-
PDF
- Split View
-
Views
-
Cite
Cite
Pieter Evenepoel, Kathleen Claes, Dirk Kuypers, Bart Maes, Yves Vanrenterghem, Impact of parathyroidectomy on renal graft function, blood pressure and serum lipids in kidney transplant recipients: a single centre study, Nephrology Dialysis Transplantation, Volume 20, Issue 8, August 2005, Pages 1714–1720, https://doi.org/10.1093/ndt/gfh892
Close - Share Icon Share
Abstract
Background. Successful kidney transplantation is believed to reverse secondary hyperparathyroidism, but persistent disease has emerged in a significant number of allograft recipients. Parathyroid hormone (PTH) is not only involved in the aetiology of calcium/phosphate abnormalities and osteitis fibrosa, but it is also a permissive factor in the occurrence of hypertension, cardiovascular damage and dyslipidaemia. In experimental renal failure, abrogation of hyperparathyroidism by administration of a calcimimetic or parathyroidectomy (PTX) attenuates progression of renal failure. To evaluate the impact of PTX on blood pressure (BP), renal graft function and serum lipids, we performed a retrospective case-controlled study in renal graft recipients.
Methods. Charts of 1647 kidney allograft recipients, transplanted between 1989 and 2004, were reviewed. Thirty-two patients with a functioning graft and a history of a successful PTX performed at least 9 months after transplantation were identified. Biochemical and clinical data available 6 months pre- and post-PTX were registered. Changes in BP, renal function and serum lipids were assessed. The data were compared with those obtained in a similar time frame in a control group closely matched for date of transplantation.
Results. Systolic BP (149.9 vs 141.7 mmHg), diastolic BP (85.6 vs 81.9 mmHg), pulse pressure (64.3 vs 58.8 mmHg), total cholesterol concentration (221.4 vs 211.1 mg/dl) and low-density lipoprotein cholesterol concentration (123.9 vs 106.7 mg/dl) improved significantly after successful PTX. Serum creatinine, conversely, significantly increased after PTX (1.75 vs 2.13 mg/dl, P<0.0001). No significant changes were observed in the control group in the same time period.
Conclusion. In patients with a functioning renal graft, BP and dyslipidaemia improve, whereas serum creatinine worsens following successful PTX. Our data are in agreement with a stimulatory effect of PTH on plasma renin activity and an inhibitory effect on lipase activity, as previously demonstrated by others. To what extent the increased serum creatinine following PTX reflects a true deterioration of the glomerular filtration rate and/or is the consequence of vitamin D-induced reduction of the renal tubular secretion of creatinine needs to be elucidated by further research.
Introduction
Secondary hyperparathyroidism (HPT) is an almost universal complication in patients with chronic renal failure. Although present knowledge is incomplete, several mechanisms have been recognized by which hyperparathyroidism is initiated and maintained in renal failure [1]. Obviously, abnormal calcium, phosphorus and vitamin D metabolism contributes to the disruption of the endocrine parathyroid hormone (PTH)–vitamin D axis in chronic renal failure [2].
Elevated concentrations of PTH play a role not only in the pathogenesis of renal bone disease, but also in the development of cardiovascular risk factors such as disturbed lipid metabolism, glucose intolerance and hypertension. Abrogation of HPT by administration of a calcimimetic or parathyroidectomy (PTX) attenuates progression of renal failure in subtotally nephrectomized rats [3].
Successful kidney transplantation corrects the endocrine and metabolic imbalances and the main abnormalities responsible for secondary HPT in the first months [2]. Nevertheless these early favourable events are not always followed by a rapid normalization of PTH secretion. Elevated PTH levels are observed in up to 25% of patients 1 year after transplantation despite adequate renal function [4]. A subgroup of these patients ultimately will require PTX.
Data on the impact of PTX on renal function, blood pressure (BP) and serum lipid concentrations are limited or non-existent in humans, especially in patients with persistent HPT after successful renal transplantation. The aim of the present observational cohort study was to elucidate this topic.
Subjects and methods
Subjects
Records from all patients who had received a kidney transplant at the University Hospital of Leuven between January 1, 1989 and January 1, 2004 were reviewed. The starting date of inclusion was deliberately set at January 1, 1989 because from then on all intact PTH (iPTH) levels in our hospital were uniformly determined by means of an immunoradiometric assay (IRMA), developed by Bouillon et al. [5]. In contrast to most other commercially available IRMAs for iPTH, this assay detects full-length human PTH but not N-terminal truncated fragments, and thereby resembles recently introduced third-generation PTH IRMAs. This also explains its lower normal range of 3–40 ng/l. Eighty-eight patients with a functioning graft who required a PTX for persistent hypercalcaemic HPT were identified. The following subjects were excluded from the final analysis: (i) patients in whom the PTX was unsuccessful, defined as a decrease of PTH <50% (n = 13); (ii) patients with a post-PTX follow-up of <6 months (n = 1); and (iii) patients with missing data (n = 18). Since renal function was arbitrarily considered to be stable by month 3 post-transplantation and considering the duration of the study periods, patients in whom the interval between transplant and PTX was <9 months (n = 24) were also excluded. The remaining patients, 16 males and 16 females, comprised the study group (group A). The causes of renal failure were: diabetes (n = 2), glomerulonephritis/vasculitis (n = 6), interstitial nephritis (n = 4), hypertensive/large-vessel disease (n = 3), cystic/hereditary/congenital (n = 10) and unknown or missing (n = 7). The mean age was 51.2±10.7 years. Twenty-nine patients received haemodialysis, while three patients were on peritoneal dialysis at the time of transplantation. The demographics of the cases did not differ from the demographics of the PTX patients that were excluded. Patients underwent surgery for the removal of adenoma if present, or otherwise subtotal PTX (fifteen-sixteenths). PTX was performed at a mean interval of 29 months (range 9–160). The patients were hospitalized for 7 days and were thereafter followed in the out-patient clinic. After PTX, most patients were given substitution therapy consisting of alfacalcidol or calcitriol and calcium salts, adapted to the serum calcaemia; this was withdrawn when necessary.
For each of the 32 PTX patients, a non-parathyroidectomized (non-PTX) renal transplant recipient matched for date of transplantation and a post-transplant follow-up exceeding the time interval between transplantation and PTX of the corresponding case was selected. The control group (group B) consisted of 17 men and 15 women with a mean age of 49.4±13.3 years. The reasons for renal failure were as follows: diabetes (n = 6), glomerulonephritis/vasculitis (n = 5), interstitial nephritis (n = 3), cystic/hereditary/congenital (n = 6) and unknown or missing (n = 12). Twenty-four patients received haemodialysis, while seven patients were on peritoneal dialysis at the time of transplantation (Table 1).
Baseline demographic and clinical data
| . | Cases . | Controls . | P-value . |
|---|---|---|---|
| Number | 32 | 32 | |
| Dialysis modality (HD/PD/pre-emptive) | 29/3/0 | 24/7/1 | NS |
| Age (years) | 51.2±10.7 | 49.4±13.3 | NS |
| Male/female (n) | 16/16 | 17/15 | NS |
| Renal diagnosis (n) | NS | ||
| Diabetic nephropathy | 2 (6.3%) | 6 (18.8%) | |
| Glomerulonephritis/vasculitis | 6 (18.8%) | 5 (15.6%) | |
| Interstitial nephritis | 4 (12.5%) | 3 (9.4%) | |
| Hypertensive/large vessel disease | 3 (9.4%) | 0 (0.0%) | |
| Cystic/heriditary/congenital diseases | 10 (31.3%) | 6 (18.8%) | |
| Miscellaneous | 2 (6.3%) | 4 (12.5%) | |
| Aetiology unknown or missing | 5 (15.6%) | 8 (25.0%) | |
| Maintenance IS (n) | NS | ||
| Steroids | 30 | 30 | |
| Cyclosporin A | 27 | 25 | |
| Tacrolimus | 6 | 7 | |
| Rapamycin | 0 | 1 | |
| Azathioprin | 7 | 5 |
| . | Cases . | Controls . | P-value . |
|---|---|---|---|
| Number | 32 | 32 | |
| Dialysis modality (HD/PD/pre-emptive) | 29/3/0 | 24/7/1 | NS |
| Age (years) | 51.2±10.7 | 49.4±13.3 | NS |
| Male/female (n) | 16/16 | 17/15 | NS |
| Renal diagnosis (n) | NS | ||
| Diabetic nephropathy | 2 (6.3%) | 6 (18.8%) | |
| Glomerulonephritis/vasculitis | 6 (18.8%) | 5 (15.6%) | |
| Interstitial nephritis | 4 (12.5%) | 3 (9.4%) | |
| Hypertensive/large vessel disease | 3 (9.4%) | 0 (0.0%) | |
| Cystic/heriditary/congenital diseases | 10 (31.3%) | 6 (18.8%) | |
| Miscellaneous | 2 (6.3%) | 4 (12.5%) | |
| Aetiology unknown or missing | 5 (15.6%) | 8 (25.0%) | |
| Maintenance IS (n) | NS | ||
| Steroids | 30 | 30 | |
| Cyclosporin A | 27 | 25 | |
| Tacrolimus | 6 | 7 | |
| Rapamycin | 0 | 1 | |
| Azathioprin | 7 | 5 |
IS = immunosuppression; HD = haemodialysis; PD = peritoneal dialysis; PTX = parathyroidectomy.
Baseline demographic and clinical data
| . | Cases . | Controls . | P-value . |
|---|---|---|---|
| Number | 32 | 32 | |
| Dialysis modality (HD/PD/pre-emptive) | 29/3/0 | 24/7/1 | NS |
| Age (years) | 51.2±10.7 | 49.4±13.3 | NS |
| Male/female (n) | 16/16 | 17/15 | NS |
| Renal diagnosis (n) | NS | ||
| Diabetic nephropathy | 2 (6.3%) | 6 (18.8%) | |
| Glomerulonephritis/vasculitis | 6 (18.8%) | 5 (15.6%) | |
| Interstitial nephritis | 4 (12.5%) | 3 (9.4%) | |
| Hypertensive/large vessel disease | 3 (9.4%) | 0 (0.0%) | |
| Cystic/heriditary/congenital diseases | 10 (31.3%) | 6 (18.8%) | |
| Miscellaneous | 2 (6.3%) | 4 (12.5%) | |
| Aetiology unknown or missing | 5 (15.6%) | 8 (25.0%) | |
| Maintenance IS (n) | NS | ||
| Steroids | 30 | 30 | |
| Cyclosporin A | 27 | 25 | |
| Tacrolimus | 6 | 7 | |
| Rapamycin | 0 | 1 | |
| Azathioprin | 7 | 5 |
| . | Cases . | Controls . | P-value . |
|---|---|---|---|
| Number | 32 | 32 | |
| Dialysis modality (HD/PD/pre-emptive) | 29/3/0 | 24/7/1 | NS |
| Age (years) | 51.2±10.7 | 49.4±13.3 | NS |
| Male/female (n) | 16/16 | 17/15 | NS |
| Renal diagnosis (n) | NS | ||
| Diabetic nephropathy | 2 (6.3%) | 6 (18.8%) | |
| Glomerulonephritis/vasculitis | 6 (18.8%) | 5 (15.6%) | |
| Interstitial nephritis | 4 (12.5%) | 3 (9.4%) | |
| Hypertensive/large vessel disease | 3 (9.4%) | 0 (0.0%) | |
| Cystic/heriditary/congenital diseases | 10 (31.3%) | 6 (18.8%) | |
| Miscellaneous | 2 (6.3%) | 4 (12.5%) | |
| Aetiology unknown or missing | 5 (15.6%) | 8 (25.0%) | |
| Maintenance IS (n) | NS | ||
| Steroids | 30 | 30 | |
| Cyclosporin A | 27 | 25 | |
| Tacrolimus | 6 | 7 | |
| Rapamycin | 0 | 1 | |
| Azathioprin | 7 | 5 |
IS = immunosuppression; HD = haemodialysis; PD = peritoneal dialysis; PTX = parathyroidectomy.
Immunosuppressive therapy in both cases and controls consisted of steroids, a calcineurin inhibitor and/or an anti-metabolite (Table 1).
The charts from both case and control patients were reviewed in detail. Information abstracted included drug regimen, clinical and laboratory data. Two study periods were arbitrarily defined: period 1 included the 6 months prior to PTX; period 2 comprised the 6 months after PTX. Controls were studied in the corresponding time span. The biochemical and clinical parameters extracted from the charts were averaged per study period.
Blood pressure
BP was measured on the arm without an arteriovenous fistula, with a mercury sphygmomanometer. Diastolic BP (DBP) was defined as the disappearance of the phase V Korotkoff sound; mean BP (MBP) was calculated as DBP + one-third of the difference between systolic BP (SBP) and DBP. Hypertension was defined as an SBP >160 mmHg and/or DBP >90 mmHg and/or the need for antihypertensive treatment.
Biochemistry
Total serum calcium, phosphorus, creatinine, total cholesterol, low-density lipoprotein (LDL) cholesterol, high-density lipoprotein (HDL) cholesterol, triglycerides and total alkaline phosphatases were measured using a computerized autoanalyser. Serum concentrations of iPTH were determined by an IRMA, as described elsewhere [5]. Creatinine clearance was calculated from a 24 h urine collection and according to the Cockcroft–Gault equation. Compliance with the salt-restricted diet was evaluated by measuring the 24 h urinary salt excretion.
Statistics and calculations
Results
Renal function
Renal function deteriorated in 21 patients (65.6%) of group A following PTX. In 11 patients, renal function was stable. The mean serum creatinine increased from 1.75±0.51 to 2.13±0.65 mg/dl (P<0.0001). The creatinine clearance decreased from 46.8±16.1 to 41.0±17.1 ml/min (P = 0.001). A similar increase of serum creatinine (1.61±0.45 vs 1.93±0.90 mg/dl) was observed in a subgroup of patients receiving no active vitamin D supplements after PTX (n = 5). In group B, renal function was stable in 26 out of the 32 patients. A deterioration was only observed in four patients (12.5%). The mean serum creatinine levels in group B was almost identical during both study periods (1.76±0.45 vs 1.74±0.47 mg/dl; period 1 vs period 2, P = NS). There were no significant changes in serum urea nitrogen and 24 h proteinuria in both cases and controls.
Blood pressure
In group A, there was a limited, though highly significant decrease of SBP, DBP and MBP after PTX (SBP 149.9±19.3 vs 141.7±13.5 mmHg; DBP 85.6±9.9 vs 81.9±7.2 mmHg; MBP 107.0±12.2 vs 101.8±8.4 mmHg; period 1 vs period 2, P<0.005 for all). The pulse pressure also improved significantly following PTX (64.3±13.7 vs 58.8±10.6 mmHg, P<0.01). Twenty-nine (91%) patients were on antihypertensive drugs before the PTX. In none of them could antihypertensive therapy be discontinued after PTX. The number of antihypertensive drugs per patient decreased from 2.2 to 2.0 (NS).
In group B, no significant changes in blood pressure were observed (SBP 141.3±17.7 vs 142.9±16.1; DBP 81.5±10.0 vs 83.6±10.0; MBP 101.4±10.8 vs 103.4± 10.3; period 1 vs period 2, P = NS). Twenty-four (75%) patients of the control group were on antihypertensive treatment. The number of antihypertensive drugs per patient amounted to 1.5 and 1.6 during period 1 and 2, respectively.
The overall compliance with the salt-restricted diet was good. No significant differences in 24 h urinary salt excretion between either study periods or groups were found.
BP, number of patients on antihypertensive therapy and mean number of antihypertensive drugs per patient were higher, although not significantly, in group A compared with group B.
Mineral metabolism
A significant decrease of the serum level of PTH, calcium and alkaline phosphatases (P<0.05, all) was observed after PTX. Serum phosphorus level, conversely, increased significantly (P<0.0001) (Table 2). The percentage of patients treated with calcium salts increased from 18.8 to 96.9 after PTX. The percentage of patients on active vitamin D treatment (calcitriol or 1-α-OHD3) increased from 9.4 to 84.4 after PTX (P<0.001) (Table 3). The mean daily dose of active vitamin D after PTX was 0.84±0.77 µg. In group B, no changes were observed in any of these parameters between the two study periods (Tables 2 and 3).
Laboratory data
| . | Cases . | . | Controls . | . | ||||
|---|---|---|---|---|---|---|---|---|
| . | Period 1 . | Period 2 . | Period 1 . | Period 2 . | ||||
| Renal function | ||||||||
| Creatinine (mg/dl) | 1.75±0.51 | 2.13±0.65a | 1.76±0.45 | 1.74±0.47b | ||||
| Creatinine Cl (ml/min) | 46.8±16.1 | 41.0±17.1a | 55.1±20.0 | 52.6±20.6 | ||||
| Urea nitrogen (mg/dl) | 50.4±24.0 | 51.0±18.9 | 58.7±24.0 | 61.7±26.3 | ||||
| 24 h urinary protein (g) | 0.24±0.51 | 0.31±0.72 | 0.24±0.27 | 0.35±0.47 | ||||
| 24 h urinary NaCl (g) | 8.0± 2.9 | 7.7±4.3 | 8.0±3.5 | 7.7±6.2 | ||||
| Blood pressure | ||||||||
| SBP (mmHg) | 149.9±19.3 | 141.7±13.5c | 144.4±21.0 | 142.9±16.2 | ||||
| DBP (mmHg) | 85.6±9.9 | 81.9±7.2c | 82.4±11.0 | 83.6±10.0 | ||||
| MBP (mmHg) | 92.9±15.1 | 87.1±11.1d | 89.5±5.0 | 87.2±14.5 | ||||
| PP (mmHg) | 64.3±13.7 | 58.8±10.6d | 62.1±18.0 | 59.4±14.8 | ||||
| Serum lipids | ||||||||
| Total cholesterol (mg/dl) | 221.4±44.1 | 211.1±50.4e | 212.4±44.1 | 215.7±32.1 | ||||
| LDL cholesterol (mg/dl) | 125.1±33.5 | 111.6±41.1c | 121.0±34.9 | 127.9±26.3 | ||||
| HDL cholesterol (mg/dl) | 58.9 ± 25.0 | 60.0±28.9 | 59.3±18.7 | 58.5±15.0 | ||||
| Triglycerides (mg/dl) | 174.1±70.3 | 183.1±93.7 | 143.1±56.9 | 155.7±73.1 | ||||
| TC/HDL ratio | 4.2±1.4 | 4.0±1.4 | 3.8±1.2 | 4.0±1.1 | ||||
| PTH, calcium metabolism | ||||||||
| iPTH (ng/l), median (IQR) | 106.4 (72.9–181.0) | 8.5 (1.8–15.5)a | 37.0 (17.8–54.1)f | 51.6 (34.1–70.6)f | ||||
| Calcium (mg/dl) | 10.7±0.6 | 9.2±0.6a | 9.9±0.6g | 10.0±0.6g | ||||
| Phosphorus (mg/dl) | 2.6±0.5 | 3.6±0.7a | 3.2±0.6h | 3.3±0.6f | ||||
| Alkaline phosphatases (U/l) | 250.3±183.3 | 187.9±119.4e | 163.3±103.1 | 162.4±72.0 | ||||
| Immunosuppression | ||||||||
| Cyclosporin A (µg/l) | 165.9±55.8 | 146±36.8 | 155.7±47.6 | 151.2±57.8 | ||||
| Tacrolimus (µg/l) | 13.6±7.3 | 8.5±3.9 | 9.0±3.5 | 9.0±1.7 | ||||
| . | Cases . | . | Controls . | . | ||||
|---|---|---|---|---|---|---|---|---|
| . | Period 1 . | Period 2 . | Period 1 . | Period 2 . | ||||
| Renal function | ||||||||
| Creatinine (mg/dl) | 1.75±0.51 | 2.13±0.65a | 1.76±0.45 | 1.74±0.47b | ||||
| Creatinine Cl (ml/min) | 46.8±16.1 | 41.0±17.1a | 55.1±20.0 | 52.6±20.6 | ||||
| Urea nitrogen (mg/dl) | 50.4±24.0 | 51.0±18.9 | 58.7±24.0 | 61.7±26.3 | ||||
| 24 h urinary protein (g) | 0.24±0.51 | 0.31±0.72 | 0.24±0.27 | 0.35±0.47 | ||||
| 24 h urinary NaCl (g) | 8.0± 2.9 | 7.7±4.3 | 8.0±3.5 | 7.7±6.2 | ||||
| Blood pressure | ||||||||
| SBP (mmHg) | 149.9±19.3 | 141.7±13.5c | 144.4±21.0 | 142.9±16.2 | ||||
| DBP (mmHg) | 85.6±9.9 | 81.9±7.2c | 82.4±11.0 | 83.6±10.0 | ||||
| MBP (mmHg) | 92.9±15.1 | 87.1±11.1d | 89.5±5.0 | 87.2±14.5 | ||||
| PP (mmHg) | 64.3±13.7 | 58.8±10.6d | 62.1±18.0 | 59.4±14.8 | ||||
| Serum lipids | ||||||||
| Total cholesterol (mg/dl) | 221.4±44.1 | 211.1±50.4e | 212.4±44.1 | 215.7±32.1 | ||||
| LDL cholesterol (mg/dl) | 125.1±33.5 | 111.6±41.1c | 121.0±34.9 | 127.9±26.3 | ||||
| HDL cholesterol (mg/dl) | 58.9 ± 25.0 | 60.0±28.9 | 59.3±18.7 | 58.5±15.0 | ||||
| Triglycerides (mg/dl) | 174.1±70.3 | 183.1±93.7 | 143.1±56.9 | 155.7±73.1 | ||||
| TC/HDL ratio | 4.2±1.4 | 4.0±1.4 | 3.8±1.2 | 4.0±1.1 | ||||
| PTH, calcium metabolism | ||||||||
| iPTH (ng/l), median (IQR) | 106.4 (72.9–181.0) | 8.5 (1.8–15.5)a | 37.0 (17.8–54.1)f | 51.6 (34.1–70.6)f | ||||
| Calcium (mg/dl) | 10.7±0.6 | 9.2±0.6a | 9.9±0.6g | 10.0±0.6g | ||||
| Phosphorus (mg/dl) | 2.6±0.5 | 3.6±0.7a | 3.2±0.6h | 3.3±0.6f | ||||
| Alkaline phosphatases (U/l) | 250.3±183.3 | 187.9±119.4e | 163.3±103.1 | 162.4±72.0 | ||||
| Immunosuppression | ||||||||
| Cyclosporin A (µg/l) | 165.9±55.8 | 146±36.8 | 155.7±47.6 | 151.2±57.8 | ||||
| Tacrolimus (µg/l) | 13.6±7.3 | 8.5±3.9 | 9.0±3.5 | 9.0±1.7 | ||||
To convert serum calcium in mg/dl to mmol/l multiply by 0.2495; to convert serum phosphorus in mg/dl to mmol/l multiply by 0.3229; to convert serum creatinine in mg/dl to µmol/l multiply by 88.4; to convert serum cholesterol in mg/dl to mmol/l multiply by 0.02586; to convert serum triglycerides in mg/dl to mmol/l multiply by 0.01129.
aP<0.0001, dP<0.001, cP<0.01, eP<0.05 period 1 vs period 2.
gP<0.0001, hP<0.001, bP<0.01, fP<0.05 cases vs controls.
iPTH = intact parathyroid hormone; BP = blood pressure; S = systolic; D = diastolic; M = mean; PP = pulse pressure; TC = total cholesterol; Cl = clearance.
Laboratory data
| . | Cases . | . | Controls . | . | ||||
|---|---|---|---|---|---|---|---|---|
| . | Period 1 . | Period 2 . | Period 1 . | Period 2 . | ||||
| Renal function | ||||||||
| Creatinine (mg/dl) | 1.75±0.51 | 2.13±0.65a | 1.76±0.45 | 1.74±0.47b | ||||
| Creatinine Cl (ml/min) | 46.8±16.1 | 41.0±17.1a | 55.1±20.0 | 52.6±20.6 | ||||
| Urea nitrogen (mg/dl) | 50.4±24.0 | 51.0±18.9 | 58.7±24.0 | 61.7±26.3 | ||||
| 24 h urinary protein (g) | 0.24±0.51 | 0.31±0.72 | 0.24±0.27 | 0.35±0.47 | ||||
| 24 h urinary NaCl (g) | 8.0± 2.9 | 7.7±4.3 | 8.0±3.5 | 7.7±6.2 | ||||
| Blood pressure | ||||||||
| SBP (mmHg) | 149.9±19.3 | 141.7±13.5c | 144.4±21.0 | 142.9±16.2 | ||||
| DBP (mmHg) | 85.6±9.9 | 81.9±7.2c | 82.4±11.0 | 83.6±10.0 | ||||
| MBP (mmHg) | 92.9±15.1 | 87.1±11.1d | 89.5±5.0 | 87.2±14.5 | ||||
| PP (mmHg) | 64.3±13.7 | 58.8±10.6d | 62.1±18.0 | 59.4±14.8 | ||||
| Serum lipids | ||||||||
| Total cholesterol (mg/dl) | 221.4±44.1 | 211.1±50.4e | 212.4±44.1 | 215.7±32.1 | ||||
| LDL cholesterol (mg/dl) | 125.1±33.5 | 111.6±41.1c | 121.0±34.9 | 127.9±26.3 | ||||
| HDL cholesterol (mg/dl) | 58.9 ± 25.0 | 60.0±28.9 | 59.3±18.7 | 58.5±15.0 | ||||
| Triglycerides (mg/dl) | 174.1±70.3 | 183.1±93.7 | 143.1±56.9 | 155.7±73.1 | ||||
| TC/HDL ratio | 4.2±1.4 | 4.0±1.4 | 3.8±1.2 | 4.0±1.1 | ||||
| PTH, calcium metabolism | ||||||||
| iPTH (ng/l), median (IQR) | 106.4 (72.9–181.0) | 8.5 (1.8–15.5)a | 37.0 (17.8–54.1)f | 51.6 (34.1–70.6)f | ||||
| Calcium (mg/dl) | 10.7±0.6 | 9.2±0.6a | 9.9±0.6g | 10.0±0.6g | ||||
| Phosphorus (mg/dl) | 2.6±0.5 | 3.6±0.7a | 3.2±0.6h | 3.3±0.6f | ||||
| Alkaline phosphatases (U/l) | 250.3±183.3 | 187.9±119.4e | 163.3±103.1 | 162.4±72.0 | ||||
| Immunosuppression | ||||||||
| Cyclosporin A (µg/l) | 165.9±55.8 | 146±36.8 | 155.7±47.6 | 151.2±57.8 | ||||
| Tacrolimus (µg/l) | 13.6±7.3 | 8.5±3.9 | 9.0±3.5 | 9.0±1.7 | ||||
| . | Cases . | . | Controls . | . | ||||
|---|---|---|---|---|---|---|---|---|
| . | Period 1 . | Period 2 . | Period 1 . | Period 2 . | ||||
| Renal function | ||||||||
| Creatinine (mg/dl) | 1.75±0.51 | 2.13±0.65a | 1.76±0.45 | 1.74±0.47b | ||||
| Creatinine Cl (ml/min) | 46.8±16.1 | 41.0±17.1a | 55.1±20.0 | 52.6±20.6 | ||||
| Urea nitrogen (mg/dl) | 50.4±24.0 | 51.0±18.9 | 58.7±24.0 | 61.7±26.3 | ||||
| 24 h urinary protein (g) | 0.24±0.51 | 0.31±0.72 | 0.24±0.27 | 0.35±0.47 | ||||
| 24 h urinary NaCl (g) | 8.0± 2.9 | 7.7±4.3 | 8.0±3.5 | 7.7±6.2 | ||||
| Blood pressure | ||||||||
| SBP (mmHg) | 149.9±19.3 | 141.7±13.5c | 144.4±21.0 | 142.9±16.2 | ||||
| DBP (mmHg) | 85.6±9.9 | 81.9±7.2c | 82.4±11.0 | 83.6±10.0 | ||||
| MBP (mmHg) | 92.9±15.1 | 87.1±11.1d | 89.5±5.0 | 87.2±14.5 | ||||
| PP (mmHg) | 64.3±13.7 | 58.8±10.6d | 62.1±18.0 | 59.4±14.8 | ||||
| Serum lipids | ||||||||
| Total cholesterol (mg/dl) | 221.4±44.1 | 211.1±50.4e | 212.4±44.1 | 215.7±32.1 | ||||
| LDL cholesterol (mg/dl) | 125.1±33.5 | 111.6±41.1c | 121.0±34.9 | 127.9±26.3 | ||||
| HDL cholesterol (mg/dl) | 58.9 ± 25.0 | 60.0±28.9 | 59.3±18.7 | 58.5±15.0 | ||||
| Triglycerides (mg/dl) | 174.1±70.3 | 183.1±93.7 | 143.1±56.9 | 155.7±73.1 | ||||
| TC/HDL ratio | 4.2±1.4 | 4.0±1.4 | 3.8±1.2 | 4.0±1.1 | ||||
| PTH, calcium metabolism | ||||||||
| iPTH (ng/l), median (IQR) | 106.4 (72.9–181.0) | 8.5 (1.8–15.5)a | 37.0 (17.8–54.1)f | 51.6 (34.1–70.6)f | ||||
| Calcium (mg/dl) | 10.7±0.6 | 9.2±0.6a | 9.9±0.6g | 10.0±0.6g | ||||
| Phosphorus (mg/dl) | 2.6±0.5 | 3.6±0.7a | 3.2±0.6h | 3.3±0.6f | ||||
| Alkaline phosphatases (U/l) | 250.3±183.3 | 187.9±119.4e | 163.3±103.1 | 162.4±72.0 | ||||
| Immunosuppression | ||||||||
| Cyclosporin A (µg/l) | 165.9±55.8 | 146±36.8 | 155.7±47.6 | 151.2±57.8 | ||||
| Tacrolimus (µg/l) | 13.6±7.3 | 8.5±3.9 | 9.0±3.5 | 9.0±1.7 | ||||
To convert serum calcium in mg/dl to mmol/l multiply by 0.2495; to convert serum phosphorus in mg/dl to mmol/l multiply by 0.3229; to convert serum creatinine in mg/dl to µmol/l multiply by 88.4; to convert serum cholesterol in mg/dl to mmol/l multiply by 0.02586; to convert serum triglycerides in mg/dl to mmol/l multiply by 0.01129.
aP<0.0001, dP<0.001, cP<0.01, eP<0.05 period 1 vs period 2.
gP<0.0001, hP<0.001, bP<0.01, fP<0.05 cases vs controls.
iPTH = intact parathyroid hormone; BP = blood pressure; S = systolic; D = diastolic; M = mean; PP = pulse pressure; TC = total cholesterol; Cl = clearance.
Therapy
| . | Cases (n = 32) . | . | Controls (n = 32) . | . | ||||
|---|---|---|---|---|---|---|---|---|
| . | Period 1 . | Period 2 . | Period 1 . | Period 2 . | ||||
| Antihypertensive (n) | ||||||||
| ACE/ATIIRB | 17 (53.1%) | 16 (50%) | 14 (43.8%) | 16 (50%) | ||||
| Diuretics | 4 (12.5%) | 3 (9.4%) | 3 (9.4%) | 3 (9.4%) | ||||
| CCB | 16 (50%) | 15 (46.9%) | 13 (40.6%) | 13 (40.6%) | ||||
| BB | 21 (65.6%) | 20 (62.5%) | 15 (46.9%) | 15 (46.9%) | ||||
| Other | 11 (34.4%) | 10 (31.3%) | 3 (9.4%) | 3 (9.4%) | ||||
| Antihypertensive therapy | 29 (90.6%) | 29 (90.6%) | 24 (75.0%) | 24 (75.0%) | ||||
| Antihypertensive drugs (per patient) | 2.2±1.1 | 2.0±1.1 | 1.5±1.3 | 1.6±1.4 | ||||
| Lipid lowering (n) | ||||||||
| Fibrates | 2 (6.3%) | 2 (6.3%) | 1 (3.1%) | 1 (3.1%) | ||||
| Statins | 10 (31.3%) | 10 (31.3%) | 5 (15.6%) | 6 (18.8%) | ||||
| Lipid-lowering therapy | 12 (37.5%) | 12 (37.5%) | 6 (18.8%) | 7 (21.9%) | ||||
| Calcium metabolism (n) | ||||||||
| Calcium salts | 6 (18.8%) | 31 (96.9%)a | 7 (21.9%) | 7 (21.9%)b | ||||
| Vitamin D | 8 (25.0%) | 27 (84.4%)a | 8 (25.0%) | 8 (25.0%)b | ||||
| Immunosuppression (daily dose, mg) | ||||||||
| Cyclosporin A | 221.1±100.7 | 222.2±75.9 | 218.4±86.7 | 221.6±64.4 | ||||
| Tacrolimus | 3.3±1.9 | 2.9±1.7 | 7.4±2.8 | 7.0±2.3 | ||||
| Mycophenolate mofetil | 1600±600 | 1700±400 | 1200±700 | 1500±500 | ||||
| Steroids | 5.8±3.2 | 5.6±2.9 | 6.1±3.3 | 5.0±2.8 | ||||
| Rapamycin | – | – | 4.3 | 3.3 | ||||
| Azathioprin | 70.9±23.3 | 69.3±34.4 | 71.8±21.7 | 51.9±32.4 | ||||
| . | Cases (n = 32) . | . | Controls (n = 32) . | . | ||||
|---|---|---|---|---|---|---|---|---|
| . | Period 1 . | Period 2 . | Period 1 . | Period 2 . | ||||
| Antihypertensive (n) | ||||||||
| ACE/ATIIRB | 17 (53.1%) | 16 (50%) | 14 (43.8%) | 16 (50%) | ||||
| Diuretics | 4 (12.5%) | 3 (9.4%) | 3 (9.4%) | 3 (9.4%) | ||||
| CCB | 16 (50%) | 15 (46.9%) | 13 (40.6%) | 13 (40.6%) | ||||
| BB | 21 (65.6%) | 20 (62.5%) | 15 (46.9%) | 15 (46.9%) | ||||
| Other | 11 (34.4%) | 10 (31.3%) | 3 (9.4%) | 3 (9.4%) | ||||
| Antihypertensive therapy | 29 (90.6%) | 29 (90.6%) | 24 (75.0%) | 24 (75.0%) | ||||
| Antihypertensive drugs (per patient) | 2.2±1.1 | 2.0±1.1 | 1.5±1.3 | 1.6±1.4 | ||||
| Lipid lowering (n) | ||||||||
| Fibrates | 2 (6.3%) | 2 (6.3%) | 1 (3.1%) | 1 (3.1%) | ||||
| Statins | 10 (31.3%) | 10 (31.3%) | 5 (15.6%) | 6 (18.8%) | ||||
| Lipid-lowering therapy | 12 (37.5%) | 12 (37.5%) | 6 (18.8%) | 7 (21.9%) | ||||
| Calcium metabolism (n) | ||||||||
| Calcium salts | 6 (18.8%) | 31 (96.9%)a | 7 (21.9%) | 7 (21.9%)b | ||||
| Vitamin D | 8 (25.0%) | 27 (84.4%)a | 8 (25.0%) | 8 (25.0%)b | ||||
| Immunosuppression (daily dose, mg) | ||||||||
| Cyclosporin A | 221.1±100.7 | 222.2±75.9 | 218.4±86.7 | 221.6±64.4 | ||||
| Tacrolimus | 3.3±1.9 | 2.9±1.7 | 7.4±2.8 | 7.0±2.3 | ||||
| Mycophenolate mofetil | 1600±600 | 1700±400 | 1200±700 | 1500±500 | ||||
| Steroids | 5.8±3.2 | 5.6±2.9 | 6.1±3.3 | 5.0±2.8 | ||||
| Rapamycin | – | – | 4.3 | 3.3 | ||||
| Azathioprin | 70.9±23.3 | 69.3±34.4 | 71.8±21.7 | 51.9±32.4 | ||||
aP<0.0001 period 1 vs period 2; bP<0.0001 cases vs controls.
ACEi = angiotensin-converting enzyme inhibitor; ATIIRB = angiotensin II receptor blocker; CCB = calcium channel blocker; BB = β-blocker.
Therapy
| . | Cases (n = 32) . | . | Controls (n = 32) . | . | ||||
|---|---|---|---|---|---|---|---|---|
| . | Period 1 . | Period 2 . | Period 1 . | Period 2 . | ||||
| Antihypertensive (n) | ||||||||
| ACE/ATIIRB | 17 (53.1%) | 16 (50%) | 14 (43.8%) | 16 (50%) | ||||
| Diuretics | 4 (12.5%) | 3 (9.4%) | 3 (9.4%) | 3 (9.4%) | ||||
| CCB | 16 (50%) | 15 (46.9%) | 13 (40.6%) | 13 (40.6%) | ||||
| BB | 21 (65.6%) | 20 (62.5%) | 15 (46.9%) | 15 (46.9%) | ||||
| Other | 11 (34.4%) | 10 (31.3%) | 3 (9.4%) | 3 (9.4%) | ||||
| Antihypertensive therapy | 29 (90.6%) | 29 (90.6%) | 24 (75.0%) | 24 (75.0%) | ||||
| Antihypertensive drugs (per patient) | 2.2±1.1 | 2.0±1.1 | 1.5±1.3 | 1.6±1.4 | ||||
| Lipid lowering (n) | ||||||||
| Fibrates | 2 (6.3%) | 2 (6.3%) | 1 (3.1%) | 1 (3.1%) | ||||
| Statins | 10 (31.3%) | 10 (31.3%) | 5 (15.6%) | 6 (18.8%) | ||||
| Lipid-lowering therapy | 12 (37.5%) | 12 (37.5%) | 6 (18.8%) | 7 (21.9%) | ||||
| Calcium metabolism (n) | ||||||||
| Calcium salts | 6 (18.8%) | 31 (96.9%)a | 7 (21.9%) | 7 (21.9%)b | ||||
| Vitamin D | 8 (25.0%) | 27 (84.4%)a | 8 (25.0%) | 8 (25.0%)b | ||||
| Immunosuppression (daily dose, mg) | ||||||||
| Cyclosporin A | 221.1±100.7 | 222.2±75.9 | 218.4±86.7 | 221.6±64.4 | ||||
| Tacrolimus | 3.3±1.9 | 2.9±1.7 | 7.4±2.8 | 7.0±2.3 | ||||
| Mycophenolate mofetil | 1600±600 | 1700±400 | 1200±700 | 1500±500 | ||||
| Steroids | 5.8±3.2 | 5.6±2.9 | 6.1±3.3 | 5.0±2.8 | ||||
| Rapamycin | – | – | 4.3 | 3.3 | ||||
| Azathioprin | 70.9±23.3 | 69.3±34.4 | 71.8±21.7 | 51.9±32.4 | ||||
| . | Cases (n = 32) . | . | Controls (n = 32) . | . | ||||
|---|---|---|---|---|---|---|---|---|
| . | Period 1 . | Period 2 . | Period 1 . | Period 2 . | ||||
| Antihypertensive (n) | ||||||||
| ACE/ATIIRB | 17 (53.1%) | 16 (50%) | 14 (43.8%) | 16 (50%) | ||||
| Diuretics | 4 (12.5%) | 3 (9.4%) | 3 (9.4%) | 3 (9.4%) | ||||
| CCB | 16 (50%) | 15 (46.9%) | 13 (40.6%) | 13 (40.6%) | ||||
| BB | 21 (65.6%) | 20 (62.5%) | 15 (46.9%) | 15 (46.9%) | ||||
| Other | 11 (34.4%) | 10 (31.3%) | 3 (9.4%) | 3 (9.4%) | ||||
| Antihypertensive therapy | 29 (90.6%) | 29 (90.6%) | 24 (75.0%) | 24 (75.0%) | ||||
| Antihypertensive drugs (per patient) | 2.2±1.1 | 2.0±1.1 | 1.5±1.3 | 1.6±1.4 | ||||
| Lipid lowering (n) | ||||||||
| Fibrates | 2 (6.3%) | 2 (6.3%) | 1 (3.1%) | 1 (3.1%) | ||||
| Statins | 10 (31.3%) | 10 (31.3%) | 5 (15.6%) | 6 (18.8%) | ||||
| Lipid-lowering therapy | 12 (37.5%) | 12 (37.5%) | 6 (18.8%) | 7 (21.9%) | ||||
| Calcium metabolism (n) | ||||||||
| Calcium salts | 6 (18.8%) | 31 (96.9%)a | 7 (21.9%) | 7 (21.9%)b | ||||
| Vitamin D | 8 (25.0%) | 27 (84.4%)a | 8 (25.0%) | 8 (25.0%)b | ||||
| Immunosuppression (daily dose, mg) | ||||||||
| Cyclosporin A | 221.1±100.7 | 222.2±75.9 | 218.4±86.7 | 221.6±64.4 | ||||
| Tacrolimus | 3.3±1.9 | 2.9±1.7 | 7.4±2.8 | 7.0±2.3 | ||||
| Mycophenolate mofetil | 1600±600 | 1700±400 | 1200±700 | 1500±500 | ||||
| Steroids | 5.8±3.2 | 5.6±2.9 | 6.1±3.3 | 5.0±2.8 | ||||
| Rapamycin | – | – | 4.3 | 3.3 | ||||
| Azathioprin | 70.9±23.3 | 69.3±34.4 | 71.8±21.7 | 51.9±32.4 | ||||
aP<0.0001 period 1 vs period 2; bP<0.0001 cases vs controls.
ACEi = angiotensin-converting enzyme inhibitor; ATIIRB = angiotensin II receptor blocker; CCB = calcium channel blocker; BB = β-blocker.
Dyslipidaemia
In group A, there was a significant decrease of total cholesterol (211.1±44.1 vs 221.4±44.1, P<0.05) and LDL cholesterol (111.6±41.1 vs 125.1±33.5, P<0.01) following PTX. Serum levels of HDL cholesterol and triglycerides remained stable (Table 2). Hypercholesterolaemia, defined as a total cholesterol >200 mg/dl, was observed in 59% of the patients before PTX. Twelve patients were taking lipid-lowering drugs. The number of patients on lipid-lowering therapy and the mean daily dose did not differ between the two study periods (Table 3).
In group B, significant changes were not observed in any of the lipid parameters between either study period. No significant differences were observed between group A and B (Tables 2 and 3).
Immunosuppression
Immunosuppressive therapy did not differ between groups and periods (Tables 1 and 3). Cyclosporin A and tacrolimus blood trough levels were not significantly different between either group and periods (Table 2).
Correlations
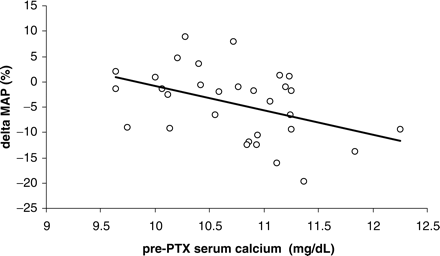
In group A, the change in BP was significantly related to pre-PTX blood pressure (r = 0.69, P<0.0001) and calcaemia (r = 0.43, P<0.05) (Figure 1), but not to pre-PTX renal function or serum iPTH level. The change in serum creatinine level correlated with none of the available clinical and biochemical parameters. BP, as opposed to renal function, was significantly correlated with cyclosporin A blood trough levels (r = 0.31, P<0.05).
Spearman correlation between the pre-parathyroidectomy (PTX) serum calcium level and the percentage change of the mean arterial pressure (MAP) following surgery (r = 0.43. P<0.05).
Discussion
The results of the present study demonstrate an improved BP and lipid profile but a deterioration of renal function in renal transplant recipients following a successful PTX. Information on the impact of PTX on BP, lipid metabolism and especially renal function is limited and often contradictory. The data are mainly derived from animal studies and human studies in primary HPT [3,6–8]. Species-related differences as well as the unknown effect of renal failure should be kept in mind as possible confounders hampering the extrapolation to the uraemic patient.
During a 15 year period, 88 patients with a functioning graft requiring a PTX for severe and persistent hypercalcaemic HPT were identified. In order to obtain a homogenous and well-controlled study population and to maximize the reliability of the data, stringent selection criteria were formulated. Thirty-two patients fulfilled these inclusion and exclusion criteria. In these renal transplant recipients, the effect of the PTX on BP, lipid metabolism and renal function was analysed retrospectively.
One of the most striking findings was the significant deterioration of renal function following PTX. A >15% increase of the serum creatinine was observed in 65.6% of the patients. This finding is in agreement with data reported by others [9,10]. An improved renal function in renal transplant recipients who underwent subtotal PTX has also been described, especially in older studies [11]. These contradictory findings are most probably related to differences in patient characteristics such as the pre-PTX serum calcium level.
Whether the observed increase of serum creatinine reflects a true deterioration of glomerular filtration rate (GFR) remains to be established. There is evidence, although not unequivocal, that active vitamin D treatment, which was commenced in most of our patients at the time of the PTX, may reduce renal tubular secretion of creatinine, and thereby affect serum creatinine levels and measurement of creatinine clearance without altering the true GFR [12]. This interference is thought to be dose dependent and to be more pronounced in patients with severe kidney disease (stages 4–5). We did not observe a correlation between the deterioration of renal function and either baseline renal function or vitamin D dose after PTX. An almost identical increase of serum creatinine was observed in patients receiving vitamin D following PTX as in those that did not. Urea nitrogen did not increase following PTX. We do not think that this observation refutes our hypothesis. Indeed, urea nitrogen is widely accepted to be a poor index of GFR since both its generation and tubule reabsorption are highly variable. Nonetheless, only prospective studies including precise and reliable measurements of graft function (e.g. determination of inulin clearance) will allow definite conclusions concerning the separate impact of PTX and active vitamin D treatment on GFR.
The renal function deterioration occurred already during the first week following the PTX (data not shown). This time course suggests an underlying haemodynamic mechanism. PTH has vasodilatory effects on pre-glomerular vessels, while efferent arterioles are constricted, presumably secondary to renin release [13]. Reversal of these effects may cause an acute deterioration of renal function. In the long term, however, these haemodynamic changes may contribute to the attenuation of the progression of renal failure, as demonstrated by others in an animal model [3,14]. A preliminary analysis of data on the long-term time course of the graft function after PTX is consistent with this hypothesis: after an initial deterioration, renal function showed a slow but steady improvement over years towards baseline. Obviously, these data need to be confirmed by a well-controlled trial. Whether a PTX affects renal graft survival remains a controversial issue. Lee et al. studied graft survival in 22 PTX renal transplant recipients and demonstrated a detrimental effect of the surgical procedure [10]. Kerby et al. conversely, did not find a difference in long-term graft survival between patients requiring PTX (n = 38) and a matched group of renal allograft recipients [15]. Obviously, only a large (multicentre) observational matched cohort study will allow definite conclusions on this issue.
A second important finding of our study was the significant decrease of BP after PTX. Studies of the effect of PTX on BP have produced contradictory results. Experimentally, PTX induces a lowering of BP in spontaneously hypertensive rats [16]. In humans too, a fall in BP following PTX has been demonstrated [9]. However, these data have not been confirmed by others [17].
The BP-lowering effect of PTX is most probably related to the normalization of the serum calcium and/or PTH levels. The mechanisms through which changes in the concentrations of serum calcium and PTH affect BP are complex and only partially understood. Hypercalcaemia may induce hypertension through an increase in cardiac output or peripheral vascular resistance, or both, or through an increased release or action, or both, of pressor substances (e.g. catecholamines or renin) [18]. PTH has been demonstrated to play an important permissive role for the hypertensive action of hypercalcaemia [18]. In the presence of PTH, more calcium may enter the vascular smooth muscle, and such an event augments the hypertensive response to the rise in serum calcium. This action counteracts the direct vasodilatatory effects of PTH, and its full expression becomes evident only in the presence of hypercalcaemia [18]. Several lines of evidence indicate that the endothelium is a target of PTH with potential impact on BP. In patients with primary hyperparathyroidism, impaired flow-mediated vasodilatation in the brachial artery is improved by PTX. In renal transplant patients, endothelial dysfunction is correlated to PTH levels, and elevated PTH concentrations have a deleterious effect on elastic properties of the arterial wall [19]. Our finding of a decreased pulse pressure following PTX is in agreement with the latter finding. In some patients, finally, the decrease in BP observed following PTX could be related to the removal of a recently identified circulating factor termed parathyroid hypertensive factor. This factor is dialysable, heat stable and potentiates different vasoconstrictors [20].
We observed a significant positive correlation between the pre-PTX serum calcium level and the decrease of BP, supporting the hypothesis that calcium plays a central role in the genesis of hypertension in patients with HPT [21].
Finally, we also observed a modest amelioration of dyslipidaemia following PTX. A beneficial effect of PTX on cholesterol levels had been observed by Ogata et al. [3] and numerous other authors [14]. The effect of PTH is probably independent of the presence of renal failure, as suggested by the observation of reversible hyperlipoproteinaemia in patients with primary HPT [8]. A decreased activity of both the lipoprotein [6] and hepatic lipase [7] has been implicated in the pathogenesis of dyslipidaemia related to HPT. These changes in lipase metabolism were corrected by PTX [6,7] and calcium channel blockade [7]. The latter, again, supports a causative role for cytosolic calcium concentrations. The observation that administration of insulin corrected the disturbed metabolism of triglyceride-rich particles indicates that the effect of PTH at least partially involves inhibition of insulin secretion or interference with its peripheral action [22].
Changes in the renin–angiotensin system and and/or cystosolic calcium concentrations are likely to play a causative role in the changes in renal function, BP and lipid metabolism following PTX. We therefore performed subgroup analyses to evaluate the effect of treatment with either a calcium channel blocker or an angiotensin-converting enzyme (ACE) inhibitor/angiotensin II receptor blocker on the outcome of a PTX. We failed to find a significant treatment effect, most probably as a result of low absolute numbers.
One could argue that the changes in blood renal function, BP and serum lipids are the consequence of non-specific effects of anaesthetic/surgery. Equally, better control of BP and serum lipids might reflect increased hospital attendance. This explanation is, however, unlikely since, in a study with a similar design including 48 renal transplant recipients with stable graft function at the time of inclusion, we did not observe significant differences in any of the above-mentioned parameters between the 6 month period before and after orthopaedic surgery (data not shown).
In conclusion, in patients with a functioning renal graft, BP and dyslipidaemia improve, whereas serum creatinine increases following successful PTX. Our data are in agreement with a stimulatory effect of PTH on plasma renin activity and an inhibitory effect on lipase activity, as previously demonstrated by others. To what extent the increased serum creatinine reflects a true deterioration of the GFR or is the consequence of active vitamin D-induced reduction of the renal tubular secretion of creatinine needs to be elucidated by further research.
Part of this study was presented at the American Society of Nephrology 2004 and published in abstract form.
Conflict of interest statement. None declared.
References
Bonarek H, Merville P, Bonarek M et al. Reduced parathyroid functional mass after successful kidney transplantation.
Messa P, Sindici C, Cannella G et al. Persistent secondary hyperparathyroidism after renal transplantation.
Ogata H, Ritz E, Odoni G, Amann K, Orth SR. Beneficial effects of calcimimetics on progression of renal failure and cardiovascular risk factors.
Evenepoel P, Claes K, Kuypers D, Maes B, Bammens B, Vanrenterghem Y. Natural history of parathyroid function and calcium metabolism after kidney transplantation: a single-centre study.
Bouillon R, Coopmans W, Degroote DE, Radoux D, Eliard PH. Immunoradiometric assay of parathyrin with polyclonal and monoclonal region-specific antibodies.
Akmal M, Kasim SE, Soliman AR, Massry SG. Excess parathyroid hormone adversely affects lipid metabolism in chronic renal failure.
Klin M, Smogorzewski M, Ni Z, Zhang G, Massry SG. Abnormalities in hepatic lipase in chronic renal failure: role of excess parathyroid hormone.
Hagstrom E, Lundgren E, Lithell H et al. Normalized dyslipidaemia after parathyroidectomy in mild primary hyperparathyroidism: population-based study over five years.
Rostaing L, Moreau-Gaudry X, Baron E et al. Changes in blood pressure and renal function following subtotal parathyroidectomy in renal transplant patients presenting with persistent hypercalcemic hyperparathyroidism.
Lee PP, Schiffmann L, Offermann G, Beige J. Effects of parathyroidectomy on renal allograft survival.
David DS, Sakai S, Brennan BL et al. Hypercalcemia after renal transplantation.
Goodman WG, Coburn JW. The use of 1,25-dihydroxyvitamin D3 in early renal failure.
Massfelder T, Parekh N, Endlich K, Saussine C, Steinhausen M, Helwig JJ. Effect of intrarenally infused parathyroid hormone-related protein on renal blood flow and glomerular filtration rate in the anaesthetized rat.
Shigematsu T, Caverzasio J, Bonjour JP. Parathyroid removal prevents the progression of chronic renal failure induced by high protein diet.
Kerby JD, Rue LW, Blair H, Hudson S, Sellers MT, Diethelm AG. Operative treatment of tertiary hyperparathryoidism; a single-center experience.
Schleiffer R, Xue H, McCarron DA, Bukoski RD. Effect of chronic and subacute parathyroidectomy on blood pressure and resistance artery contractility in the spontaneously hypertensive rat.
Almirall J, Lopez T, Comerma I, Garcia E, Marques G. Effect of parathyroidectomy on blood pressure in dialysis patients.
Massry SG, Iseki K, Campese VM. Serum calcium, parathyroid hormone, and blood pressure.
Barenbrock M, Hausberg M, Kosch M, Kisters K, Hoeks AP, Rahn KH. Effect of hyperparathyroidism on arterial distensibility in renal transplant recipients.
Lewanczuk RZ, Resnick LM, Ho MS, Benishin CG, Shan J, Pang PK. Clinical aspects of parathyroid hypertensive factor.
Vaziri ND, Ni Z, Wang Q, Oveisi F, Zhou XJ. Downregulation of nitric oxide synthase in chronic renal insufficiency: role of excess PTH.
- dyslipidemias
- parathyroid hormones
- hypertension
- ldl cholesterol lipoproteins
- renal transplantation
- hyperparathyroidism
- hyperparathyroidism, secondary
- renal function
- systolic blood pressure
- blood pressure
- creatinine
- kidney failure
- glomerular filtration rate
- calcium phosphates
- cardiovascular system
- osteitis fibrosa disseminata
- parathyroidectomy
- time factors
- tissue transplants
- vitamins
- kidney
- lipase
- lipids
- transplantation
- persistence
- diastolic blood pressure
- tubular secretion
- creatinine tests, serum
- total cholesterol
- calcimimetic agents
- pulse pressure
- allografting
- causality
- plasma renin activity





Comments