-
PDF
- Split View
-
Views
-
Cite
Cite
Boguslaw Tomanek, Umar Iqbal, Barbara Blasiak, Abedelnasser Abulrob, Homam Albaghdadi, John R. Matyas, Dragana Ponjevic, Garnette R. Sutherland, Evaluation of brain tumor vessels specific contrast agents for glioblastoma imaging, Neuro-Oncology, Volume 14, Issue 1, January 2012, Pages 53–63, https://doi.org/10.1093/neuonc/nor183
Close - Share Icon Share
Abstract
A mouse model of glioblastoma multiforme was used to determine the accumulation of a targeted contrast agent in tumor vessels. The contrast agent, consisting of superparamagnetic iron oxide coated with dextran, was functionalized with an anti-insulin-like-growth-factor binding protein 7 (anti-IGFBP7) single domain antibody. The near infrared marker, Cy5.5, was also attached for an in vivo fluorescence study. A 9.4T magnetic resonance imaging (MRI) system was used for in vivo studies on days 10 and 11 following tumor inoculation. T2 relaxation time was used to measure the accumulation of the contrast agent in the tumor. Changes in tumor to brain contrast because of active targeting were compared with a nontargeted contrast agent. Effective targeting was confirmed with near infrared measurements and fluorescent microscopic analysis. The results showed that there was a statistically significant (P < .01) difference in normalized T2 between healthy brain and tumor tissue 10 min, 1 h, and 2 h point postinjection of the anti-IGFBP7 single domain antibody targeted and nontargeted iron oxide nanoparticles. A statistical difference remained in animals treated with targeted nanoparticles 24 h postinjection only. The MRI, near infrared imaging, and fluorescent microscopy studies showed corresponding spatial and temporal changes. We concluded that the developed anti-IGFBP7-iron oxide single domain antibody-targeted MRI contrast agent selectively binds to abnormal vessels within a glioblastoma. T2-weighted MRI and near infrared imaging are able to detect the targeting effects in brain tumors.
Among primary brain tumors, glioblastoma multiforme is among the most common and most difficult to treat.1,2 The mean survival rate is only 9 months following diagnosis and has not increased over the past several decades.3,4 Relatively poor prognosis of theses tumors can be attributed to late tumor detection and limited response to conventional therapies. Glioblastoma multiforme is heterogeneous, invades adjacent brain parenchyma, and travels down normal brain connections. Furthermore, the tumor has a leaky blood-brain barrier (BBB) and releases vasomodulatory cytokines and several matrix metalloproteinases.5,6 These factors, among others, contribute to poorly defined tumor margins, brain edema, and considerable difficulty obtaining a gross tumor resection, inevitably resulting in tumor recurrence.7 Thus, early and efficient diagnosis, together with the ability to follow developing therapeutic strategies, is necessary to improve outcome.
Traditional diagnostic imaging techniques, such as CT, positron emission tomography, or conventional magnetic resonance imaging (MRI), including T1, T2-weighted and gadolinium-enhanced studies, have been used in glioma diagnosis and monitoring.8,9 These imaging techniques, however, are unable to detect small portions of tumor, in particular that portion which invades the normal brain or individual tumor cells travelling down fiber tracks.7 Furthermore, although anatomical MRI provides some information about disease localization, tissue histology is required for molecular characterization, including the expression of receptors that could be targeted by new pharmaceuticals.
Recently introduced molecular MRI10 uses cell-specific proteins for targeted contrast agents, which may be comprised of superparamagnetic nanoparticles (NP) binding to specific cell sites by biologically active targeting moieties.11 The application of this technique was possible because of the recent advances in nanotechnology that allow reproducible production of NPs with controlled physical and chemical properties including size, shape, and surface properties.12–17 Inherent high magnetic moments of NPs and their small size allow for efficient magnetic manipulation, enabling synthesis with biological objects, such as antibodies,18 viruses,19 liposomes,20 or macrophages.21 These contrast agents reduce T2 relaxation time of targeted cells,22,23 enabling detection with MRI. Both large magnetic moments of NPs and frequency of cell-specific protein expression are important characteristics allowing sufficient MR signal changes for detection using MRI.
The most frequently used paramagnetic NPs in molecular MRI are based on iron oxide.24–26 These particles alone can be used for localization of abnormal lymph nodes because they are selectively taken up by the reticulo-endothelial system and by macrophages.27,28 Iron oxide contrast agents can also be used to assess inflammatory processes and drug delivery.29,30 After systemic application, circulating NPs are preferentially phagocytosed by monocytes before clearance within the reticuloendothelial system of the liver, spleen, and lymph nodes. Small superparamagnetic iron oxide NPs have been used to detect liver lesions,31,32 and ultra small NPs have been used to image the vasculature and vessel-rich cancers with MRI because of their long circulation in the bloodstream.33
To develop a method for gliomoblastoma detection, the diagnostic efficacy of a new contrast agent constructed from an iron oxide NP functionalized with single-domain antibodies to target antigen-expressing brain tumor vessels was evaluated. The target chosen was the insulin-like growth factor binding protein 7 (IGFBP7), which is a selective and abundantly expressed biomarker of human GBM vessels. IGFBP7 is a 31 kDa secreted protein that shares structural homology with a family of IGFBP-related proteins that includes IGFBP1-6.34 Several studies demonstrated that IGFBP7 is over-expressed in tumor blood vessels, with little or no expression in normal blood vessels.35–37 VEGFR-238 and αvβ3 integrin39 are also highly expressed targets on tumor endothelial cells that have been exploited for molecular imaging. In contrast to IGFBP7, however, the expression of VEGFR is heterogenous depending on the size of the tumor,40 and the expression of αvβ3 integrin is also heterogenous because it is also associated with nontumor angiogenic vessels and tumor cells.41 IGFBP7 expression appears to be highly selective for tumor vessels only with a homogenous pattern of expression.42 This approach required development of conjugation of cell-specific single domain antibodies to NPs. Because the diagnostic efficacy of molecular imaging can be enhanced by application of multimodal contrast agents,43 the paramagnetic core of the contrast agent was also bound to an infrared marker to produce a multifunctional particle. The efficacy of the contrast agent was evaluated using MR and infrared imaging in an animal model of glioblastoma. Histology and fluorescence microscopy were performed to confirm accumulation of the contrast agent in the tumor.
Materials and Methods
Selection of NPs
Commercially available iron oxide NPs were used (Nanotech-Ocean). The NP consists of the Fe3O4 core of mean diameter of 20 nm embedded in a dextran matrix.44
Dynamic Light Scattering Measurements
Dynamic light scattering of 20 nm core nontargeted iron oxide NPs, and anti-IGFBP7 single domain antibody-targeted iron oxide NPs was performed using a Zetasizer Nano (Malvern Instruments). These experiments were performed at 23°C on samples dispersed (1:100 v/v) in milliQ water (Millipore). Size distribution was determined by intensity-weighted data output.
Pharmacokinetics
A total of 0.2 mL of the Cy5.5 labeled NP (nontargeted) was injected via the tail vein in normal CD-1 mice; 50 µL volume blood samples were collected in heparinized tubes by creating a small nick in the tail vein. Collection was performed over a period of 24 h (i.e., 10 min, 30 min, 1 h, 2 h, 4 h, and 24 h). Samples were then analyzed for Cy5.5 labeled NPs using a fluorescent plate reader with an excitation wavelength of 670 nm and an emission wavelength of 690 nm, and quantified using a standard curve of known Cy5.5 labeled NPs concentrations (mg Fe/mL) in whole blood. Pharmacokinetic parameters were calculated using the 5.2 version WinNonlin software package (Pharsight). A 2-compartment IV-Bolus model was selected for pharmacokinetic modeling because it best represented the actual data. This model is described by the following equation: C(t) = A exp (−αt) + B exp (−βt), where C(t) represents the agent's concentration in serum. A and B represent the zero time intercept of the alpha phase and beta phase, respectively, α and β are disposition rate constants, and α > β. The area under the serum concentration-time curve was calculated with the equation AUC0−∞ = D/V/K10, where D is dose given, V is the apparent distribution volume and K10 is the elimination rate constant. Total clearance was determined from the equation Cl/F = D/AUC0−∞.
Tumor Cell Preparation
The U87MGdEGFRvIII cell line, which overexpresses the EGFR type III variant and is highly malignant, was derived from a human tumor known to express high levels of VEGF and EGFR.45,46 The EGFR is highly expressed in approximately half of patients with glioblastoma. The cell line for the study was generously provided by Dr. W.L. Cavenee (Ludwig Institute for Cancer Research). U87MG cells were cultured in a Dulbecco's Modified Eagle's Medium solution supplemented with 10% fetal calf serum and maintained in a humidified 5% CO2 atmosphere at 37°C. Cells were harvested by trypsinization in ethylenediaminetetraacetic acid/trypsin, washed in phosphate-buffered saline, and centrifuged 3 times at 200G. Viability was assessed using a 0.4% trypan blue exclusion test. After cell density was determined, cells were brought into suspension at a final concentration of 5 × 104/3 μL. The solution was kept on ice until injection. It was agitated gently at room temperature to ensure homogeneity prior to taking up in the syringe. Then 3 μL injection was done slowly while retracting the syringe needle back up the injection track.
Brain Tumor Model
Animal procedures were performed according to a protocol approved by a local animal care committee. Nude male CD-1 mice, 4 to 6 weeks of age, were obtained from Charles River Laboratories. The animals were housed in cages in groups of 3, maintained on a 12 h light/dark schedule with a temperature of 22°C and a relative humidity of 50 ± 5%. Food and water were available ad libitum.
The mice were anesthetized by intraperitoneal injection of a mixture of ketamine (8–120 mg/kg) and xylzine (6 mg/kg) and placed in a stereotactic head frame. Tumor cells were inoculated using procedures described previously.47 The scalp was shaved and swabbed with iodine and alcohol. The skin was incised and a 0.18-mm diameter hole was drilled in the skull. Approximately 5 × 104 U87MGdEGFRvIII glioma cells, suspended in a total volume of 5 μL, were injected into the frontal lobe of each mouse with a chromatography syringe at a depth of 2.5–3 mm, 1 mm anterior, and 1.8 mm lateral to the bregma using a Kopf stereotactic apparatus (Kopf Instruments). Subsequently, the bony calvarium was sealed by a droplet of bone wax to prevent reflux and the skin was sutured. After the surgery, animals were allowed to recover from the anesthesia and were placed in the cages.
Bioconjugation of Nanoparticles with Single-Domain Antibody
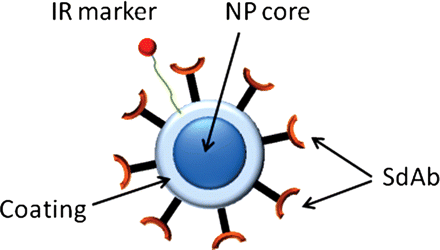
The contrast agent used in the study is composed of an iron oxide NP core, coated with dextran, labeled with Cy5.5, and functionalized with single domain antibody (Fig. 1). The near infrared dye, Cy5.5-hydrazide (GE Healthcare) was first conjugated to free COOH on the NPs. To achieve this, 0.2 mL of NPs was mixed with 0.2 mL of activation buffer (Ocean Nanotech) and 25 μg of Cy5.5-hydrazide. Immediately, 100 μL of a 4 mg/mL solution of EDC in activation buffer was added, and the mixture was flushed with nitrogen gas. The reaction was incubated at room temperature for 2 h, and the free unreacted dye was purified using Amicon 10k columns (Millipore). Bioconjugation of the single domain antibody to the iron oxide NP was done according to the manufacturer instructions (Ocean Nanotech). In brief, 0.2 mL of NPs (1 mg of iron) was mixed with 0.4 mL of activation buffer (Ocean Nanotech). To this mixture, 100 μL EDC (3 mg/mL in activation buffer; Sigma) and 100 μL of Sulfo-NHS (2 mg/mL in activation buffer; Fischer) was added and incubated at RT for 30 min; 2 mL of coupling buffer (Ocean Nanotech) was the added, followed by 1 mg of single domain antibody protein in 500 μL and reacted at room temperature for 2 h. Unbound protein was purified using an Amicon 50K column (Millipore), and bioconjugated NPs were resuspended in a wash buffer. To estimate the number of single domain antibody per nanoparticle, protein concentration measurements (A280 nm) were compared between the single domain antibody targeted and nontargeted NPs. The difference in protein concentration was then used to estimate the number of single domain antibodies bound relative to the number of NPs present in the solution (as specified by the manufacturer).
A schematic representation of the targeted contrast agent. The contrast agent consists of the superparamagnetic Fe3O4 core of mean diameter of 20 nm embedded in a dextran coating (NantoTech Ocean, USA). The core is labelled with infra-red marker (Cy 5.5.) and functionalized with anti-IGFPB7 single domain antibodies.
Animal MRI
While T2-weighted MRI was used to quantify the accumulation of targeted and nontargeted NPs, gradient echo (GE)-based sequences provide better contrast-to-noise ratio when superparamagnetic contrast agents49 are used. However, it should be noted that GE-based pulse sequences provide better contrast-to-noise ratio (CNR), but they are system dependent as they are sensitive to susceptibility effects, magnet shimming, and eddy currents caused by switching gradients. These effects increase with the strength of the magnetic field and therefore were not used for quantification in our studies at 9.4T. However, considering possible applications of the proposed contrast agents at the lower, clinically relevant fields (e.g., 1.5T and 3T), we have also collected a MRI using a GE flow compensation (GE FC) method50 with the following parameters: FOV = 2 × 2 cm, slice thickness 1 mm, TR = 50 ms, TE = 7 ms, 50 kHz bandwidth, 15 degree flip angle, matrix size 128 × 128. The same animal setup was used as for T2-weighted MRI.
In Vivo Near-Infrared Fluorescence Imaging
For near infrared imaging, a separate set of intracranial tumor-bearing mice was used. The mice were anesthetized with 1.5% isoflurane administered with a face mask. Anti-IGFBP7 single domain antibody-targeted and nontargeted iron oxide NPs labeled with Cy5.5 were administered via the tail vein. Mice (n = 5 per group) were imaged using small animal time-domain eXplore Optix preclinical imager MX2 (Advanced Research Technologies) at time intervals (10 min, 2 h, and 24 h) after NPs injections. In all imaging experiments, a 670-nm pulsed laser diode with a repetition frequency of 80 MHz and a time resolution of 12 ps was used for excitation. The fluorescence emission at 700 nm was collected by a highly sensitive time-correlated single photon counting system and detected through a fast photomultiplier tube offset by 3 mm for diffuse optical topography reconstruction. Each animal was positioned prone on a plate that was then placed on a heated base (36°C) in the imaging system. A 2-dimensional scanning region encompassing the head was selected via a top-reviewing real-time digital camera. The optimal elevation of the animal was verified via a side viewing digital camera. The animal was then moved into the imaging chamber where a laser excitation beam controlled by galvomirrors was moved over the selected region of interest. Laser power and counting time per pixel were optimized at 30 μW and 0.5 s, respectively, and these values were maintained constant during the entire experiment. The raster scan interval of 1 mm was held constant during the acquisition of each frame; 1024 points were scanned for each region of interest. The data were recorded as temporal point-spread functions, and the images were reconstructed as fluorescence concentration maps. Explore Optix OptiView software program (Advanced Research Technologies) was used to estimate fluorescence intensity and for the reconstruction of topography and optical sectioning. At the end of the experiment, mice bearing intracranial U87MG.EGFRvIII brain tumors were i.v. injected with 40 µg of fluorescein-labeled tomato lectin 10 min prior to being sacrificed, to stain the brain vessels for further analysis using fluorescent microscopy. All animals were then perfused with heparanized saline, and organs were excised and imaged ex vivo using the eXplore Optix preclinical imager.
Fluorescence Microscopy
The brains and organs were fixed in paraformaldehyde overnight, after which coronal sections (50 µm) were produced using a Vibratome sectioning instrument (Ted Pella). Tissue sections were stored at 4°C in low molarity PBS with 0.1% NaN3. Tissue sections were mounted on Superfrost Plus microscope slides (Fisher Scientific) using mounting media containing 2 µg/mL Hoescht nuclei stain (Sigma). All sections were then visualized under an Olympus 1X 81 inverted motorized microscope (Olympus). InVivo and ImagePro 6.2 software (Olympus) were used to acquire and analyze images. The Cy 5.5 filter set used in the study had an excitation of 670 nm and emission of 700 nm (Olympus, Markham, ON, Canada).
Results
Characterization of the Iron Oxide NPs
Dynamic light scattering of the nontargeted iron oxide NP revealed an average hydrodynamic diameter of 33.24 nm (20 nm core plus dextran coating) with a polydispersity index (PdI) of 0.172. Bioconjugation of single domain antibody to the iron oxide NP increased the average hydrodynamic diameter to 42.23 nm with a PdI of 0.375. It was estimated that there were ∼20 sdAbs per NPs. In vivo pharmacokinetic analysis of Cy5.5 labeled NPs injected intravenously via the mouse tail followed by repeated blood sampling indicated an elimination half life of ∼7.5 h and an apparent volume of distribution (Vss) of 14 mL. The renal clearance of the nanoparticles was estimated to be 0.0242 mL/min. These pharmacokinetic data indicate that the dextran coated NP using in this study is cleared slowly over the course of 2 days.
MR Relaxation Measurements In Vivo
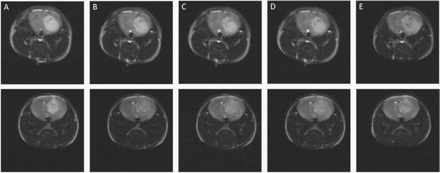
Representative T2-weighted MR images of the mouse tumor model using spin-echo (SE) pulse sequence prior to and after injection of targeted and nontargeted contrast agents are shown in Fig. 2. Figure 3 shows MR images prior to and after injection of targeted contrast agent obtained with GE FC pulse sequence for comparison with T2-weighted MRI.
In vivo T2-weighted MRI of a CD-1 nude brain tumor mouse model. (A) Before, (B) 10 min, (C) 1 h, (D) 2 h, and (E) 24 h post-injection of the targeted (top row) and nontargeted (bottom row) superparamagnetic Fe3O4 nanoparticles. A spin echo pulse sequence with the following parameters was used: TR = 5000 ms, TE = 60 ms, FOV = 2 × 2 cm, matrix size 128 × 128, and slice thickness of 1 mm.
In vivo 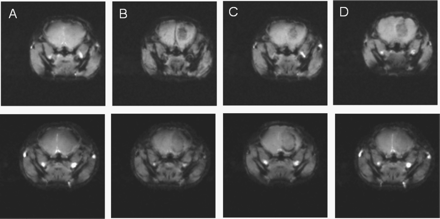 MRI of a CD-1 nude brain tumor mouse model. (A) Before, (B) 20 min, (C) 1 h, and (D) 24 h postinjection of targeted (top row) and nontargeted (bottom row) superparamagnetic Fe3O4 nanoparticles. A gradient echo flow compensation method was used with the following parameters: FOV = 2 × 2 cm, slice thickness 1 mm, TR = 50 ms, TE = 7 ms, 50 kHz bandwidth, 15 degree flip angle and matrix size 128 × 128.
MRI of a CD-1 nude brain tumor mouse model. (A) Before, (B) 20 min, (C) 1 h, and (D) 24 h postinjection of targeted (top row) and nontargeted (bottom row) superparamagnetic Fe3O4 nanoparticles. A gradient echo flow compensation method was used with the following parameters: FOV = 2 × 2 cm, slice thickness 1 mm, TR = 50 ms, TE = 7 ms, 50 kHz bandwidth, 15 degree flip angle and matrix size 128 × 128.
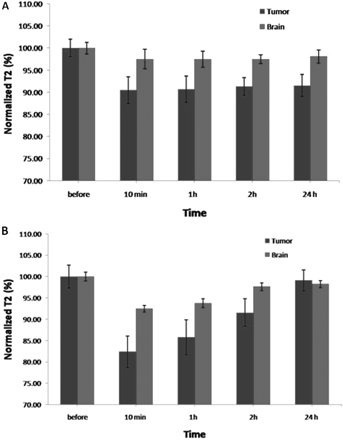
T2 relaxation values were measured using SE pulse sequence within the tumor and normal brain regions following injection of the targeted and nontargeted contrast agents. Normalized T2 was then calculated to accommodate the initial brain and tumor T2 variation among animals. Figure 4 shows changes in normalized T2 relaxation time in the tumor and normal contralateral brain postinjection of the targeted (Fig. 4A) and nontargeted (Fig. 4B) NPs.
Normalized T2 relaxation times of tumor and brain measured before and 10 min, 1 h, 2 h, and 24 h postinjection of (A) the targeted and (B) nontargeted NPs.
For the targeted NPs, normalized T2 of tumor decreased when compared with normalized T2 before injection at 10 min, 1 h, 2 h, and 24 h after injection by (9.5 ± 3.0%), (9.3 ± 3.1%), (8.6 ± 2.7%), and (8.5 ± 2.5%), respectively. For the normal brain, the respective values were (2.5 ± 2.2%), (2.5 ± 1.8%), (2.5 ± 1%), and (1.5 ± 1.0%). For the nontargeted NPs, normalized T2 of tumor decreased when compared to normalized T2 before injection at 10 min, 1 h, 2 h, and 24 h after injection by (17.6 ± 3.7%), (14.2 ± 4.1%), (8.5 ± 3.2%), and (0.8 ± 2.5%), respectively. For the normal brain, the respective values were (7.5 ± 0.8%), (6.3 ± 1%), (2.3 ± 0.9%), and (1.2 ± 0.8%). Normalized tumor T2 was statistically different between animals treated with targeted and nontargeted NPs 10 min (P < .025) and 24 h (P < .048) after injection. There was no significant difference 1 h (P < .157) and 2 h (P < .475) after injection. Normalized brain T2 was statistically different between animals treated with targeted and nontargeted NPs 10 min (P < .005) after injection. There was no significant difference 1 h (P < .018), 2 h (P < .447), and 24 h (P < .235) after injection.
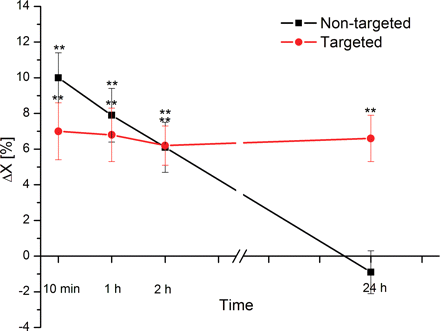
To determine accumulation of the contrast agent in both the tumor and the brain, the differences in normalized T2 relaxation of tumor and brain were calculated at each time point after injection and are provided in Fig. 5.
The difference in normalized T2 relaxation of tumor and brain calculated at time intervals postinjection of the targeted and nontargeted NPs.
For the nontargeted NPs, the difference between brain and tumor normalized T2 at 10 min, 1 h, 2 h, and 24 h after injection were (10.0 ± 1.4%), (7.9 ± 1.5%), (6.1 ± 1.4%), and (−0.9 ± 1.2%), respectively. For the targeted NPs, the corresponding values were (7.0 ± 1.6%), (6.8 ± 1.5%), (6.2 ± 1.1%), and (6.6 ± 1.3%).
The targeted NPs showed a statistically significant (P < .01) difference between brain and tumor normalized T2 at each time point after injection. The nontargeted NPs showed a statistically significant (P < .01) difference between normalized T2 of brain and tumor at 10 min, 1 h, and 2 h postinjection, but no significant difference 24 h (P > .12) postinjection.
Optical Imaging
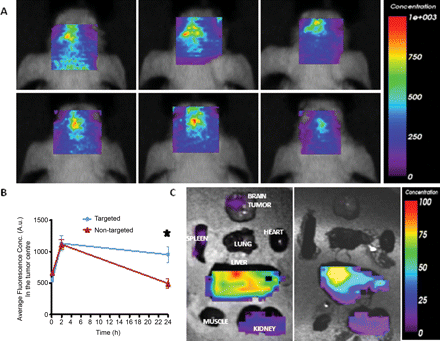
In vivo optical imaging showed an increase in the accumulation of both targeted and nontargeted contrast agent in the mouse brain tumor (Fig. 6A) between the 10 min and 2 h time points after intravenous injection. Quantification of the accumulation of the contrast agent in the brain is shown in Fig. 6B. At the 24 h time point, there was a statistically significant retention of the anti-IGFBP7 single domain antibody-targeted iron oxide NP (954 ± 223 A.U.) compared with nontargeted iron oxide NPs (494 ± 123 A.U.) in the brain tumor. The nontargeted NP signal demonstrated considerable clearance from the brain tumor region at this later time point.
Targeting of anti-IGFBP7sdAb-targeted Gd-sULVs in an orthotopic brain tumor model. (A) In vivo optical imaging of the head biodistribution of anti-IGFBP7 single domain antibody-targeted (top panels) and nontargeted (bottom panels) iron oxide-Cy5.5 NPs injected in mice bearing orthotopic glioblastoma tumors at various time points (10 min, 2 h, and 24 h). (B) Graph showing changes of the average fluorescence concentration in the brain tumor region in vivo at indicated times after the injection of either anti-IGFBP7 single domain antibody-targeted or nontargeted-NPs-Cy5.5. Data are expressed as mean ± SEM for n = 5 animals. *Indicates significant difference between anti-IGFBP7 single domain antibody-targeted and nontargeted-NP-Cy5.5 (P < .01). (C) Ex-vivo optical imaging of the organ biodistribution of anti-IGFBP7 single domain antibody-targeted (left panel) and nontargeted (right panel) NPs-Cy5.5 24 h after injection in mice bearing orthotopic glioblastoma tumors.
Ex vivo optical imaging comparing the distribution of the anti-IGFBP7 single domain antibody targeted NPs to the nontargeted NPs at 24 h indicated a similar organ distribution profile. The liver possessed the highest optical signal in the body, which suggests that this is the main route of elimination for both the targeted and nontargeted NPs. In the brain tumor, there was a detectable signal originating from the brain tumor for the targeted NP but not for the nontargeted NP. Saline perfusion of the animal appeared to wash out much of the nonspecific accumulation of the nontargeted NP seen in the in vivo optical imaging at 24 h. The targeted NP was retained within the tumor, presumably due to the antibody-antigen interaction at the level of the tumor vessel. The kidneys also possessed some optical signals, which is due to the clearance of the Cy5.5 dye from the body after of the NP in the liver. There was no nonspecific accumulation of either the targeted or nontargeted NPs in any other organs, which suggests a good toxicological profile for these NPs.
Fluorescence Microscopy
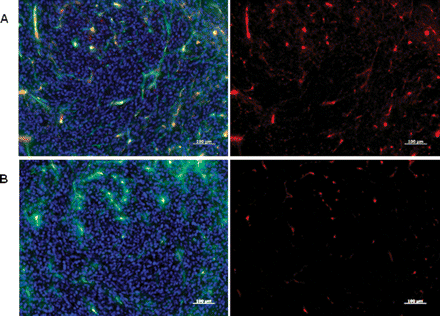
Fluorescence microscopy detected contrast agent accumulation within the brain tumor vessels (Fig. 7) in brain sections derived from tumor bearing mice 24 h after injection of both nontargeted and targeted NPs. There was no detectable presence of contrast agent within the healthy brain (i.e., contralateral area to the tumor; data not shown). The level of the Cy5.5 labeled targeted NP was higher and clearly localized to all sections of the tumor vessels, in contrast to the nontargeted NP, which displayed some fluorescent signals and patchy association to some tumor vessels. We anticipate that this result suggests that due to the small size of the dextran-coated iron oxide NP (30 nm), the NP may get trapped in tumor vessels, presumably in the areas where the blood-brain barrier is abnormal, as shown previously in a mouse model of glioblastoma.40
Fluorescent microscopic images of mouse glioblastoma multiforme tumor sections obtained 24 h after intravenous injection of anti-IGFBP7 single domain antibody-targeted (A) or nontargeted (B) iron oxide NPs-Cy5.5 (red). Images reveal the strong association of the targeted NP to tumor vessels compared to the nontargeted NP. Mice were also injected with 40 µg of FITC-labeled tomato lectin 10 minutes before sacrifice to stain blood vessels in vivo. Lectin staining (green) co-localizes with the NP-Cy5.5 signal (red) in overlay images to produce yellow-orange. Cell nuclei are stained with DAPI (blue). Scale bar: 100 μm.
Discussion
The iron oxide-based contrast agent, targeted using a single domain antibody against IGFBP7, has been evaluated in a brain tumor model in CD-1 nude mice. In vivo MRI of these tumors injected with the targeted contrast agent showed prolonged shortening of T2 relaxation within the tumor, unlike nontargeted contrast agent. Furthermore, single domain antibody attached to the iron oxide NPs selectively bind IGFBP7, a target expressed in abnormal blood vessels associated with glioblastoma multiforme.42 The accumulation of the contrast agent in the tumor was demonstrated with in vivo optical imaging and MRI, and its tumor vessel localization was confirmed with immunohistochemistry of brain sections.
We attribute the higher decrease in normalized T2 at earlier time points when nontargeted NPs were used to the differences in permeability of the brain-blood barrier (BBB) to the targeted and nontargeted NPs. The total size of the nontargeted NPs, measured with DLS, is ∼30 nm, which is small enough to pass through areas of leaky BBB, that is widespread in the glioma model used in this study.11 It appears, however, that the targeted NPs did not exhibit passive accumulation into the tumor (through leaky vasculature) as they first contact their target (IGFBP7) in tumor vessels and appear to stay bound to their target in these vessels for up to 24 h. Therefore, we anticipate that the nontargeted NPs enter the tumor through passive targeting causing first a strong T2 decrease (just after injection) but then clearing quickly, whereas the targeted NPs bind to the tumor vessels and remain bound.
The study also showed that iron oxide NPs can be successfully conjugated to targeting moieties for increased MRI specificity and to fluorescent molecules for bimodality. The synthesized contrast agent enables selective accumulation of the iron oxide NPs at sites of high target expression with sufficient density, allowing detection with MRI.
Although T2-weighted images were used in the study to ensure system-independent quantification of the results, other pulse sequences such as gradient echo or susceptibility weighted imaging could be applied. Indeed, as seen from the example in Fig. 3, imaging provides better CNR than T2-weighted pulse sequence. It is also worth to mentioning, that the tumor tissue is almost invisible in
MRI prior to injection of the targeted and nontargeted NPs. However, further studies are needed to find the optimal pulse sequence for superparamagnetic-based contrast agents of glioma MRI at the high field.
The MRI contrast efficacy of the iron oxide NPs may be improved by new synthesis methods. The iron oxide NPs may be coated, for example, with silica, enabling more efficient attachment of biomolecules and reducing toxicity.51 The silica-coated iron oxide NPs may be functionalized with hydroxyl or amine groups and then pegylated, slowing renal clearance and uptake by the reticuloendothelial system, thereby optimizing blood circulation half-life.52 Furthermore, magnetic moments of iron oxide NPs can be increased by enhancing the NP's core with Co, Pt, or other strong paramagnetic elements, which would further reduce T2 relaxation time, thereby improving MR contrast.53,54
Single domain antibody-targeted NPs were functionalized with the near infrared fluorescent probe Cy5.5 to render bimodal imaging contrast for in vivo MR and optical imaging. In vivo optical imaging demonstrates that IGFBP7-targeted iron oxide NPs tagged with Cy5.5 can selectively detect intracranial glioblastoma tumor vessels. Fluorescence microscopy confirmed the presence of IGFBP7 targeted iron oxide nanoparticles in the tumor vessels sections.
The results of the study suggest therapeutic implications. For example, such particles injected 24 h prior to surgical intervention may enhance visualization on preoperative MRI or intra-operative MRI. During dissection the surgeon could visualize fluorescing tumor vessels. This may be particularly important in vessel-rich tumor-brain interfaces potentially increasing the extent of tumor resection. Furthermore, as IGFBP7 is selectively expressed in glioblastoma multiforme vessels, the developed particles could provide diagnostic specificity and a predictive marker for anti-angiogenic treatment response.
In conclusion, this study has demonstrated that it is indeed possible to construct tumor vessel-specific bimodal nanoparticles capable of selectively binding to glioblastoma vessels in a mouse tumor model. The result is important in respect to not only the diagnosis and detection of tumor vessels but also possibly aiding surgical strategies and monitoring pharmaceutical therapeutic interventions. In addition, the principles learned in the development of such particles may be applied to other cancers and disease processes that have selective protein overexpression.
Conflict of interest statement. None declared.
Funding
The work was supported by the Canadian Institutes of Health Research Team Grant RMF-79031 and FP7-PEOPLE-IRSES-2008.