31 | 15 |
| CAD | 170 | 23 | 14 | 41 | 12 | 65 | 55 | 65 | 24 |
| Proteinuria with DM | 119 | 21 | 24 | 58 | 23 | 51 | 64 | 44 | 19 |
| Proteinuria without DM | 178 | 22 | 12 | 52 | 13 | 44 | 55 | 27 | 17 |
| Condition . | No. . | EPO % . | Iron % . | ACEI % . | ARB % . | BB % . | CCB % . | Aspirin % . | HMG % . |
|---|---|---|---|---|---|---|---|---|---|
| All patients | 619 | 20 | 13 | 44 | 13 | 46 | 52 | 37 | 16 |
| Diabetic | 231 | 21 | 17 | 48 | 15 | 48 | 53 | 47 | 19 |
| Hypertension | 555 | 19 | 13 | 45 | 14 | 50 | 55 | 39 | 17 |
| Anaemia | 270 | 45 | 20 | 45 | 14 | 46 | 53 | 31 | 15 |
| CAD | 170 | 23 | 14 | 41 | 12 | 65 | 55 | 65 | 24 |
| Proteinuria with DM | 119 | 21 | 24 | 58 | 23 | 51 | 64 | 44 | 19 |
| Proteinuria without DM | 178 | 22 | 12 | 52 | 13 | 44 | 55 | 27 | 17 |
ACEI = ACE inhibitor; ARB = angiotensin receptor blocker; BB = β-blocker; CAD = coronary artery disease; CCB = calcium channel blocker; DM = diabetes mellitus; EPO = erythropoietic agent (epoetin or darbepoetin); HMG = HMG-CoA reductase inhibitor.
Percentage of patients on selected medications by condition
| Condition . | No. . | EPO % . | Iron % . | ACEI % . | ARB % . | BB % . | CCB % . | Aspirin % . | HMG % . |
|---|---|---|---|---|---|---|---|---|---|
| All patients | 619 | 20 | 13 | 44 | 13 | 46 | 52 | 37 | 16 |
| Diabetic | 231 | 21 | 17 | 48 | 15 | 48 | 53 | 47 | 19 |
| Hypertension | 555 | 19 | 13 | 45 | 14 | 50 | 55 | 39 | 17 |
| Anaemia | 270 | 45 | 20 | 45 | 14 | 46 | 53 | 31 | 15 |
| CAD | 170 | 23 | 14 | 41 | 12 | 65 | 55 | 65 | 24 |
| Proteinuria with DM | 119 | 21 | 24 | 58 | 23 | 51 | 64 | 44 | 19 |
| Proteinuria without DM | 178 | 22 | 12 | 52 | 13 | 44 | 55 | 27 | 17 |
| Condition . | No. . | EPO % . | Iron % . | ACEI % . | ARB % . | BB % . | CCB % . | Aspirin % . | HMG % . |
|---|---|---|---|---|---|---|---|---|---|
| All patients | 619 | 20 | 13 | 44 | 13 | 46 | 52 | 37 | 16 |
| Diabetic | 231 | 21 | 17 | 48 | 15 | 48 | 53 | 47 | 19 |
| Hypertension | 555 | 19 | 13 | 45 | 14 | 50 | 55 | 39 | 17 |
| Anaemia | 270 | 45 | 20 | 45 | 14 | 46 | 53 | 31 | 15 |
| CAD | 170 | 23 | 14 | 41 | 12 | 65 | 55 | 65 | 24 |
| Proteinuria with DM | 119 | 21 | 24 | 58 | 23 | 51 | 64 | 44 | 19 |
| Proteinuria without DM | 178 | 22 | 12 | 52 | 13 | 44 | 55 | 27 | 17 |
ACEI = ACE inhibitor; ARB = angiotensin receptor blocker; BB = β-blocker; CAD = coronary artery disease; CCB = calcium channel blocker; DM = diabetes mellitus; EPO = erythropoietic agent (epoetin or darbepoetin); HMG = HMG-CoA reductase inhibitor.
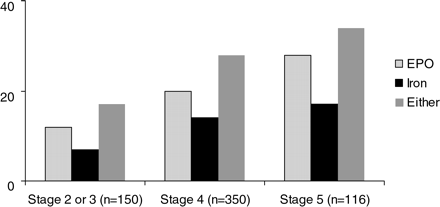
Monitoring of iron indices was infrequent within 6 months of enrolment into the study. Serum iron was available in 10% of all patients and only 14% of those who were anaemic. Serum ferritin was available in only 6% of all and 9% of those who were anaemic, and transferrin saturation monitoring was even more infrequent (0.6% overall and 1.5% among anaemic subjects). Overall, the frequency of iron prescription was 13%. Only 20% of anaemic patients were receiving iron at baseline. The frequency of epoetin prescription was 20% overall and 45% among those who were anaemic. In general, there were similar degrees of prescription of both agents regardless of underlying co-morbidity. There was some variability in prescription of these agents, however, depending on baseline GFR. More patients in CKD stages 4 and 5 were receiving epoetin, iron or either than patients in stages 2 and 3 CKD. Patients that were treated vs not treated with iron had different GFRs (20.8 vs 24.3 ml/min/1.73 m2, respectively) (Table 3, Figure 1), with significantly higher GFR values if they were untreated. The proportion of patients treated with iron increased as GFR declined through stage 5. There were marginally significant differences in the haemoglobin of patients treated vs untreated with epoetin (P = 0.05) or either epoetin or iron (P = 0.02). Untreated patients had higher haemoglobin values. The significance of the results in Table 3 is maintained after the data were adjusted for site effect. Higher GFR, haemoglobin and serum albumin were associated with lower odds of being prescribed epoetin (Table 4, χ2 = 31.9, P = 0.0008). Positive predictors of epoetin therapy were white race [odds ratio (OR) = 1.73] and presence of diabetes (OR = 1.15). White race (OR = 2.6) and diabetic (OR = 2.1) patients were more likely to be treated by iron alone (Table 5, χ2 = 23.0, P = 0.003). We did similar logistic models among anaemic patients, but found no significant predictors. Among anaemic patients, 51, 55 and 57% had not received epoetin within 6 months of enrolment in CKD stage 2 and 3, stage 4 and stage 5, respectively (P = 0.88). The proportions of phosphate binders in CKD stage 3, 4 and 5 patients were 13, 18 and 27%, respectively (P = 0.02).
Mean GFR and haemoglobin in all patients by medication
| Medication . | GFR (ml/min/1.73 m2) . | . | . | Hb (g/dl) . | . | . | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| . | Treated . | Untreated . | P-value . | Treated . | Untreated . | P-value . | ||||
| Epoetin | 20.8 | 24.3 | 0.0002 | 11.5 | 11.9 | 0.05 | ||||
| Iron | 21.0 | 24.0 | 0.01 | 11.5 | 11.9 | 0.10 | ||||
| Either | 21.3 | 24.4 | 0.0005 | 11.5 | 11.9 | 0.02 | ||||
| Medication . | GFR (ml/min/1.73 m2) . | . | . | Hb (g/dl) . | . | . | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| . | Treated . | Untreated . | P-value . | Treated . | Untreated . | P-value . | ||||
| Epoetin | 20.8 | 24.3 | 0.0002 | 11.5 | 11.9 | 0.05 | ||||
| Iron | 21.0 | 24.0 | 0.01 | 11.5 | 11.9 | 0.10 | ||||
| Either | 21.3 | 24.4 | 0.0005 | 11.5 | 11.9 | 0.02 | ||||
Mean GFR and haemoglobin in all patients by medication
| Medication . | GFR (ml/min/1.73 m2) . | . | . | Hb (g/dl) . | . | . | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| . | Treated . | Untreated . | P-value . | Treated . | Untreated . | P-value . | ||||
| Epoetin | 20.8 | 24.3 | 0.0002 | 11.5 | 11.9 | 0.05 | ||||
| Iron | 21.0 | 24.0 | 0.01 | 11.5 | 11.9 | 0.10 | ||||
| Either | 21.3 | 24.4 | 0.0005 | 11.5 | 11.9 | 0.02 | ||||
| Medication . | GFR (ml/min/1.73 m2) . | . | . | Hb (g/dl) . | . | . | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| . | Treated . | Untreated . | P-value . | Treated . | Untreated . | P-value . | ||||
| Epoetin | 20.8 | 24.3 | 0.0002 | 11.5 | 11.9 | 0.05 | ||||
| Iron | 21.0 | 24.0 | 0.01 | 11.5 | 11.9 | 0.10 | ||||
| Either | 21.3 | 24.4 | 0.0005 | 11.5 | 11.9 | 0.02 | ||||
Proportion of epoetin and intravenous iron use in all patients by GFR stage. GFR = glomerular filtration rate. Stage 2 = GFR 89–60 ml/min/1.73 m2. Stage 3 = GFR 59-30 ml/min/1.73 m2. Stage 4 = GFR 29-15 ml/min/1.73 m2. Stage 5 = GFR<15 ml/min/1.73 m2. Note: since there are only two patients in stage 2, we combined those individuals in the group with stage 3 CKD.
Predictors of epoetin therapy
| Factor . | OR (95% CI) . | P-value . |
|---|---|---|
| Age (per 10 years) | 0.93 (0.77–1.13) | 0.45 |
| White race | 1.73 (0.84–3.59) | 0.14 |
| Male sex | 0.64 (0.36–1.13) | 0.13 |
| Diabetes | 1.15 (0.64–2.05) | 0.65 |
| CAD | 1.76 (0.90–3.45) | 0.10 |
| GFR (per CKD stage) | 0.95 (0.92–0.98) | 0.001 |
| Hb (per 1 g/dl) | 0.96 (0.81–1.14) | 0.65 |
| Albumin (per 0.1 g/dl) | 0.63 (0.34–1.18) | 0.15 |
| Factor . | OR (95% CI) . | P-value . |
|---|---|---|
| Age (per 10 years) | 0.93 (0.77–1.13) | 0.45 |
| White race | 1.73 (0.84–3.59) | 0.14 |
| Male sex | 0.64 (0.36–1.13) | 0.13 |
| Diabetes | 1.15 (0.64–2.05) | 0.65 |
| CAD | 1.76 (0.90–3.45) | 0.10 |
| GFR (per CKD stage) | 0.95 (0.92–0.98) | 0.001 |
| Hb (per 1 g/dl) | 0.96 (0.81–1.14) | 0.65 |
| Albumin (per 0.1 g/dl) | 0.63 (0.34–1.18) | 0.15 |
CAD = coronary artery disease; CI = confidence interval; GFR = glomerular filtration rate; Hb = haemoglobin; OR = odds ratio.
Predictors of epoetin therapy
| Factor . | OR (95% CI) . | P-value . |
|---|---|---|
| Age (per 10 years) | 0.93 (0.77–1.13) | 0.45 |
| White race | 1.73 (0.84–3.59) | 0.14 |
| Male sex | 0.64 (0.36–1.13) | 0.13 |
| Diabetes | 1.15 (0.64–2.05) | 0.65 |
| CAD | 1.76 (0.90–3.45) | 0.10 |
| GFR (per CKD stage) | 0.95 (0.92–0.98) | 0.001 |
| Hb (per 1 g/dl) | 0.96 (0.81–1.14) | 0.65 |
| Albumin (per 0.1 g/dl) | 0.63 (0.34–1.18) | 0.15 |
| Factor . | OR (95% CI) . | P-value . |
|---|---|---|
| Age (per 10 years) | 0.93 (0.77–1.13) | 0.45 |
| White race | 1.73 (0.84–3.59) | 0.14 |
| Male sex | 0.64 (0.36–1.13) | 0.13 |
| Diabetes | 1.15 (0.64–2.05) | 0.65 |
| CAD | 1.76 (0.90–3.45) | 0.10 |
| GFR (per CKD stage) | 0.95 (0.92–0.98) | 0.001 |
| Hb (per 1 g/dl) | 0.96 (0.81–1.14) | 0.65 |
| Albumin (per 0.1 g/dl) | 0.63 (0.34–1.18) | 0.15 |
CAD = coronary artery disease; CI = confidence interval; GFR = glomerular filtration rate; Hb = haemoglobin; OR = odds ratio.
Predictors of iron therapy
| Factor . | OR (95% CI) . | P-value . |
|---|---|---|
| Age (per 10 years) | 0.91 (0.74–1.13) | 0.40 |
| White race | 2.63 (1.08–6.42) | 0.03 |
| Male sex | 0.78 (0.42–1.48) | 0.45 |
| Diabetes | 2.11 (1.12–3.96) | 0.02 |
| CAD | 0.96 (0.46–2.02) | 0.92 |
| GFR (per CKD stage) | 0.97 (0.94–1.01) | 0.11 |
| Hb (per 1 g/dl) | 0.86 (0.70–1.06) | 0.15 |
| Albumin (per 0.1 g/dl) | 0.60 (0.31–1.17) | 0.13 |
| Factor . | OR (95% CI) . | P-value . |
|---|---|---|
| Age (per 10 years) | 0.91 (0.74–1.13) | 0.40 |
| White race | 2.63 (1.08–6.42) | 0.03 |
| Male sex | 0.78 (0.42–1.48) | 0.45 |
| Diabetes | 2.11 (1.12–3.96) | 0.02 |
| CAD | 0.96 (0.46–2.02) | 0.92 |
| GFR (per CKD stage) | 0.97 (0.94–1.01) | 0.11 |
| Hb (per 1 g/dl) | 0.86 (0.70–1.06) | 0.15 |
| Albumin (per 0.1 g/dl) | 0.60 (0.31–1.17) | 0.13 |
CAD = coronary artery disease; CI = confidence interval; GFR = glomerular filtration rate; Hb = haemoglobin; OR = odds ratio.
Predictors of iron therapy
| Factor . | OR (95% CI) . | P-value . |
|---|---|---|
| Age (per 10 years) | 0.91 (0.74–1.13) | 0.40 |
| White race | 2.63 (1.08–6.42) | 0.03 |
| Male sex | 0.78 (0.42–1.48) | 0.45 |
| Diabetes | 2.11 (1.12–3.96) | 0.02 |
| CAD | 0.96 (0.46–2.02) | 0.92 |
| GFR (per CKD stage) | 0.97 (0.94–1.01) | 0.11 |
| Hb (per 1 g/dl) | 0.86 (0.70–1.06) | 0.15 |
| Albumin (per 0.1 g/dl) | 0.60 (0.31–1.17) | 0.13 |
| Factor . | OR (95% CI) . | P-value . |
|---|---|---|
| Age (per 10 years) | 0.91 (0.74–1.13) | 0.40 |
| White race | 2.63 (1.08–6.42) | 0.03 |
| Male sex | 0.78 (0.42–1.48) | 0.45 |
| Diabetes | 2.11 (1.12–3.96) | 0.02 |
| CAD | 0.96 (0.46–2.02) | 0.92 |
| GFR (per CKD stage) | 0.97 (0.94–1.01) | 0.11 |
| Hb (per 1 g/dl) | 0.86 (0.70–1.06) | 0.15 |
| Albumin (per 0.1 g/dl) | 0.60 (0.31–1.17) | 0.13 |
CAD = coronary artery disease; CI = confidence interval; GFR = glomerular filtration rate; Hb = haemoglobin; OR = odds ratio.
Discussion
Despite the availability of considerable data that describe the prescription patterns of medications in patients with ESRD, particularly those treated with haemodialysis [1,2,9,10], there are very few data that describe the characteristics in pre-dialysis patients. This information is an important tool for use in epidemiological studies, for comparative purposes between treatment modalities, and as a means of examining outcomes. This information was collected for a pre-dialysis cohort in the current study comprised of a large number of patients from several geographically distinct areas within the USA, and thus may be viewed as being reasonably representative of prescription patterns in the out-patient nephrology clinic setting within the USA.
This study identified several interesting phenomena regarding the prescription of medications in a CKD cohort. Epoetin and iron appear to be under-prescribed in anaemic patients, with only 45 and 20% receiving these agents at baseline. This finding has been noted in dialysis patients, and national initiatives have been implemented using continual quality assessment techniques in an effort to improve the situation [11]. This process has been largely successful in terms of increasing average haemoglobin and iron status values [12]. The current study suggests that there is also room for significant improvement in terms of anaemia management. The underuse of these agents was also compounded by a low degree of monitoring of iron indices. For example, serum ferritin and transferrin saturation values were available for only 9 and 1.5% of anaemic patients, respectively. Despite these findings, the mean haemoglobin at baseline was 11.8 g/dl, albeit with a large SD, which suggests that a substantial proportion of patients had values below the lower end of the target range, i.e. 11 g/dl.
Higher GFR values were associated with a lower probability of being treated with epoetin or parenteral iron, and patients not receiving these agents had higher haemoglobin values. This phenomenon probably represents confounding by indication, demonstrating that more epoetin and iron are given to patients with worse anaemia, rather than that their use is associated with poorer haemoglobin outcomes. Other interesting findings indicate that women, white and diabetic patients were more likely to be prescribed these agents. The reasons for those findings are unclear, since it suggests that those groups have more severe or difficult to treat anaemia in CKD.
Only 24% of patients with coronary artery disease were prescribed HMG-CoA reductase inhibitors. That statins have a profound influence on the outcomes of non-CKD patients with dyslipidaemias is clear, and has led to guidelines for detection and treatment [13]. Extrapolation of these data has resulted in the recent adoption of K/DOQI clinical practice guidelines for the management of dyslipidaemias in the CKD population, with the inference that aggressive control of serum lipid aberrations is also appropriate for these patients [14]. A recent analysis of data from haemodialysis patients from many countries has also shown that the prescription of statins, even in situations where there are clear indications for use, is suboptimal [10]. For example, statins were prescribed for <28% of patients with a total cholesterol >200 mg/dl, or with a history of myocardial infarction or coronary artery disease. The K/DOQI clinical practice guidelines were published after data collection for the RRI-CKD study was complete, however, suggesting that there was less awareness of this need at the time. Presumably, there is a need for significant education to ensure that more patients receive this treatment.
While 58 and 23% of diabetic patients with proteinuria had prescriptions for ACE inhibitors and angiotensin receptor blockers, respectively, overall only 44 and 13% were prescribed these medications. Both ACE inhibitors and angiotensin receptor blockers may slow the rate of progression of kidney disease [15], and both have been classified as preferred agents within the recent guidelines for use of antihypertensive agents, even in patients without hypertension, to slow the progression of CKD. One implication of these findings is that a greater proportion of the CKD stage 2–4 population should be taking these classes of drugs in both proteinuric and non-proteinuric nephropathies [16]. Other antihypertensive agents were not substituted for ACE inhibitors and/or angiotensin receptor blockers in this cadre, since the use of β-blockers and calcium channel blockers was low overall, and in those patients with proteinuria with or without diabetes.
Other recent studies have demonstrated a very low prescription rate of targeted medications within specific kidney disease populations. In one of these, a retrospective review of 602 patients in five out-patient nephrology clinics was conducted to determine the appropriateness of CKD management [4]. The mean GFR of those patients was 22 ml/min/1.73 m2, with a mean age of 63 years, which is very similar to that in our study (60 years). They also noted suboptimal treatment of their patients, with iron indices obtained for only 18% (≤14% in our study), parathyroid hormone (PTH) values for 15% and lipid studies in less than half. Medication management was also less than desirable, with only 59% of patients with a haematocrit of 30% having received epoetin. Further, ACE inhibitors were used in only 65% of diabetic patients. These data are very similar to some of those collected in our study. In Tonelli's study, 304 CKD patients (mean age 61 years, GFR = 30 ml/min) in Canada were followed to determine the prevalence of coronary risk factors and the extent of use of cardioprotective medications [5]. They noted that 34% were on β-blockers, 27% on aspirin and 65% on ACE inhibitors or angiotensin receptor blockers. Only 18% were on statins. The authors again concluded that there should be increased use of these medications within this patient population.
A number of limitations of this observational study must be considered. There is a potential discrepancy between the prescription of drugs compared with the actual consumption of them by patients. The phenomenon of adherence is more profound for some types of medication than others. For example, it is likely that there is a lower adherence rate to those types of oral medications that need to be taken long term, such as antihypertensives and those for treating dyslipidaemias, compared with agents administered by injection by a health care provider in a clinic setting, such as epoetin and intravenous iron [17]. Secondly, the exact appropriateness of medication prescription is difficult to assess. Thus, in a patient who is hypertensive and who is prescribed a blood pressure-lowering agent, the prescription may remain inappropriate if the correct dose is not prescribed and particularly if the patient does not achieve target blood pressure control. An additional shortcoming of the study is the point prevalence nature of the medication-related data. It cannot be assumed that the prescription characteristics of a particular medication for a given patient remain unchanged over the course of follow-up. Of interest, too, is the similarity of ages in patients in different stages of CKD: none of the mean ages in each stage was different from the collective mean of 60.6 years. Data from NHANES show that, in the general population, GFR decreases with advancing age [18]. However, since patients in the RRI-CKD cadre had all been referred to nephrologists for care, those with lower GFR values would tend to be over-represented in the study.
With the recent realization of the very large potential number of people in the US population with early CKD or at risk for its development, emphasis is required at two levels. One of these is to increase the detection of such individuals as early as possible, perhaps using screening programmes such as KEEP [19]. A second approach would be to optimize the care that CKD patients receive when they have been diagnosed. Recent emphasis has called for early referral of CKD patients to nephrologists, since this approach has been demonstrated to improve patient outcomes and result in earlier preparation for an initiation of dialysis [20]. This study identifies a further component of the overall care plan, namely to improve the use of prescription medications that are known to impact co-morbidities and outcomes of complications of CKD based on currently available knowledge about use of medications in this group of patients. The recent Medicare Modernization Act in the USA will require that prescription drug plans for stage 5 CKD patients have a continuous quality improvement programme, to ensure appropriate use of prescription medications. The current study suggests that there is a need for increased awareness among primary care physicians and nephrologists alike of the importance of early detection and treatment of complications associated with CKD along with well known renoprotective strategies.
We are grateful to the study coordinators at each participating site for their diligent data collection: University of Michigan, Kerri Briesmiester, AAS, BBA, Laura Davidson, BA, Christine Kehrer, RDMS, RVP, CCRP; University of North Carolina at Chapel Hill, Catherine G. Lambeth, RN, Melissa Caughey, BS, RVT, RDCS; Albany Medical Center, Diane Delmonico RN, BSN; Metabolism Associates (New Haven, CT), Christine Turcio. This project was funded by the Renal Research Institute, LLC, New York, NY, and Amgen, Inc., Thousand Oaks, CA. Data from this paper were presented in part as an abstract at the National Kidney Foundation's 2003 Clinical Meeting, Dallas, TX.
Conflict of interest statement. None declared.
References
United States Renal Data System 1998 Annual Data Report. Medication use among dialysis patients in the DMMS.
Manley HJ, McClaran ML, Overbay DK et al. Factors associated with medication-related problems in ambulatory hemodialysis patients.
Abramson JL, Jurkovitz CT, Vaccarino V, Weintraub WS, McClellan W. Chronic kidney disease, anemia, and incident stroke in a middle-aged, community-based population: the ARIC study.
Kausz AT, Khan SS, Abichandani R et al. Management of patients with chronic renal insufficiency in the northeastern United States.
Tonelli M, Bohm C, Pandeya S et al. Cardiac risk factors and the use of cardioprotective medications in patients with chronic renal insufficiency.
Levey AS, Bosch JP, Lewis JB et al. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group.
Perlman RL, Kiser M, Finkelstein F et al. The Longitudinal Chronic Kidney Disease Study: a prospective cohort study of predialysis renal failure.
Young EW, Goodkin DA, Mapes DL et al. The Dialysis Outcomes and Practice Patterns Study (DOPPS): an international hemodialysis study.
Bailie GR, Mason NA, Bragg-Gresham JL, Gillespie BW, Young EW. Analgesic prescription patterns among hemodialysis patients in the DOPPS: potential for underprescription.
Mason NA, Bailie GR, Satayathum S et al. HMG-coenzyme A reductase inhibitor use is associated with mortality reduction in hemodialysis patients.
National Kidney Foundation.
Expert Panel on Detection, Evaluation and Treatment of High Blood Cholesterol in Adults. Executive Summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III).
National Kidney Foundation.
Parving HH, Hovind P. Microalbuminuria in type 1 and type 2 diabetes mellitus: evidence with angiotensin converting enzyme inhibitors and angiotensin II receptor blockers for treating early and preventing clinical nephropathy.
National Kidney Foundation.
Vik SA, Maxwell CJ, Hogan DB. Measurement, correlates, and health outcomes of medication adherence among seniors.
Coresh J, Astor BC, Greene T et al. Prevalence of chronic kidney disease and decreased kidney function in the adult US population: third National Health and Nutrition Examination Survey.
Kidney Early Evaluation Program Annual Data Report. National Kidney Foundation.
Author notes
1Albany Nephrology Pharmacy (ANephRx) Group, Albany College of Pharmacy, 2Albany College of Medicine, Albany, NY, 3Kidney Epidemiology and Cost Center, 6University Renal Research and Education Association and 8Division of Nephrology, University of Michigan, Ann Arbor, MI, 4University of North Carolina-Chapel Hill, NC, 5Metabolism Associates, New Haven, CT and 7Nephrology Management Group, Sunnyvale, CA, USA
- anemia
- angiotensin-converting enzyme inhibitors
- antihypertensive agents
- aspirin
- proteinuria
- calcium channel blockers
- coronary arteriosclerosis
- iron
- statins
- kidney diseases
- diabetes mellitus
- antilipemic agents
- angiotensin receptor antagonists
- kidney failure, chronic
- diabetes mellitus, type 2
- erythropoietin
- glomerular filtration rate
- hemoglobin
- cardiovascular system
- demography
- nephrology
- prescriptions, drug
- morbidity
- serum albumin
- lipid-lowering therapy
- drug usage





Comments