-
PDF
- Split View
-
Views
-
Cite
Cite
Arsalan N. Habib, Bradley C. Baird, John K. Leypoldt, Alfred K. Cheung, Alexander S. Goldfarb-Rumyantzev, The association of lipid levels with mortality in patients on chronic peritoneal dialysis, Nephrology Dialysis Transplantation, Volume 21, Issue 10, October 2006, Pages 2881–2892, https://doi.org/10.1093/ndt/gfl272
Close - Share Icon Share
Abstract
Background. The role of traditional risk factors, including plasma lipids, in the pathogenesis of cardiovascular (CV) disease in chronic dialysis patients is unclear. Previous studies have suggested that lower serum total cholesterol (TC) is associated with higher mortality in patients on chronic haemodialysis (HD). Whether this relationship is specific to the HD population or is common to the uraemic state is unclear. The present study evaluated the association of serum TC and triglycerides with clinical outcomes in chronic peritoneal dialysis (PD) patients.
Methods. Data of 1053 PD patients from the United States Renal Data System (USRDS) prospective Dialysis Morbidity and Mortality Study Wave 2 were examined. Cox regression was used to evaluate the relationship between lipid levels and mortality.
Results. Patients with TC levels ≤125 mg/dl (3.24 mmol/l) had a statistically significant increased risk of an all-cause mortality, including those taking or not taking lipid-modifying medications, compared with the reference of 176–225 mg/dl (4.54–5.83 mmol/l). In stratified analysis, this association was demonstrated in patients with serum albumin >3.0 g/dl (30 g/l), but not with albumin ≤3.0 g/dl.
Compared with patients with triglyceride levels of 201–300 mg/dl (2.27–3.39 mmol/l), a statistically significant reduction of all-cause, but not CV, mortality was observed in patients with triglyceride levels of 101–200 mg/dl (1.14–2.26 mmol/l), as well as in the subgroup with serum albumin levels <3.0 g/dl (30 g/l) and triglycerides of ≤100 mg/dl (1.13 mmol/l) and 101–200 mg/dl (1.14–2.26 mmol/l).
Conclusions. While confounding factors and causal pathways have not been clearly identified, aggressive lowering of plasma cholesterol in PD patients is not supported by this study, however, treatment of hypertriglyceridaemia may be warranted with triglyceride levels >200 mg/dl (2.26 mmol/l).
Introduction
The prevalence of cardiovascular (CV) disease is much higher in patients with end-stage renal disease (ESRD) than in the general population and is largely responsible for the high mortality rate seen in ESRD [ 1 ]. Establishing the nature of the associations between the predictors of CV disease and clinical outcome in this population is important, since they will guide further direction of investigation and perhaps guide therapy.
The role of some conventional CV risk factors in the ESRD population has been recently evaluated and has yielded unexpected results [ 2–9 ]. In particular, serum lipid levels, a modifiable CV risk factor in the general population, are often abnormal in ESRD patients. Peritoneal dialysis (PD) patients have higher serum levels of total and low-density lipoprotein (LDL) cholesterol, and both haemodialysis (HD) and PD patients have elevated serum levels of total triglycerides, apolipoprotein-B (Apo-B) and apolipoprotein-E (Apo-E), while high-density lipoprotein (HDL) cholesterol levels are significantly lower [ 10 , 11 ] compared with the general population.
In several studies involving HD patients, low-serum total cholesterol (TC) levels were paradoxically associated with increased mortality [ 5 , 12 , 13 ]. It is not clear if this phenomenon is associated with uraemia or is specific for patients on a particular type of renal replacement therapy. Added to the uncertainty is the different lipid profiles that are associated with various dialysis modalities [ 10 ]. Specifically, PD patients have a markedly different lipid profile when compared with HD patients [ 14 ], which may result from the PD procedure itself [ 15 ]. There has been little analysis of the relationship between dyslipidaemia and clinical outcome of PD patients. Therefore, the aim of this study was to examine the association of TC and triglycerides with all-cause and CV mortality in patients undergoing chronic PD, our hypothesis being that high triglycerides and low-TC are associated with adverse clinical outcomes in this patient population.
Methods
Source of data
Data from the United States Renal Data System (USRDS) database collected during the prospective Dialysis Morbidity and Mortality Study (DMMS) Wave 2 study ( n = 4500) was used for the present analysis. The DMMS Wave 2 study started at the beginning of 1996, with patients enrolled in 1996 and 1997. The DMMS Wave 2 laboratory data was obtained from 259 facilities reporting to USRDS, most with only a few patients. Lipid measurement methods were not standardized across individual laboratories. However, we feel that in this broad range of facilities different laboratory procedures are likely well-represented. The mean follow-up time of the subpopulation analysed in this report was 23 ± 14 months. Patient records were censored at death, kidney transplant, last dialysis or at the end of the study (31 December 1999), whichever was earliest. A total of 1788 patients were treated with PD at the time of enrolment. After applying the inclusion and exclusion criteria described below, and eliminating the records with a missing study start date, 1053 patients remained in the final data set and constituted the cohort for the present analysis.
Inclusion criteria
Patients who survived more than 90 days since the onset of ESRD at the time of enrolment in the DMMS Wave 2 study were included in this analysis. This criterion was selected as the investigators were interested only in ESRD patients, not acute renal failure patients who later recovered, or those very ill patients who died rapidly due to unrelated conditions, because that would potentially bias the results. Therefore, the time at risk for survival analysis begins 90 days after starting dialysis.
Exclusion criteria
The following exclusion criteria were applied: (i) patients <18 years of age, (ii) those who recovered kidney function and no longer required dialysis at any time during the follow-up and (iii) patients in whom the cholesterol and triglyceride measurements were not available.
Independent variables
TC and total triglyceride levels were the primary independent variables. The information on the fasting state of TC or triglycerides for lipid analysis is not indicated in the DMMS Wave 2 study data available through USRDS. Our attempts to find this information were unsuccessful (USRDS, private communication). Only a few patients in the DMMS Wave 2 study had LDL and HDL cholesterol levels reported, therefore we elected not to analyse these variables since they were available in only a very small subset of the study population because the analysis would probably be biased and potentially misleading. Cholesterol levels were stratified based on the following values: ≤125 mg/dl (3.24 mmol/l), 126–175 mg/dl (3.25–4.53 mmol/l), 176–225 mg/dl (4.54–5.83 mmol/l), 226–275 mg/dl (5.84–7.12 mmol/l) and >275 mg/dl (7.13 mmol/l). The category of 176–225 mg/dl (4.54–5.83 mmol/l) was used as the reference as the mean TC in the study population was 208 mg/dl (5.39 mmol/l). Triglyceride levels were stratified into the following categories: ≤100 mg/dl (1.13 mmol/l), 101–200 mg/dl (1.14–2.26 mmol/l), 201–300 mg/dl (1.14–2.26 mmol/l), 301–400 mg/dl (3.40–4.52 mmol/l), and >400 mg/dl (4.53 mmol/l). The category of 201–300 was used as the reference as the mean triglyceride levels for the study population was 212 mg/dl (2.40 mmol/l). The reference categories for TC and triglycerides were chosen not only because they encompassed the study population mean values, but also because the investigators were interested in the effects of extremes from the mean.
Covariates
The following baseline variables were used in the Cox models as covariates: age, gender, race, weight, height, primary cause of ESRD (diabetes, hypertension, glomerulonephritis or other), haemoglobin, serum albumin, serum calcium–phosphate product, serum bicarbonate, residual kidney creatinine clearance, PD parameters [dialysate effluent volume, dialysis creatinine clearance, dialysate-to-plasma (D/P) creatinine ratio after a 4-h dwell], use of lipid-modifying medications and comorbidity characteristics [diabetes mellitus, coronary artery disease (CAD), congestive heart failure (CHF), left ventricular hypertrophy (LVH), cerebrovascular disease (CVD) and peripheral vascular disease (PVD)]. Smoking history and history of neoplasm were not included in the final models, because of non-significant results in preliminary multivariate analysis and a substantial number of missing values.
D/P creatinine ratio at 4 h was derived from a 24-h dialysate collection using a formula that was developed utilizing simultaneous measurement of 24-h and 4-h dialysate creatinine and D/P ratio. The quartile cut-points for D/P ratio at 4 h in our study population were very similar to those reported by Twardowski et al . [ 16 ].
Dialysate concentration of glucose is not indicated in the DMMS Wave 2 data, therefore it was not used in the analysis. In addition, the glucose-free solutions were not widely available at the time of data collection (mid-1990s).
The duration of ESRD prior to the entry into the study was not used in the multivariate models since only incident PD patients were enrolled in the DMMS Wave 2 study. Virtually all durations between ESRD onset and study start date were 30–120 days in the data set. Only about 1% of the patients had a pre-study ESRD history exceeding 120 days.
The final statistical models included only those variables that (i) were shown to have a significant association with the outcome in the initial multivariate model or (ii) were not found to be significant predictors but were deemed to have potential physiological confounding effect (e.g. gender, height and weight).
Outcome measures
The primary outcome variables were the all-cause mortality and CV mortality. Deaths were classified as CV-related or non-CV related. CV-related deaths include hypertensive disease (ICD-9 [International Classification of Diseases] 401–405), ischaemic heart disease (ICD-9 410–414), other heart disease (ICD-9 420–429) and CVD (ICD-9 430–438). Data on the cause of death was obtained from the USRDS patient file which reported death certificates.
Statistical analysis
Cox regression models with fixed covariates were used to test if lipid levels were independent predictors of mortality and to evaluate the relationship between these variables. The SAS statistical package (SAS Institute, Cary, NC, USA) was used to perform these analyses.
Eliminating unreliable data
Specific values of variables that were deemed unrealistic were eliminated. Values that were considered unlikely, but physiologically possible, were retained. The limits for specific variables were set a priori as follows: height 100–215 cm, weight 23–180 kg, systolic blood pressure 70–220 mmHg, diastolic blood pressure 30–125 mmHg, haemoglobin 4–20 g/dl, serum phosphate 0–20 mg/dl (0–6.46 mmol/l), serum bicarbonate 5–45 mEq/l (mmol/l), serum creatinine 0.6–20.0 mg/dl (45.8–1526 µmol/l), PD drain volume 2–40 l/24 h and dialysate creatinine concentration >0.
Missing data
The investigators imputed continuous variables that were missing >20% of the entries. Regression analysis was used to impute height and weight. Those variables missing more than 20% were imputed using the method described by Dupont [ 17 ].
Testing the proportional hazards assumption
We tested the proportionality assumption by employing the technique based on the introduction of interaction terms in the model. The test is based on the fact that if the interaction terms have a significant association with the outcome, the proportionality assumption is violated. Our analysis yielded the P -value of 0.57. P -values <0.05 would have suggested a problem with the assumption.
Results
Patient characteristics
Baseline characteristics of the study population are presented in Table 1 . Continuous variables are presented as a mean ± SD. The average age was 57.2 ± 15.3 years; 52% were male and 72.2% were white. The primary cause of ESRD was diabetes, accounting for 45%. According to the DMMS study design, baseline characteristics were collected at 2 months after the onset of ESRD. CV diseases were prevalent in this population. Over 80% were taking anti-hypertensive medications. The mean TC was 208 ± 59 mg/dl (5.39 ± 1.53 mmol/l), while the mean total triglyceride level was 212 ± 163 mg/dl (2.40 ± 1.84 mmol/l). The percentage of patients in each cholesterol and triglyceride category is presented in Table 1 . Most of the patients (86.4%) were not taking lipid-modifying agents; 13.4% were taking one agent and only 0.2% were taking two agents.
In the subgroup treated with lipid-lowering medications ( n = 143) only 10 patients (7%) were using agents (gemfibrozil or niacin) other than 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase inhibitors.
Events during follow-up
Enrolment in the DMMS Wave 2 study was performed over a 2-year period (1996–97) and follow-up ended on 31 December 1999 for all patients unless censored or they reached the primary outcome. Actual follow-up of the enrolled patients was 22.6 ± 13.8 months (range 0.03–46 months). The number of patients who completed the study (administrative censoring) was 224 patients (21.3%); 218 (20.7%) received a kidney transplant and 93 (8.8%) were lost to follow-up. There were a total of 518 (49.2%) deaths during follow-up, of which 339 were identified as CV deaths.
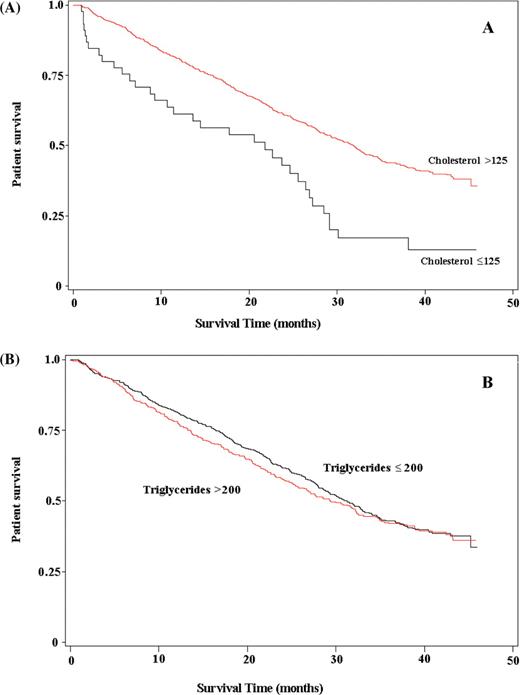
Kaplan–Meier analysis of TC and triglycerides are seen in Figure 1 (A and B), with an observed decrease in patient survival seen with TC levels of ≤125 mg/dl (3.24 mmol/l) when compared with >125mg/dl (3.24 mmol/l). No difference in patient survival was observed between the two triglyceride categories of >200 mg/dl and ≤200mg/dl (2.26 mmol/l).
Association between TC and mortality
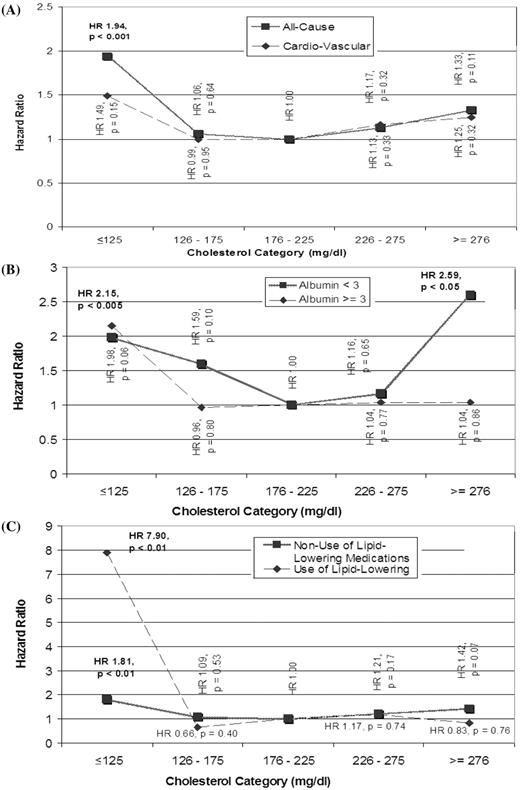
Overall, the hazard ratio (HR) of all-cause mortality for the various categories of cholesterol level showed a reverse J-shaped curve ( Figure 2 A, Table 2 ). The only statistically significant HR, however, was associated with the lowest cholesterol category of ≤125 mg/dl (3.24 mmol/l), compared with the reference category of 176–225 mg/dl (4.54–5.83 mmol/l) [HR = 1.94 (1.31–2.89); P < 0.001]. Analysis of CV causes of death revealed no statistically significant HR for any of the cholesterol categories, compared with the reference category. However, the presence of a reverse J-shaped curve was again noted ( Figure 2 A), with the lowest cholesterol category showing a trend towards increased CV mortality [HR = 1.49 (0.87–2.57); P = 0.15].
To investigate the potential effect modification of the results by nutritional status or inflammation, expressed as serum albumin concentration, subgroup analysis was performed based on serum albumin levels of ≤3.0 g/dl or >3.0 g/dl (30 g/l). In patients with albumin levels ≤3 g/dl ( n = 185), there continued to be a reverse J-shaped relationship between TC and all-cause mortality ( Figure 2 B, Table 2 ); the increase in risk [HR = 2.59 (1.19–5.61); P < 0.05] for the cholesterol category of >275 mg/dl (7.13 mmol/l), compared with the chosen reference, was statistically significant. For those patients with albumin levels >3.0 g/dl (30 g/l) ( n = 810), the lowest cholesterol category had the highest HR for all-cause mortality [HR = 2.15 (1.29–3.59); P < 0.005], while the other cholesterol categories were not statistically different from the reference ( Figure 2 B, Table 2 ).
The treatment of some patients for dyslipidaemia was a potential confounder, which prompted the analysis of mortality stratified by lipid-modifying medications ( Figure 2 C, Table 2 ). In both the subgroups who were taking [HR = 7.90 (1.73–36.02); P < 0.01; n = 137] or not taking [HR = 1.81 (1.18–2.76); P < 0.01; n = 858] lipid-modifying medications, the lowest cholesterol category was associated with a statistically significant increased risk of all-cause mortality. This increased risk in the lowest cholesterol category was most pronounced in those taking lipid-modifying medications by over 4-fold. However, these results should be interpreted with caution, as there were only six patients in this subgroup.
Association between total triglycerides and mortality
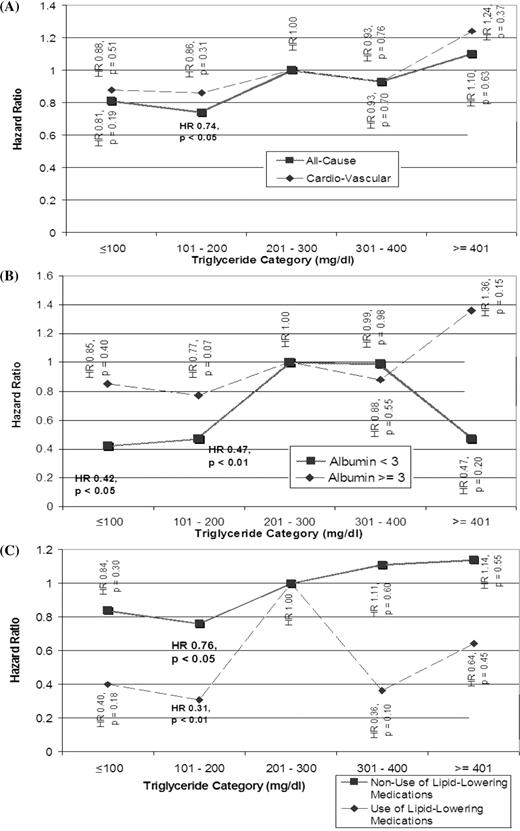
There was a general trend relating higher triglycerides to higher all-cause mortality, although only one triglyceride category [101–200 mg/dl (1.14–2.26 mmol/l)] was associated with a statistically significant different risk of all-cause mortality [HR = 0.74 (0.58–0.94); P < 0.05] compared with the reference ( Figure 3 A, Table 3 ). A similar trend relating higher triglycerides to CV mortality was observed, although none of the categories achieved a statistically significant difference in risk compared with the reference category ( Figure 3 A, Table 3 ). In the subgroup of patients with albumin ≤ 3 g/dl (30 g/l) ( n = 185), triglycerides ≤100 mg/dl (1.13 mmol/l) [HR = 0.42 (0.20–0.87); P < 0.05], triglycerides 101–200 mg/dl (1.14–2.26 mmol/l) [HR = 0.47 (0.27–0.81); P < 0.01] were significantly associated with lower risks of all-cause mortality ( Figure 3 B, Table 3 ). In the subgroup with albumin >3.0 g/dl (30 g/l)( n = 810), none of the triglyceride categories achieved significant association with all-cause mortality.
In the both subgroups who were taking [HR = 0.31 (0.12–0.75); P < 0.01; n = 137] or not taking [HR = 0.76 (0.59–0.99); P < 0.05; n = 858] lipid-modifying medications, the triglyceride category of 101–200 mg/dl (1.14–2.26 mmol/l) was associated with a statistically significant reduction in the all-cause mortality risk ( Figure 3 C, Table 3 ).
All of the above stratified subgroup analysis was performed using the endpoint of CV mortality and there were no statistically significant results noted (results not shown).
Examining distributional assumptions
Variables with skewed distribution may cause disproportionate weighting in multivariate analysis, i.e. the data may contain extreme values that might bias the results of the analysis from the actual trend. To address this issue log-transformation of the covariates can be performed. In our analyses, we repeated Cox modelling with covariates having skewed distribution log-transformed. The association between the primary variables of interest and the outcome remained largely unchanged. Primary variables of interest (e.g. cholesterol and triglyceride levels) were categorized, and therefore were not log-transformed as log-transformation would add no statistical benefit or change the analysis.
Analysis of the trend in HRs
The association between lipid categories and the outcome in this study seems to be non-linear; therefore we attempted to fit both linear and logarithmically transformed functions to demonstrate the trend in cholesterol and triglyceride levels’ association with all-cause mortality. None of these models of the trend showed statistical significance, likely due to the small number of data points (data not shown).
Discussion
The present study evaluated the relationship between lipidaemia and all-cause or CV mortality of PD patients. Based on our study in the PD population and prior studies involving HD patients, the reported relationships between TC and mortality are more likely due to the uraemic state rather than the specific dialysis modality. We demonstrated statistical significance between TC levels ≤25 mg/dl (3.24 mmol/l) and increased mortality compared with the reference group [TC of 176-225 mg/dl (4.54 – 5.83 mmol/l)]. The group with TC levels >225 mg/dl (5.83 mmol/l) also showed a trend towards increased all-cause mortality, although this was not statistically significant. These reverse J-shaped data resemble those seen in HD patients [ 4 ].
A paradoxical association between TC levels and mortality in HD patients has been demonstrated [ 5 ], indicating that HD patients with high TC tend to have lower mortality. This phenomenon can be explained by either true association (i.e. effect of uraemia/ESRD or HD) or by statistical distortion due to statistical confounding (e.g. by malnutrition or inflammation) or late-life and reverse-causation biases [ 7 , 18 ]. Phenomena explaining a J-curve association between mortality risk and disease risk factors can be explained by statistical confounding that artificially elevates mortality risk at low risk-factor levels in longitudinal studies [ 18 ].
Previous studies of serum from PD and HD patients have documented decreased HDL cholesterol, increased small dense LDL particles, elevated triglycerides and alterations in Apo-B, compared with the general population [ 11 , 19 ] and that the initiation of PD itself may alter the patients’ lipid profiles [ 15 ]. This uraemic dyslipidaemia may contribute to the increased mortality seen in the dialysis population. In the general population, there are published guidelines for treating patients with high total or LDL cholesterol [ 20 ]. Our study, as well as other reports [ 21 ] demonstrated that only a small percentage of PD patients are treated with lipid-modifying medications, a practice some have termed ‘therapeutic nihilism’ [ 21 ] but others may justify based on the lack of data showing improved mortality with treatment [ 9 ]. In the Deutsche Diabetes Dialyse Studie (4D study) [ 9 , 22 ] treatment of diabetic patients on HD with the HMG-CoA reductase inhibitor atorvastatin showed no significant difference in mortality compared with placebo, despite dramatic reductions of total and LDL cholesterol. In fact, the data showed an increased rate of cerebrovascular accidents in the study population on atorvastatin [ 9 ].
Like the 4D study, the results of our analysis brings up the issue of potential harm of lipid-modifying medications in the dialysis population ( Figure 2 C). The group on lipid-modifying medications and a TC of ≤125 mg/dl (3.24 mmol/l) consisted of only six patients, yet these results do not support the idea that treating PD patients to lower cholesterol levels is of benefit. Potential explanations include the protective effect of higher cholesterol levels from bacterial infection as an inhibitor of endotoxin [ 23 , 24 ]. A higher rate of infection could explain the increased HR seen in our study population with the lowest cholesterol category. Lowering TC may not be the goal in uraemic patients; rather the focus may need to shift elsewhere. Results of the 4D study suggest that the relationship between lipid metabolism and CV disease in uraemic patients is quite different from the general population [ 9 ].
Both inflammation and malnutrition can affect lipid levels and mortality [ 5 , 7 , 12 , 13 ]. In the prospective study by Liu et al . [ 7 ] the authors used a stratification based on serum albumin, interleukin-6 (IL-6) and C-reactive protein (CRP) levels to evaluate patients with different nutritional and inflammatory status separately. In the absence of hypoalbuminaemia or inflammation, the association of TC level and mortality was similar to that in the general population. The DMMS data set did not contain values of serum IL-6, CRP or other inflammatory markers except for albumin; therefore, we used serum albumin, a negative acute phase reactant, as an indicator of malnutrition and/or inflammation. It should be noted that some studies have suggested that inflammation is a more important determinant of serum albumin level than albumin synthesis rate and nutrition [ 25 ]. The cut-point of 3.0 g/dl (30 g/l) for stratification by albumin was selected to identify a subpopulation that had more advanced malnutrition and/or inflammation. Hypoalbuminaemia did not seem to play a significant role in the relationship between TC and mortality as the biphasic relationship held true despite stratifying for albumin levels. Only a total of 185 patients had a serum albumin ≤3 g/dl (30 g/l) and they were subdivided into the five categories of cholesterol and triglycerides, leaving a small number of patients in each category; however, the trend was apparent and probably meaningful. Interestingly, those patients with normal albumin still had increased mortality with lower cholesterol levels.
Reference ranges in our study were chosen as they encompassed the mean values of TC and triglycerides in the study population. We chose to analyse TC and triglyceride levels in the categories presented, as we were interested in the effects of the extremes of these values. Reducing the number of categories would obligate a larger number of patients in these categories and would likely dilute the effect of the extreme values.
In the entire study population, the analysis of TC was the opposite of what is expected in the general population, but similar to previous observations [ 5 , 7 , 12 , 13 ] in which low TC was associated with an increased risk of all-cause mortality. When our analysis was stratified based on serum albumin level, the group with albumin ≤3.0 g/dl (30 g/l) (roughly corresponding to ‘inflammation’ in the study of Liu et al . [ 7 ]) showed results similar to the general population where elevated cholesterol is associated with increased mortality, but opposite to that of Liu et al . [ 7 ]. In contrast, the group with normal albumin (roughly corresponding to ‘no inflammation’ in the study of Liu et al . [ 7 ]) showed a relationship between cholesterol and mortality which is different both from the general population and Liu et al . [ 7 ]. These two subgroups of albumin ≤3.0 g/dl (30 g/l) with TC >275 mg/dl (7.13 mmol/l) and albumin >3.0 g/dl and TC ≤125 mg/dl (3.24 mmol/l) had small sample sizes (23 and 28, respectively); thus, their results should be interpreted with caution. The differences seen in our study and that of Liu et al .'s [ 7 ] may be related to differences in the study population as our population included entirely PD patients vs only 19.1% ( n = 157) in Liu et al . [ 7 ]. Our population included 17.5% categorized as malnutrition/inflammation while in Liu et al . [ 7 ] it was 75%; we used an albumin cut-point of ≤3.0 g/dl (30 glL) while Liu et al . [ 7 ] used 3.6 g/dl (36 g/l). In addition, the pathogenesis of hyperlipidaemia may also be different in PD and HD patients leading to the observed differences in each population's lipid profiles [ 10 , 14 , 15 ].
While analysing the data we did not have information about active or recent acute illnesses (e.g. peritonitis) in the DMMS Wave 2 study population. This is important because it can potentially confound the results of the analysis by affecting both lipid levels and survival. Unfortunately, this information was not available to us at the time of analysis. In addition, patients enrolled in this study are incident PD patients, which means that the vast majority of them started dialysis between 30 and 120 days prior to study start. Therefore, while we do not know if subjects might indeed have had an episode of peritonitis prior to being enrolled in the study, we believe that the probability is quite low. In fact, in a recent study by Lim et al . [ 26 ] the authors demonstrated average time between the initiation of PD and the onset of first peritonitis to be on average between 9.9 and 19.3 months in different patient populations.
Patients on lipid-modifying medications might be different from those not treated with these agents by their physician, which represents a potential selection bias and may confound the results. Even if the Cox model is adjusted for the use of medications, residual confounding might still be present. To diminish the potential confounding by treatment with lipid-modifying medications we analysed patient subgroups who were, or were not, treated with lipid-modifying medications. A total of 143 patients were taking lipid-modifying medications and they were subdivided into the five categories of cholesterol and triglycerides, resulting in a small number of patients in each category. These categories were defined a priori before the data analysis. Consequently, only six patients ended up in the lowest cholesterol category and the results of the analysis should be interpreted very carefully. However, we believe our study demonstrated that in both the subgroups of patients, TC levels ≤125 mg/dl (3.24 mmol/l) were associated with increased relative risks for all-cause mortality, but this increased risk was most striking in those taking lipid-modifying medications by an over 4-fold increase. This striking difference could be explained by the small number of patients who were taking lipid-modifying medications and were in the lowest cholesterol category. Since the DMMS Wave 2 study data did not contain HDL or LDL subfractions, the role of these lipoproteins in PD patient mortality cannot be deduced from this study.
Elevated serum levels of triglyceride-containing lipoprotein remnant particles are the hallmark of uraemic dyslipidaemia [ 11 ]. Studies have shown that lipoprotein metabolism in chronic kidney disease patients changes even before initiation of renal replacement therapy, as early as a fall in glomerular filtration rate to <50 ml/min [ 27 ]. Previous studies have also shown that changes in lipid metabolism, including hypertriglyceridaemia, can occur at the initiation of PD or HD [ 28 ]. Several different mechanisms may explain an association of elevated triglycerides with increased CV events including increased levels of small dense LDL particles, decreased levels of HDL cholesterol or HDL-associated enzymes [ 29 ]. Little is known about the relationship between triglycerides and clinical outcomes in PD or HD patients [ 30 , 31 ]. Our analysis suggests that low-to-normal levels of serum triglycerides [≤200 mg/dl (2.26 mmol/l)] were protective whether the patients were treated with lipid-modifying medications or not. The only subgroup that did not seem to show statistically significant benefit from a lower triglyceride level was patients with an albumin >3.0 g/dl (30 g/l), although there was a trend towards lower mortality. Therefore, in the PD population, triglycerides appear to behave in a manner more consistent with the general population, where lower levels are associated with lower mortality [ 32–34 ].
The present analysis has several limitations. First, as this was a retrospective data analysis, all the caveats of a retrospective study must be taken into account. Second, as our study was not adjusted for CRP, IL-6 or other inflammatory markers other than albumin, it could still have residual confounding by systemic inflammation. Third, as we were interested in analysing the extreme values of TC and triglycerides, the division of each category resulted in a small number of patients in some of these categories. Fourth, USRDS data does not provide information about the fasting states of patients who underwent lipid blood tests, which would especially affect results of triglycerides. Fifth, the majority of patients did not have HDL or LDL levels reported and as a result data analysis using these parameters could not be accurately performed. Finally, the role of lipids may be obscured by uraemia-related risk factors that are currently unknown and could not be used for adjustment in the statistical models.
The presented data on TC do not support aggressive lowering of total cholesterol. In contrast, treatment of hypertriglyceridaemia may be warranted with levels >200 mg/dl, as levels below 200 mg/dl (2.26 mmol/l) were beneficial in the current analysis. The results of confirmatory prospective clinical trials would be valuable. These data also support the important role of triglyceride-containing lipoprotein remnant particles in the overall mortality of dialysis patients.
Baseline characteristics of the study population a
| Age (years) | 57.2 (15.3) |
| Gender (male) | 52% |
| Race | |
| White | 72.2% |
| African-American | 18.7% |
| Asian | 5.3% |
| Native American | 1.4% |
| Unknown | 0.1% |
| Other | 2.3% |
| Primary cause of ESRD (diabetes, hypertension, glomerulonephritis or other) | 45, 22, 9 or 24% |
| Height (cm) | 168.7 (10.3) |
| Weight (kg) | 72.7 (17.4) |
| Body surface area (m 2 ) | 1.82 (0.23) |
| Body mass index (kg/m 2 ) | 25.5 (5.4) |
| Duration of ESRD prior to the study start (days) | 67.7 (22.4) |
| History of diabetes | 51% |
| History of coronary artery disease | 35% |
| History of congestive heart failure | 32% |
| History of left ventricular hypertrophy | 33% |
| History of cerebrovascular disease | 10% |
| History of peripheral vascular disease | 19% |
| Cause of death | |
| Cardiovascular disease | 50.4% |
| Cerebrovascular disease | 6.6% |
| Malignancy | 3.0% |
| Infection | 18.0% |
| Other | 22.0% |
| Systolic blood pressure (mmHg) | 141.7 (20.3) |
| Diastolic blood pressure (mmHg) | 79.9 (12.0) |
| Number of anti-hypertensive medications | 1.6 (1.2) |
| Haemoglobin (g/dl) | 10.7 (1.8) |
| Serum albumin (g/dl) | 3.41 (0.57) |
| Serum calcium (mg/dl) | 8.72 (1.47) |
| Serum phosphate (mg/dl) | 5.51 (1.75) |
| Serum calcium × phosphate product | 47.64 (16.06) |
| Serum bicarbonate (mmol/l) | 23.4 (4.7) |
| Total cholesterol (mg/dl) (for mmol/l multiply by 0.0259) | 208 (59) |
| Percent of patients in cholesterol category ≤125 mg/dl | 4.3% |
| Percent of patients in cholesterol category 126–175 mg/dl | 25.1% |
| Percent of patients in cholesterol category 176–225 mg/dl (reference) | 38.0% |
| Percent of patients in cholesterol category 226–275 mg/dl | 23.0% |
| Percent of patients in cholesterol category ≥275 mg/dl | 9.7% |
| Total triglycerides (mg/dl) (for mmol/l multiply by 0.0113) | 212 (163) |
| Percent of patients in triglyceride category ≤100 mg/dl | 15.4% |
| Percent of patients in triglyceride category 101–200 mg/dl | 45.4% |
| Percent of patients in triglyceride category 201–300 mg/dl (reference) | 23.7% |
| Percent of patients in triglyceride category 301–400 mg/dl | 8.1% |
| Percent of patients in triglyceride category ≥401 mg/dl | 7.5% |
| Taking one and two different lipid-modifying medications | 13%, <1% |
| Residual kidney creatinine clearance (ml/min) | 6.9 (4.3) |
| D/P creatinine ratio in 24 h dialysate collection | 0.66 (0.17) |
| D/P creatinine ratio in 4 h dialysate collection (estimated) | 0.70 (0.19) |
| Dialysis clearance of creatinine (l/week) | 45.39 (13.01) |
| Volume drained (l/24 h) | 10.01 (2.65) |
| Dialysate volume (l/24 h) | 9.19 (2.34) |
| Age (years) | 57.2 (15.3) |
| Gender (male) | 52% |
| Race | |
| White | 72.2% |
| African-American | 18.7% |
| Asian | 5.3% |
| Native American | 1.4% |
| Unknown | 0.1% |
| Other | 2.3% |
| Primary cause of ESRD (diabetes, hypertension, glomerulonephritis or other) | 45, 22, 9 or 24% |
| Height (cm) | 168.7 (10.3) |
| Weight (kg) | 72.7 (17.4) |
| Body surface area (m 2 ) | 1.82 (0.23) |
| Body mass index (kg/m 2 ) | 25.5 (5.4) |
| Duration of ESRD prior to the study start (days) | 67.7 (22.4) |
| History of diabetes | 51% |
| History of coronary artery disease | 35% |
| History of congestive heart failure | 32% |
| History of left ventricular hypertrophy | 33% |
| History of cerebrovascular disease | 10% |
| History of peripheral vascular disease | 19% |
| Cause of death | |
| Cardiovascular disease | 50.4% |
| Cerebrovascular disease | 6.6% |
| Malignancy | 3.0% |
| Infection | 18.0% |
| Other | 22.0% |
| Systolic blood pressure (mmHg) | 141.7 (20.3) |
| Diastolic blood pressure (mmHg) | 79.9 (12.0) |
| Number of anti-hypertensive medications | 1.6 (1.2) |
| Haemoglobin (g/dl) | 10.7 (1.8) |
| Serum albumin (g/dl) | 3.41 (0.57) |
| Serum calcium (mg/dl) | 8.72 (1.47) |
| Serum phosphate (mg/dl) | 5.51 (1.75) |
| Serum calcium × phosphate product | 47.64 (16.06) |
| Serum bicarbonate (mmol/l) | 23.4 (4.7) |
| Total cholesterol (mg/dl) (for mmol/l multiply by 0.0259) | 208 (59) |
| Percent of patients in cholesterol category ≤125 mg/dl | 4.3% |
| Percent of patients in cholesterol category 126–175 mg/dl | 25.1% |
| Percent of patients in cholesterol category 176–225 mg/dl (reference) | 38.0% |
| Percent of patients in cholesterol category 226–275 mg/dl | 23.0% |
| Percent of patients in cholesterol category ≥275 mg/dl | 9.7% |
| Total triglycerides (mg/dl) (for mmol/l multiply by 0.0113) | 212 (163) |
| Percent of patients in triglyceride category ≤100 mg/dl | 15.4% |
| Percent of patients in triglyceride category 101–200 mg/dl | 45.4% |
| Percent of patients in triglyceride category 201–300 mg/dl (reference) | 23.7% |
| Percent of patients in triglyceride category 301–400 mg/dl | 8.1% |
| Percent of patients in triglyceride category ≥401 mg/dl | 7.5% |
| Taking one and two different lipid-modifying medications | 13%, <1% |
| Residual kidney creatinine clearance (ml/min) | 6.9 (4.3) |
| D/P creatinine ratio in 24 h dialysate collection | 0.66 (0.17) |
| D/P creatinine ratio in 4 h dialysate collection (estimated) | 0.70 (0.19) |
| Dialysis clearance of creatinine (l/week) | 45.39 (13.01) |
| Volume drained (l/24 h) | 10.01 (2.65) |
| Dialysate volume (l/24 h) | 9.19 (2.34) |
a Continuous variables are presented as means (SD).
Baseline characteristics of the study population a
| Age (years) | 57.2 (15.3) |
| Gender (male) | 52% |
| Race | |
| White | 72.2% |
| African-American | 18.7% |
| Asian | 5.3% |
| Native American | 1.4% |
| Unknown | 0.1% |
| Other | 2.3% |
| Primary cause of ESRD (diabetes, hypertension, glomerulonephritis or other) | 45, 22, 9 or 24% |
| Height (cm) | 168.7 (10.3) |
| Weight (kg) | 72.7 (17.4) |
| Body surface area (m 2 ) | 1.82 (0.23) |
| Body mass index (kg/m 2 ) | 25.5 (5.4) |
| Duration of ESRD prior to the study start (days) | 67.7 (22.4) |
| History of diabetes | 51% |
| History of coronary artery disease | 35% |
| History of congestive heart failure | 32% |
| History of left ventricular hypertrophy | 33% |
| History of cerebrovascular disease | 10% |
| History of peripheral vascular disease | 19% |
| Cause of death | |
| Cardiovascular disease | 50.4% |
| Cerebrovascular disease | 6.6% |
| Malignancy | 3.0% |
| Infection | 18.0% |
| Other | 22.0% |
| Systolic blood pressure (mmHg) | 141.7 (20.3) |
| Diastolic blood pressure (mmHg) | 79.9 (12.0) |
| Number of anti-hypertensive medications | 1.6 (1.2) |
| Haemoglobin (g/dl) | 10.7 (1.8) |
| Serum albumin (g/dl) | 3.41 (0.57) |
| Serum calcium (mg/dl) | 8.72 (1.47) |
| Serum phosphate (mg/dl) | 5.51 (1.75) |
| Serum calcium × phosphate product | 47.64 (16.06) |
| Serum bicarbonate (mmol/l) | 23.4 (4.7) |
| Total cholesterol (mg/dl) (for mmol/l multiply by 0.0259) | 208 (59) |
| Percent of patients in cholesterol category ≤125 mg/dl | 4.3% |
| Percent of patients in cholesterol category 126–175 mg/dl | 25.1% |
| Percent of patients in cholesterol category 176–225 mg/dl (reference) | 38.0% |
| Percent of patients in cholesterol category 226–275 mg/dl | 23.0% |
| Percent of patients in cholesterol category ≥275 mg/dl | 9.7% |
| Total triglycerides (mg/dl) (for mmol/l multiply by 0.0113) | 212 (163) |
| Percent of patients in triglyceride category ≤100 mg/dl | 15.4% |
| Percent of patients in triglyceride category 101–200 mg/dl | 45.4% |
| Percent of patients in triglyceride category 201–300 mg/dl (reference) | 23.7% |
| Percent of patients in triglyceride category 301–400 mg/dl | 8.1% |
| Percent of patients in triglyceride category ≥401 mg/dl | 7.5% |
| Taking one and two different lipid-modifying medications | 13%, <1% |
| Residual kidney creatinine clearance (ml/min) | 6.9 (4.3) |
| D/P creatinine ratio in 24 h dialysate collection | 0.66 (0.17) |
| D/P creatinine ratio in 4 h dialysate collection (estimated) | 0.70 (0.19) |
| Dialysis clearance of creatinine (l/week) | 45.39 (13.01) |
| Volume drained (l/24 h) | 10.01 (2.65) |
| Dialysate volume (l/24 h) | 9.19 (2.34) |
| Age (years) | 57.2 (15.3) |
| Gender (male) | 52% |
| Race | |
| White | 72.2% |
| African-American | 18.7% |
| Asian | 5.3% |
| Native American | 1.4% |
| Unknown | 0.1% |
| Other | 2.3% |
| Primary cause of ESRD (diabetes, hypertension, glomerulonephritis or other) | 45, 22, 9 or 24% |
| Height (cm) | 168.7 (10.3) |
| Weight (kg) | 72.7 (17.4) |
| Body surface area (m 2 ) | 1.82 (0.23) |
| Body mass index (kg/m 2 ) | 25.5 (5.4) |
| Duration of ESRD prior to the study start (days) | 67.7 (22.4) |
| History of diabetes | 51% |
| History of coronary artery disease | 35% |
| History of congestive heart failure | 32% |
| History of left ventricular hypertrophy | 33% |
| History of cerebrovascular disease | 10% |
| History of peripheral vascular disease | 19% |
| Cause of death | |
| Cardiovascular disease | 50.4% |
| Cerebrovascular disease | 6.6% |
| Malignancy | 3.0% |
| Infection | 18.0% |
| Other | 22.0% |
| Systolic blood pressure (mmHg) | 141.7 (20.3) |
| Diastolic blood pressure (mmHg) | 79.9 (12.0) |
| Number of anti-hypertensive medications | 1.6 (1.2) |
| Haemoglobin (g/dl) | 10.7 (1.8) |
| Serum albumin (g/dl) | 3.41 (0.57) |
| Serum calcium (mg/dl) | 8.72 (1.47) |
| Serum phosphate (mg/dl) | 5.51 (1.75) |
| Serum calcium × phosphate product | 47.64 (16.06) |
| Serum bicarbonate (mmol/l) | 23.4 (4.7) |
| Total cholesterol (mg/dl) (for mmol/l multiply by 0.0259) | 208 (59) |
| Percent of patients in cholesterol category ≤125 mg/dl | 4.3% |
| Percent of patients in cholesterol category 126–175 mg/dl | 25.1% |
| Percent of patients in cholesterol category 176–225 mg/dl (reference) | 38.0% |
| Percent of patients in cholesterol category 226–275 mg/dl | 23.0% |
| Percent of patients in cholesterol category ≥275 mg/dl | 9.7% |
| Total triglycerides (mg/dl) (for mmol/l multiply by 0.0113) | 212 (163) |
| Percent of patients in triglyceride category ≤100 mg/dl | 15.4% |
| Percent of patients in triglyceride category 101–200 mg/dl | 45.4% |
| Percent of patients in triglyceride category 201–300 mg/dl (reference) | 23.7% |
| Percent of patients in triglyceride category 301–400 mg/dl | 8.1% |
| Percent of patients in triglyceride category ≥401 mg/dl | 7.5% |
| Taking one and two different lipid-modifying medications | 13%, <1% |
| Residual kidney creatinine clearance (ml/min) | 6.9 (4.3) |
| D/P creatinine ratio in 24 h dialysate collection | 0.66 (0.17) |
| D/P creatinine ratio in 4 h dialysate collection (estimated) | 0.70 (0.19) |
| Dialysis clearance of creatinine (l/week) | 45.39 (13.01) |
| Volume drained (l/24 h) | 10.01 (2.65) |
| Dialysate volume (l/24 h) | 9.19 (2.34) |
a Continuous variables are presented as means (SD).
Kaplan–Meier analysis of the patient survival by cholesterol categories ( A ) and triglyceride categories ( B ). For TC multiply by 0.0259 and for triglycerides multiply by 0.0113 for conversion to mmol/l.
The results of Cox modelling of total cholesterol (mg/dl) a
| . | n . | HR . | 95% CI . | P . |
|---|---|---|---|---|
| Entire patient group, all-cause mortality | ||||
| Total cholesterol ≤125 | 45 | 1.94 | 1.31–2.89 | <0.001 |
| Total cholesterol 126–175 | 264 | 1.06 | 0.83–1.35 | 0.64 |
| Total cholesterol 176–225 | 400 | 1 | Reference | Reference |
| Total cholesterol 226–275 | 242 | 1.13 | 0.88–1.47 | 0.33 |
| Total cholesterol ≥275 | 102 | 1.33 | 0.94–1.88 | 0.11 |
| Entire patient group, cardiovascular mortality | ||||
| Total cholesterol ≤125 | 45 | 1.49 | 0.87–2.57 | 0.15 |
| Total cholesterol 126–175 | 264 | 0.99 | 0.74–1.34 | 0.95 |
| Total cholesterol 176–225 | 400 | 1 | Reference | Reference |
| Total cholesterol 226–275 | 242 | 1.17 | 0.86–1.60 | 0.32 |
| Total cholesterol ≥275 | 102 | 1.25 | 0.81–1.91 | 0.32 |
| Patients with albumin >3 g/dl, all-cause mortality | ||||
| Total cholesterol ≤125 | 28 | 2.15 | 1.29–3.60 | <0.005 |
| Total cholesterol 126–175 | 220 | 0.96 | 0.72–1.28 | 0.80 |
| Total cholesterol 176–225 | 328 | 1 | Reference | Reference |
| Total cholesterol 226–275 | 204 | 1.04 | 0.78–1.40 | 0.77 |
| Total cholesterol ≥275 | 79 | 1.04 | 0.68–1.58 | 0.86 |
| Patients with albumin ≤3 g/dl, all–cause mortality | ||||
| Total cholesterol ≤125 | 17 | 1.98 | 0.96–4.09 | 0.07 |
| Total cholesterol 126–175 | 44 | 1.59 | 0.91–2.78 | 0.10 |
| Total cholesterol 176–225 | 72 | 1 | Reference | Reference |
| Total cholesterol 226–275 | 38 | 1.16 | 0.61–2.20 | 0.65 |
| Total cholesterol ≥275 | 23 | 2.59 | 1.19–5.61 | <0.05 |
| Patients taking lipid-modifying medication, all-cause mortality | ||||
| Total cholesterol ≤125 | 6 | 7.90 | 1.73–36.02 | <0.01 |
| Total cholesterol 126–175 | 25 | 0.66 | 0.25–1.73 | 0.40 |
| Total cholesterol 176–225 | 53 | 1 | Reference | Reference |
| Total cholesterol 226–275 | 40 | 1.17 | 0.47–2.93 | 0.74 |
| Total cholesterol ≥275 | 19 | 0.83 | 0.25–2.72 | 0.76 |
| Patients not taking lipid-modifying medication, all-cause mortality | ||||
| Total cholesterol ≤125 | 41 | 1.81 | 1.18–2.76 | <0.01 |
| Total cholesterol 126–175 | 239 | 1.09 | 0.84–1.41 | 0.54 |
| Total cholesterol 176–225 | 345 | 1 | Reference | Reference |
| Total cholesterol 226–275 | 202 | 1.21 | 0.92–1.60 | 0.17 |
| Total cholesterol ≥275 | 83 | 1.42 | 0.97–2.07 | 0.07 |
| . | n . | HR . | 95% CI . | P . |
|---|---|---|---|---|
| Entire patient group, all-cause mortality | ||||
| Total cholesterol ≤125 | 45 | 1.94 | 1.31–2.89 | <0.001 |
| Total cholesterol 126–175 | 264 | 1.06 | 0.83–1.35 | 0.64 |
| Total cholesterol 176–225 | 400 | 1 | Reference | Reference |
| Total cholesterol 226–275 | 242 | 1.13 | 0.88–1.47 | 0.33 |
| Total cholesterol ≥275 | 102 | 1.33 | 0.94–1.88 | 0.11 |
| Entire patient group, cardiovascular mortality | ||||
| Total cholesterol ≤125 | 45 | 1.49 | 0.87–2.57 | 0.15 |
| Total cholesterol 126–175 | 264 | 0.99 | 0.74–1.34 | 0.95 |
| Total cholesterol 176–225 | 400 | 1 | Reference | Reference |
| Total cholesterol 226–275 | 242 | 1.17 | 0.86–1.60 | 0.32 |
| Total cholesterol ≥275 | 102 | 1.25 | 0.81–1.91 | 0.32 |
| Patients with albumin >3 g/dl, all-cause mortality | ||||
| Total cholesterol ≤125 | 28 | 2.15 | 1.29–3.60 | <0.005 |
| Total cholesterol 126–175 | 220 | 0.96 | 0.72–1.28 | 0.80 |
| Total cholesterol 176–225 | 328 | 1 | Reference | Reference |
| Total cholesterol 226–275 | 204 | 1.04 | 0.78–1.40 | 0.77 |
| Total cholesterol ≥275 | 79 | 1.04 | 0.68–1.58 | 0.86 |
| Patients with albumin ≤3 g/dl, all–cause mortality | ||||
| Total cholesterol ≤125 | 17 | 1.98 | 0.96–4.09 | 0.07 |
| Total cholesterol 126–175 | 44 | 1.59 | 0.91–2.78 | 0.10 |
| Total cholesterol 176–225 | 72 | 1 | Reference | Reference |
| Total cholesterol 226–275 | 38 | 1.16 | 0.61–2.20 | 0.65 |
| Total cholesterol ≥275 | 23 | 2.59 | 1.19–5.61 | <0.05 |
| Patients taking lipid-modifying medication, all-cause mortality | ||||
| Total cholesterol ≤125 | 6 | 7.90 | 1.73–36.02 | <0.01 |
| Total cholesterol 126–175 | 25 | 0.66 | 0.25–1.73 | 0.40 |
| Total cholesterol 176–225 | 53 | 1 | Reference | Reference |
| Total cholesterol 226–275 | 40 | 1.17 | 0.47–2.93 | 0.74 |
| Total cholesterol ≥275 | 19 | 0.83 | 0.25–2.72 | 0.76 |
| Patients not taking lipid-modifying medication, all-cause mortality | ||||
| Total cholesterol ≤125 | 41 | 1.81 | 1.18–2.76 | <0.01 |
| Total cholesterol 126–175 | 239 | 1.09 | 0.84–1.41 | 0.54 |
| Total cholesterol 176–225 | 345 | 1 | Reference | Reference |
| Total cholesterol 226–275 | 202 | 1.21 | 0.92–1.60 | 0.17 |
| Total cholesterol ≥275 | 83 | 1.42 | 0.97–2.07 | 0.07 |
a Only primary variables of interest are indicated here. For total cholesterol in mmol/l multiply by 0.0259. The Cox models were also adjusted for the following covariates: age, gender, race, weight, height, primary cause of ESRD (diabetes, hypertension, glomerulonephritis or other), haemoglobin, serum albumin, serum calcium–phosphate product, serum bicarbonate, residual kidney creatinine clearance, PD parameters (dialysate effluent volume, dialysis creatinine clearance, D/P creatinine ratio after a 4 h dwell), use of lipid-modifying medications and comorbidity characteristics (diabetes mellitus, CAD, CHF, LVH, CVD and PVD).
The results of Cox modelling of total cholesterol (mg/dl) a
| . | n . | HR . | 95% CI . | P . |
|---|---|---|---|---|
| Entire patient group, all-cause mortality | ||||
| Total cholesterol ≤125 | 45 | 1.94 | 1.31–2.89 | <0.001 |
| Total cholesterol 126–175 | 264 | 1.06 | 0.83–1.35 | 0.64 |
| Total cholesterol 176–225 | 400 | 1 | Reference | Reference |
| Total cholesterol 226–275 | 242 | 1.13 | 0.88–1.47 | 0.33 |
| Total cholesterol ≥275 | 102 | 1.33 | 0.94–1.88 | 0.11 |
| Entire patient group, cardiovascular mortality | ||||
| Total cholesterol ≤125 | 45 | 1.49 | 0.87–2.57 | 0.15 |
| Total cholesterol 126–175 | 264 | 0.99 | 0.74–1.34 | 0.95 |
| Total cholesterol 176–225 | 400 | 1 | Reference | Reference |
| Total cholesterol 226–275 | 242 | 1.17 | 0.86–1.60 | 0.32 |
| Total cholesterol ≥275 | 102 | 1.25 | 0.81–1.91 | 0.32 |
| Patients with albumin >3 g/dl, all-cause mortality | ||||
| Total cholesterol ≤125 | 28 | 2.15 | 1.29–3.60 | <0.005 |
| Total cholesterol 126–175 | 220 | 0.96 | 0.72–1.28 | 0.80 |
| Total cholesterol 176–225 | 328 | 1 | Reference | Reference |
| Total cholesterol 226–275 | 204 | 1.04 | 0.78–1.40 | 0.77 |
| Total cholesterol ≥275 | 79 | 1.04 | 0.68–1.58 | 0.86 |
| Patients with albumin ≤3 g/dl, all–cause mortality | ||||
| Total cholesterol ≤125 | 17 | 1.98 | 0.96–4.09 | 0.07 |
| Total cholesterol 126–175 | 44 | 1.59 | 0.91–2.78 | 0.10 |
| Total cholesterol 176–225 | 72 | 1 | Reference | Reference |
| Total cholesterol 226–275 | 38 | 1.16 | 0.61–2.20 | 0.65 |
| Total cholesterol ≥275 | 23 | 2.59 | 1.19–5.61 | <0.05 |
| Patients taking lipid-modifying medication, all-cause mortality | ||||
| Total cholesterol ≤125 | 6 | 7.90 | 1.73–36.02 | <0.01 |
| Total cholesterol 126–175 | 25 | 0.66 | 0.25–1.73 | 0.40 |
| Total cholesterol 176–225 | 53 | 1 | Reference | Reference |
| Total cholesterol 226–275 | 40 | 1.17 | 0.47–2.93 | 0.74 |
| Total cholesterol ≥275 | 19 | 0.83 | 0.25–2.72 | 0.76 |
| Patients not taking lipid-modifying medication, all-cause mortality | ||||
| Total cholesterol ≤125 | 41 | 1.81 | 1.18–2.76 | <0.01 |
| Total cholesterol 126–175 | 239 | 1.09 | 0.84–1.41 | 0.54 |
| Total cholesterol 176–225 | 345 | 1 | Reference | Reference |
| Total cholesterol 226–275 | 202 | 1.21 | 0.92–1.60 | 0.17 |
| Total cholesterol ≥275 | 83 | 1.42 | 0.97–2.07 | 0.07 |
| . | n . | HR . | 95% CI . | P . |
|---|---|---|---|---|
| Entire patient group, all-cause mortality | ||||
| Total cholesterol ≤125 | 45 | 1.94 | 1.31–2.89 | <0.001 |
| Total cholesterol 126–175 | 264 | 1.06 | 0.83–1.35 | 0.64 |
| Total cholesterol 176–225 | 400 | 1 | Reference | Reference |
| Total cholesterol 226–275 | 242 | 1.13 | 0.88–1.47 | 0.33 |
| Total cholesterol ≥275 | 102 | 1.33 | 0.94–1.88 | 0.11 |
| Entire patient group, cardiovascular mortality | ||||
| Total cholesterol ≤125 | 45 | 1.49 | 0.87–2.57 | 0.15 |
| Total cholesterol 126–175 | 264 | 0.99 | 0.74–1.34 | 0.95 |
| Total cholesterol 176–225 | 400 | 1 | Reference | Reference |
| Total cholesterol 226–275 | 242 | 1.17 | 0.86–1.60 | 0.32 |
| Total cholesterol ≥275 | 102 | 1.25 | 0.81–1.91 | 0.32 |
| Patients with albumin >3 g/dl, all-cause mortality | ||||
| Total cholesterol ≤125 | 28 | 2.15 | 1.29–3.60 | <0.005 |
| Total cholesterol 126–175 | 220 | 0.96 | 0.72–1.28 | 0.80 |
| Total cholesterol 176–225 | 328 | 1 | Reference | Reference |
| Total cholesterol 226–275 | 204 | 1.04 | 0.78–1.40 | 0.77 |
| Total cholesterol ≥275 | 79 | 1.04 | 0.68–1.58 | 0.86 |
| Patients with albumin ≤3 g/dl, all–cause mortality | ||||
| Total cholesterol ≤125 | 17 | 1.98 | 0.96–4.09 | 0.07 |
| Total cholesterol 126–175 | 44 | 1.59 | 0.91–2.78 | 0.10 |
| Total cholesterol 176–225 | 72 | 1 | Reference | Reference |
| Total cholesterol 226–275 | 38 | 1.16 | 0.61–2.20 | 0.65 |
| Total cholesterol ≥275 | 23 | 2.59 | 1.19–5.61 | <0.05 |
| Patients taking lipid-modifying medication, all-cause mortality | ||||
| Total cholesterol ≤125 | 6 | 7.90 | 1.73–36.02 | <0.01 |
| Total cholesterol 126–175 | 25 | 0.66 | 0.25–1.73 | 0.40 |
| Total cholesterol 176–225 | 53 | 1 | Reference | Reference |
| Total cholesterol 226–275 | 40 | 1.17 | 0.47–2.93 | 0.74 |
| Total cholesterol ≥275 | 19 | 0.83 | 0.25–2.72 | 0.76 |
| Patients not taking lipid-modifying medication, all-cause mortality | ||||
| Total cholesterol ≤125 | 41 | 1.81 | 1.18–2.76 | <0.01 |
| Total cholesterol 126–175 | 239 | 1.09 | 0.84–1.41 | 0.54 |
| Total cholesterol 176–225 | 345 | 1 | Reference | Reference |
| Total cholesterol 226–275 | 202 | 1.21 | 0.92–1.60 | 0.17 |
| Total cholesterol ≥275 | 83 | 1.42 | 0.97–2.07 | 0.07 |
a Only primary variables of interest are indicated here. For total cholesterol in mmol/l multiply by 0.0259. The Cox models were also adjusted for the following covariates: age, gender, race, weight, height, primary cause of ESRD (diabetes, hypertension, glomerulonephritis or other), haemoglobin, serum albumin, serum calcium–phosphate product, serum bicarbonate, residual kidney creatinine clearance, PD parameters (dialysate effluent volume, dialysis creatinine clearance, D/P creatinine ratio after a 4 h dwell), use of lipid-modifying medications and comorbidity characteristics (diabetes mellitus, CAD, CHF, LVH, CVD and PVD).
Cox model HR of patient mortality. For TC in mmol/l multiply by 0.0259. ( A ) All-cause and CV mortality stratified by TC; ( B ), all-cause mortality stratified by TC and albumin level and ( C ) all-cause mortality stratified by TC and use of lipid-lowering medications.
The results of Cox modelling of triglycerides (mg/dl) a
| . | n . | HR . | 95% CI . | P . |
|---|---|---|---|---|
| Entire patient group, all-cause mortality | ||||
| Triglycerides ≤100 | 162 | 0.81 | 0.59–1.11 | 0.19 |
| Triglycerides 101–200 | 478 | 0.74 | 0.58–0.94 | <0.05 |
| Triglycerides 201–300 | 249 | 1 | Reference | Reference |
| Triglycerides 301–400 | 85 | 0.93 | 0.64–1.35 | 0.70 |
| Triglycerides ≥401 | 79 | 1.10 | 0.75–1.61 | 0.63 |
| Entire patient group, CV mortality | ||||
| Triglycerides ≤100 | 162 | 0.88 | 0.59–1.30 | 0.51 |
| Triglycerides 101–200 | 478 | 0.86 | 0.63–1.16 | 0.31 |
| Triglycerides 201–300 | 249 | 1 | Reference | Reference |
| Triglycerides 301–400 | 85 | 0.93 | 0.58–1.50 | 0.76 |
| Triglycerides ≥401 | 79 | 1.24 | 0.78–1.97 | 0.37 |
| Patients with albumin >3 g/dl, all-cause mortality | ||||
| Triglycerides ≤100 | 124 | 0.85 | 0.59–1.23 | 0.40 |
| Triglycerides 101–200 | 385 | 0.77 | 0.58–1.02 | 0.07 |
| Triglycerides 201–300 | 209 | 1 | Reference | Reference |
| Triglycerides 301–400 | 73 | 0.88 | 0.58–1.34 | 0.55 |
| Triglycerides ≥401 | 68 | 1.36 | 0.89–2.08 | 0.15 |
| Patients with albumin ≤3 g/dl, all-cause mortality | ||||
| Triglycerides ≤100 | 38 | 0.42 | 0.20–0.87 | <0.05 |
| Triglycerides 101–200 | 93 | 0.47 | 0.27–0.81 | <0.01 |
| Triglycerides 201–300 | 40 | 1 | Reference | Reference |
| Triglycerides 301–400 | 12 | 0.99 | 0.40–2.46 | 0.98 |
| Triglycerides ≥401 | 11 | 0.47 | 0.15–1.47 | 0.20 |
| Patients taking lipid-modifying medication, all cause mortality | ||||
| Triglycerides ≤100 | 18 | 0.40 | 0.10–1.54 | 0.18 |
| Triglycerides 101–200 | 57 | 0.31 | 0.12–0.75 | 0.01 |
| Triglycerides 201–300 | 34 | 1 | Reference | Reference |
| Triglycerides 301–400 | 16 | 0.36 | 0.11–1.21 | 0.01 |
| Triglycerides ≥401 | 18 | 0.64 | 0.20–2.04 | 0.45 |
| Patients not taking lipid-modifying medication, all-cause mortality | ||||
| Triglycerides ≤100 | 144 | 0.84 | 0.60–1.17 | 0.30 |
| Triglycerides 101–200 | 421 | 0.76 | 0.59–0.99 | <0.05 |
| Triglycerides 201–300 | 215 | 1 | Reference | Reference |
| Triglycerides 301–400 | 69 | 1.11 | 0.75–1.66 | 0.60 |
| Triglycerides ≥401 | 61 | 1.14 | 0.75–1.74 | 0.55 |
| . | n . | HR . | 95% CI . | P . |
|---|---|---|---|---|
| Entire patient group, all-cause mortality | ||||
| Triglycerides ≤100 | 162 | 0.81 | 0.59–1.11 | 0.19 |
| Triglycerides 101–200 | 478 | 0.74 | 0.58–0.94 | <0.05 |
| Triglycerides 201–300 | 249 | 1 | Reference | Reference |
| Triglycerides 301–400 | 85 | 0.93 | 0.64–1.35 | 0.70 |
| Triglycerides ≥401 | 79 | 1.10 | 0.75–1.61 | 0.63 |
| Entire patient group, CV mortality | ||||
| Triglycerides ≤100 | 162 | 0.88 | 0.59–1.30 | 0.51 |
| Triglycerides 101–200 | 478 | 0.86 | 0.63–1.16 | 0.31 |
| Triglycerides 201–300 | 249 | 1 | Reference | Reference |
| Triglycerides 301–400 | 85 | 0.93 | 0.58–1.50 | 0.76 |
| Triglycerides ≥401 | 79 | 1.24 | 0.78–1.97 | 0.37 |
| Patients with albumin >3 g/dl, all-cause mortality | ||||
| Triglycerides ≤100 | 124 | 0.85 | 0.59–1.23 | 0.40 |
| Triglycerides 101–200 | 385 | 0.77 | 0.58–1.02 | 0.07 |
| Triglycerides 201–300 | 209 | 1 | Reference | Reference |
| Triglycerides 301–400 | 73 | 0.88 | 0.58–1.34 | 0.55 |
| Triglycerides ≥401 | 68 | 1.36 | 0.89–2.08 | 0.15 |
| Patients with albumin ≤3 g/dl, all-cause mortality | ||||
| Triglycerides ≤100 | 38 | 0.42 | 0.20–0.87 | <0.05 |
| Triglycerides 101–200 | 93 | 0.47 | 0.27–0.81 | <0.01 |
| Triglycerides 201–300 | 40 | 1 | Reference | Reference |
| Triglycerides 301–400 | 12 | 0.99 | 0.40–2.46 | 0.98 |
| Triglycerides ≥401 | 11 | 0.47 | 0.15–1.47 | 0.20 |
| Patients taking lipid-modifying medication, all cause mortality | ||||
| Triglycerides ≤100 | 18 | 0.40 | 0.10–1.54 | 0.18 |
| Triglycerides 101–200 | 57 | 0.31 | 0.12–0.75 | 0.01 |
| Triglycerides 201–300 | 34 | 1 | Reference | Reference |
| Triglycerides 301–400 | 16 | 0.36 | 0.11–1.21 | 0.01 |
| Triglycerides ≥401 | 18 | 0.64 | 0.20–2.04 | 0.45 |
| Patients not taking lipid-modifying medication, all-cause mortality | ||||
| Triglycerides ≤100 | 144 | 0.84 | 0.60–1.17 | 0.30 |
| Triglycerides 101–200 | 421 | 0.76 | 0.59–0.99 | <0.05 |
| Triglycerides 201–300 | 215 | 1 | Reference | Reference |
| Triglycerides 301–400 | 69 | 1.11 | 0.75–1.66 | 0.60 |
| Triglycerides ≥401 | 61 | 1.14 | 0.75–1.74 | 0.55 |
a Only primary variables of interest are indicated here. For triglycerides in mmol/l multiply by 0.0113. The Cox models were also adjusted for the following covariates: age, gender, race, weight, height, primary cause of ESRD (diabetes, hypertension, glomerulonephritis or other), haemoglobin, serum albumin, serum calcium–phosphate product, serum bicarbonate, residual kidney creatinine clearance, PD parameters (dialysate effluent volume, dialysis creatinine clearance, D/P creatinine ratio after a 4 h dwell), use of lipid-modifying medications and comorbidity characteristics (diabetes mellitus, CAD, CHF, LVH, CVD and PVD).
The results of Cox modelling of triglycerides (mg/dl) a
| . | n . | HR . | 95% CI . | P . |
|---|---|---|---|---|
| Entire patient group, all-cause mortality | ||||
| Triglycerides ≤100 | 162 | 0.81 | 0.59–1.11 | 0.19 |
| Triglycerides 101–200 | 478 | 0.74 | 0.58–0.94 | <0.05 |
| Triglycerides 201–300 | 249 | 1 | Reference | Reference |
| Triglycerides 301–400 | 85 | 0.93 | 0.64–1.35 | 0.70 |
| Triglycerides ≥401 | 79 | 1.10 | 0.75–1.61 | 0.63 |
| Entire patient group, CV mortality | ||||
| Triglycerides ≤100 | 162 | 0.88 | 0.59–1.30 | 0.51 |
| Triglycerides 101–200 | 478 | 0.86 | 0.63–1.16 | 0.31 |
| Triglycerides 201–300 | 249 | 1 | Reference | Reference |
| Triglycerides 301–400 | 85 | 0.93 | 0.58–1.50 | 0.76 |
| Triglycerides ≥401 | 79 | 1.24 | 0.78–1.97 | 0.37 |
| Patients with albumin >3 g/dl, all-cause mortality | ||||
| Triglycerides ≤100 | 124 | 0.85 | 0.59–1.23 | 0.40 |
| Triglycerides 101–200 | 385 | 0.77 | 0.58–1.02 | 0.07 |
| Triglycerides 201–300 | 209 | 1 | Reference | Reference |
| Triglycerides 301–400 | 73 | 0.88 | 0.58–1.34 | 0.55 |
| Triglycerides ≥401 | 68 | 1.36 | 0.89–2.08 | 0.15 |
| Patients with albumin ≤3 g/dl, all-cause mortality | ||||
| Triglycerides ≤100 | 38 | 0.42 | 0.20–0.87 | <0.05 |
| Triglycerides 101–200 | 93 | 0.47 | 0.27–0.81 | <0.01 |
| Triglycerides 201–300 | 40 | 1 | Reference | Reference |
| Triglycerides 301–400 | 12 | 0.99 | 0.40–2.46 | 0.98 |
| Triglycerides ≥401 | 11 | 0.47 | 0.15–1.47 | 0.20 |
| Patients taking lipid-modifying medication, all cause mortality | ||||
| Triglycerides ≤100 | 18 | 0.40 | 0.10–1.54 | 0.18 |
| Triglycerides 101–200 | 57 | 0.31 | 0.12–0.75 | 0.01 |
| Triglycerides 201–300 | 34 | 1 | Reference | Reference |
| Triglycerides 301–400 | 16 | 0.36 | 0.11–1.21 | 0.01 |
| Triglycerides ≥401 | 18 | 0.64 | 0.20–2.04 | 0.45 |
| Patients not taking lipid-modifying medication, all-cause mortality | ||||
| Triglycerides ≤100 | 144 | 0.84 | 0.60–1.17 | 0.30 |
| Triglycerides 101–200 | 421 | 0.76 | 0.59–0.99 | <0.05 |
| Triglycerides 201–300 | 215 | 1 | Reference | Reference |
| Triglycerides 301–400 | 69 | 1.11 | 0.75–1.66 | 0.60 |
| Triglycerides ≥401 | 61 | 1.14 | 0.75–1.74 | 0.55 |
| . | n . | HR . | 95% CI . | P . |
|---|---|---|---|---|
| Entire patient group, all-cause mortality | ||||
| Triglycerides ≤100 | 162 | 0.81 | 0.59–1.11 | 0.19 |
| Triglycerides 101–200 | 478 | 0.74 | 0.58–0.94 | <0.05 |
| Triglycerides 201–300 | 249 | 1 | Reference | Reference |
| Triglycerides 301–400 | 85 | 0.93 | 0.64–1.35 | 0.70 |
| Triglycerides ≥401 | 79 | 1.10 | 0.75–1.61 | 0.63 |
| Entire patient group, CV mortality | ||||
| Triglycerides ≤100 | 162 | 0.88 | 0.59–1.30 | 0.51 |
| Triglycerides 101–200 | 478 | 0.86 | 0.63–1.16 | 0.31 |
| Triglycerides 201–300 | 249 | 1 | Reference | Reference |
| Triglycerides 301–400 | 85 | 0.93 | 0.58–1.50 | 0.76 |
| Triglycerides ≥401 | 79 | 1.24 | 0.78–1.97 | 0.37 |
| Patients with albumin >3 g/dl, all-cause mortality | ||||
| Triglycerides ≤100 | 124 | 0.85 | 0.59–1.23 | 0.40 |
| Triglycerides 101–200 | 385 | 0.77 | 0.58–1.02 | 0.07 |
| Triglycerides 201–300 | 209 | 1 | Reference | Reference |
| Triglycerides 301–400 | 73 | 0.88 | 0.58–1.34 | 0.55 |
| Triglycerides ≥401 | 68 | 1.36 | 0.89–2.08 | 0.15 |
| Patients with albumin ≤3 g/dl, all-cause mortality | ||||
| Triglycerides ≤100 | 38 | 0.42 | 0.20–0.87 | <0.05 |
| Triglycerides 101–200 | 93 | 0.47 | 0.27–0.81 | <0.01 |
| Triglycerides 201–300 | 40 | 1 | Reference | Reference |
| Triglycerides 301–400 | 12 | 0.99 | 0.40–2.46 | 0.98 |
| Triglycerides ≥401 | 11 | 0.47 | 0.15–1.47 | 0.20 |
| Patients taking lipid-modifying medication, all cause mortality | ||||
| Triglycerides ≤100 | 18 | 0.40 | 0.10–1.54 | 0.18 |
| Triglycerides 101–200 | 57 | 0.31 | 0.12–0.75 | 0.01 |
| Triglycerides 201–300 | 34 | 1 | Reference | Reference |
| Triglycerides 301–400 | 16 | 0.36 | 0.11–1.21 | 0.01 |
| Triglycerides ≥401 | 18 | 0.64 | 0.20–2.04 | 0.45 |
| Patients not taking lipid-modifying medication, all-cause mortality | ||||
| Triglycerides ≤100 | 144 | 0.84 | 0.60–1.17 | 0.30 |
| Triglycerides 101–200 | 421 | 0.76 | 0.59–0.99 | <0.05 |
| Triglycerides 201–300 | 215 | 1 | Reference | Reference |
| Triglycerides 301–400 | 69 | 1.11 | 0.75–1.66 | 0.60 |
| Triglycerides ≥401 | 61 | 1.14 | 0.75–1.74 | 0.55 |
a Only primary variables of interest are indicated here. For triglycerides in mmol/l multiply by 0.0113. The Cox models were also adjusted for the following covariates: age, gender, race, weight, height, primary cause of ESRD (diabetes, hypertension, glomerulonephritis or other), haemoglobin, serum albumin, serum calcium–phosphate product, serum bicarbonate, residual kidney creatinine clearance, PD parameters (dialysate effluent volume, dialysis creatinine clearance, D/P creatinine ratio after a 4 h dwell), use of lipid-modifying medications and comorbidity characteristics (diabetes mellitus, CAD, CHF, LVH, CVD and PVD).
Cox model HR of patient mortality. For triglycerides in mmol/l multiply by 0.0113. ( A ) All-cause and CV mortality stratified by triglycerides; ( B ), all-cause mortality stratified by triglycerides and albumin level and ( C ) all-cause mortality stratified by triglycerides and use of lipid-lowering medications.
Acknowledgements
This study was supported in part by the Dialysis Research Foundation (Ogden, UT). The data reported herein were provided by the USRDS. The interpretation and reporting of these data are the responsibility of the authors and in no way should be considered as official policy or interpretation of the US Government.
Conflict of interest statement . None declared.
References
Foley RN, Parfrey PS, Sarnak MJ. (
Port FK, Hulbert-Shearon TE, Wolfe RA, Bloembergen WE, Golper TA, Agodoa LY, Young EW. (
Zager PG, Nikolic J, Brown RH, et al. (
Nishizawa Y, Shoji T, Ishimura E, Inaba M, Morii H. (
Iseki K, Yamazato M, Tozawa M, Takishita S. (
Fleischmann EH, Bower JD, Salahudeen AK. (
Liu Y, Coresh J, Eustace JA, et al. (
Kalantar-Zadeh K, Kilpatrick RD, McAllister CJ, Greenland S, Kopple JD. (
Wanner C, Krane V, Marz W, et al. (
Fytili CI, Progia EG, Panagoutsos SA, et al. (
Quaschning T, Krane V, Metzger T, Wanner C. (
Coresh J, Longenecker JC, Miller ER 3rd, Young HJ, Klag MJ. (
Lowrie EG and Lew NL. (
Kronenberg F, Lingenhel A, Neyer U, et al. (
O'Riordan E, O'Donoghue DJ, Kalra PA, Foley RN, Waldek S. (
Twardowski ZJ, Nolph KD, Prowant BF, Ryan L, Moore HL, Nielsen MP. (
Dupont W. (
Greenberg JA. (
Avram MM, Goldwasser P, Burrell DE, Antignani A, Fein P, Mittman N. (
Grundy SM, Cleeman JI, Merz CN, et al. (
Fox CS, Longenecker JC, Powe NR, et al. (
Wanner C, Krane V, Marz W, et al. (
Kalantar-Zadeh K and Anker SD. (
Ravnskov U. (
Kaysen GA, Dubin JA, Muller HG, Rosales L, Levin NW, Mitch WE. (
Lim WH, Johnson DW, McDonald SP. (
Wanner C. (
Johansson AC. (
Liberopoulos EN, Papavasiliou E, Miltiadous GA, et al. (
Cressman MD, Hoogwerf BJ, Schreiber MJ, Cosentino FA. (
Tschope W, Koch M, Thomas B, Ritz E. (
Austin MA. (
Austin MA, McKnight B, Edwards KL, et al. (





Comments