-
PDF
- Split View
-
Views
-
Cite
Cite
M. Gugatschka, H. Dobnig, A. Fahrleitner-Pammer, P. Pietschmann, S. Kudlacek, A. Strele, B. Obermayer-Pietsch, Molecularly-defined lactose malabsorption, milk consumption and anthropometric differences in adult males, QJM: An International Journal of Medicine, Volume 98, Issue 12, December 2005, Pages 857–863, https://doi.org/10.1093/qjmed/hci140
Close - Share Icon Share
Abstract
Background: Lactose malabsorption (LM) may be associated with reduced skeletal calcium content. Diagnosis to date has been based on indirect methods, with a high false-negative rate. Identification of the LCT polymorphism led to development of a PCR-based test.
Aim: To evaluate the PCR-based test compared to a combination the hydrogen breath test and the lactose tolerance test, and investigate anthropometrical differences, changes in bone mineral density and oral calcium intake according to LCT polymorphism and milk-drinking habits.
Methods: All participants (n = 278) underwent clinical examination, with measurement of height, weight and bone density (DXA), and were genotyped for LCT polymorphism (LCT CC or LCT TT: CC is associated with LM). A subgroup (n = 51) had a hydrogen breath test and a lactose tolerance test, in addition to genotyping.
Results: Detection of LM by LCT polymorphism was highly significant (p = 0.001). The correlation between LCT genotype and self-reported milk-intolerance or dislike of milk with was slight, but the correlation with functional tests was highly significant. Non-milk-drinkers were lighter (−5 kg) and significantly shorter (−4 cm) than milk-drinkers (p = 0.07 and 0.04, respectively). Total calcium consumption was lower among non-milk-drinkers by about 18% (p = 0.03).
Discussion: Genotyping is an economic, quick and convenient method for diagnosing lactose malabsorption, with results comparable to existing tests. Sufficient calcium consumption may be relevant to body growth, as milk-drinkers were taller. Negative calcium bone balance may be prevented when provision is made for adequate calcium intake.
Introduction
Lactose malabsorption (LM) is a world-wide autosomal recessive condition, characterized by decreased levels of intestinal lactase. The genetic background was unclear, until a single-nucleotide polymorphism (SNP) (C/T-dimorphism) on chromosome 2q21 (the LCT polymorphism) was reported by a Finnish group in 2002.1 Individuals homozygous for the C genotype (LCT CC) have almost undetectable levels of intestinal lactase production compared to LCT TC or LCT TT individuals, and variable symptoms of lactose malabsorption. These symptoms include abdominal pain, bloating, flatulence, diarrhoea or even extra-abdominal manifestations such as acne vulgaris or depression.2 The frequency of LM ranges from 2% in Scandinavia to 20–25% in Caucasian populations in Europe and the US, to about 80% in African-Americans and nearly 100% in South-East Asian populations.3
Conventional diagnostic methods for LM, such as the hydrogen breath test, have a 20% false-negative rate, due to hydrogen non-excretion. The diagnostic sensitivity of the lactose tolerance test is about the same.4
The present study investigated the correlations between a molecularly defined lactose malabsorption test based on the LCT polymorphism, a conventional hydrogen breath test and a lactose tolerance test. The relationships between the LCT polymorphism and clinical and biochemical signs of LM, and differences in skeletal parameters and milk-drinking habits in a group of middle-aged men, were also studied.
Methods
We invited 502 unrelated men from (i) a population-based study by the Austrian Study Group on Normative Values on Bone Metabolism,5(ii) individuals attending our out-patient clinic, to undergo an examination of bone metabolism and nutritional and constitutional factors; 278 responded. After application of the exclusion criteria (liver or kidney disease, primary hyperparathyroidism, long-term use of corticosteroids, other possible causes of secondary osteoporosis, consumption of bone-active medication, alcoholism), 228 men (mean age 56 ± 12 years) remained in the study cohort and were genotyped. All men were of Caucasian ethnicity. Clinical examination comprised measurements of body weight and height, as well as documentation of both parameters at the age of 20 years. In a second phase, 51 men (30 LCT CC and 21 LCT TT) were willing to undergo a second trial, including a conventional hydrogen breath test, as well as a lactose tolerance test. All patients gave their written informed consent. Study procedures were approved by the Ethics Committee of the Medical University of Graz, Austria.
Lifestyle factors
A questionnaire was used to estimate calcium intake from dairy and other food products in milligrams per week, smoking and drinking habits, any family history of osteoporosis, extent of current exercise, and current or former profession. All patients were asked whether they knew of lactose intolerance and whether they tolerated milk. Individuals who answered the latter question with ‘yes’ were classified as ‘milk-drinkers’.
Bone mass measurements
Bone mineral density (BMD, g/cm2) was measured at the lumbar spine (L1–L4) and the hip by dual-energy-X-ray absorptiometry (Hologic QDR 4000 plus), always by the same technician. The reference population of the computer software was based on the NHANES database. Osteoporosis was defined as a T score below −2.5 SD of the young mean.6
Hydrogen breath test
After an overnight fast, each patient ingested 50 g lactose dissolved in 200 ml water. End-expiratory hydrogen concentrations were measured at baseline and after 15, 30, 45, 60, 90, 150 and 180 min (technical equipment: Hzwei-Atemtest). Lactose malabsorption was diagnosed when the difference between breath hydrogen concentration at baseline and maximum exceeded 20 parts per million according to international standards (ppm).4
Lactose tolerance test
Parallel to the H2 breath test, serum blood glucose levels were measured at baseline and 30 minutes after ingestion of 50 g lactose. A rise in blood glucose of >18 mg/dl was chosen as the cut-off value for normal lactose digestion, indicating a non-pathological lactose tolerance test; lower levels indicated lactose malabsorption.4
Biochemical measurements
Blood samples were drawn using a Vacutainer system (Greiner) and the sera were aliquoted. Routine laboratory data were analysed on the same day, using enzymic tests on a Hitachi 917 autoanalyser (Roche); they included total serum calcium (normal range 2.20–2.65 mmol/l), phosphate (normal range 2.6–4.5 mg/dl), alkaline phosphatase (normal range 25–105 U/l), TSH (normal range 0.1–4.0 mU/l), fT3, fT4, serum creatinine and GGT. Blood samples for bone markers were stored at −20°C for later analysis and comprised 25(OH)vitamin D3 (normal range 19.0–58.0 ng/ml; Inkstar), osteocalcin (normal range 1–35 ng/ml; CIS-Bio-International) as well as serum C-terminal-linked telopeptides of type I collagen (CrossLaps, normal range 1400–4500 pmol/l, Osteometer).
Genotyping
Genomic DNA was extracted from samples of peripheral venous blood according to standard procedures. SNP genotyping was performed using a microplate fluorometer (Fluoroskan Ascent, Thermo Electron Corp.). Identification of C/T exchanges was done by an allele-specific polymerase chain reaction (PCR) as previously published10 (Amplifluor SNPs HT Genotyping System FAM-SR, Serologicals Corp.). The polymorphic site was amplified by using 5'-GTTCCTTTGAGGCCAGGGA-3' as specific primer for LCT-T and 5'-TTCCTTTGAGGCCAGGGG-3' as specific primer for LCT-C.
Statistics
All analyses used SPSS for the PC (release 12.0.1, 2003, SPSS Inc.). Data were tested for normality with the Kolmogorov-Smirnov test. Student's t-tests or Mann-Whitney U-tests were used to compare two groups; analysis of covariance calculated differences between relevant clinical variables in the three genotype groups. Categorical variables were analysed using Pearson's χ2 analysis. All tests were performed as two-sided using a significance level of p = 0.05.
Results
Distribution of the LCT genotypes was 27% CC, 55% TC and 18% TT. General anthropometric characteristics did not differ significantly among the three groups. Table 1 compares general and anthropometric data, BMD, biochemical measurements, amounts of calcium consumed, and frequencies of self-reported LM and dislike of milk according to LCT genotype.
General, anthropometric, biochemical and nutritional data by LCT genotype
| . | LCT genotype . | . | . | p . | ||
|---|---|---|---|---|---|---|
| . | TT (42) . | TC (124) . | CC (62) . | . | ||
| Age (years) | 57 ± 14 | 56 ± 11 | 56 ± 12 | 0.57 | ||
| Height (cm) | 177 ± 6.6 | 177 ± 6 | 176 ± 7.8 | 0.65 | ||
| Weight (kg) | 85.6 ± 13.1 | 82.8 ± 11.3 | 81.7 ± 12.2 | 0.25 | ||
| BMI(kg/m2) | 27.2 ± 3.5 | 26.6 ± 3.5 | 26.3 ± 3.5 | 0.49 | ||
| Self-reported lactose intolerance | 1 (2.4%) | 4 (3.6%) | 1 (1.6%) | 0.81 | ||
| Milk tolerance | 39 (95%) | 107 (87%) | 48 (79%) | 0.058 | ||
| Pathological breath test | 1 (4%) | – | 27 (90%) | 0.001 (χ2) | ||
| Pathological lactose tolerance test | 0 (0%) | – | 20 (66.7%) | 0.001 (χ2) | ||
| Total calcium intake (mg/day) | 710 ± 200 | 670 ± 240 | 700 ± 240 | 0.64 | ||
| Calcium from fresh milk (mg/day) | 23 ± 55 | 26 ± 70 | 18 ± 40 | 0.95 | ||
| Calcium from yoghurt (mg/day) | 56 ± 66 | 62 ± 60 | 6057 | 0.84 | ||
| CrossLaps (pmol/l) | 0.15 ± 0.1 | 0.15 ± 0.1 | 0.17 ± 0.1 | 0.88 | ||
| Osteocalcin (ng/ml) | 18.8 ± 7.3 | 21.4 ± 7.3 | 21.4 ± 7.3 | 0.16 | ||
| 25(OH) vitamin D3 (ng/ml) | 33.53 ± 10.2 | 34.4 ± 11.8 | 31.6 ± 12.6 | 0.34 | ||
| Spinal bone density(L1–L4) Z score | 0.119 ± 1.52 | −0.288 ± 1.32 | −0.170 ± 1.18 | 0.26 | ||
| Femoral bone density (total) Z score | 0.329 ± 0.90 | 0.294 ± 0.80 | 0.325 ± 0.78 | 0.99 | ||
| . | LCT genotype . | . | . | p . | ||
|---|---|---|---|---|---|---|
| . | TT (42) . | TC (124) . | CC (62) . | . | ||
| Age (years) | 57 ± 14 | 56 ± 11 | 56 ± 12 | 0.57 | ||
| Height (cm) | 177 ± 6.6 | 177 ± 6 | 176 ± 7.8 | 0.65 | ||
| Weight (kg) | 85.6 ± 13.1 | 82.8 ± 11.3 | 81.7 ± 12.2 | 0.25 | ||
| BMI(kg/m2) | 27.2 ± 3.5 | 26.6 ± 3.5 | 26.3 ± 3.5 | 0.49 | ||
| Self-reported lactose intolerance | 1 (2.4%) | 4 (3.6%) | 1 (1.6%) | 0.81 | ||
| Milk tolerance | 39 (95%) | 107 (87%) | 48 (79%) | 0.058 | ||
| Pathological breath test | 1 (4%) | – | 27 (90%) | 0.001 (χ2) | ||
| Pathological lactose tolerance test | 0 (0%) | – | 20 (66.7%) | 0.001 (χ2) | ||
| Total calcium intake (mg/day) | 710 ± 200 | 670 ± 240 | 700 ± 240 | 0.64 | ||
| Calcium from fresh milk (mg/day) | 23 ± 55 | 26 ± 70 | 18 ± 40 | 0.95 | ||
| Calcium from yoghurt (mg/day) | 56 ± 66 | 62 ± 60 | 6057 | 0.84 | ||
| CrossLaps (pmol/l) | 0.15 ± 0.1 | 0.15 ± 0.1 | 0.17 ± 0.1 | 0.88 | ||
| Osteocalcin (ng/ml) | 18.8 ± 7.3 | 21.4 ± 7.3 | 21.4 ± 7.3 | 0.16 | ||
| 25(OH) vitamin D3 (ng/ml) | 33.53 ± 10.2 | 34.4 ± 11.8 | 31.6 ± 12.6 | 0.34 | ||
| Spinal bone density(L1–L4) Z score | 0.119 ± 1.52 | −0.288 ± 1.32 | −0.170 ± 1.18 | 0.26 | ||
| Femoral bone density (total) Z score | 0.329 ± 0.90 | 0.294 ± 0.80 | 0.325 ± 0.78 | 0.99 | ||
Data are means ± SD, or numbers (%), as appropriate.
General, anthropometric, biochemical and nutritional data by LCT genotype
| . | LCT genotype . | . | . | p . | ||
|---|---|---|---|---|---|---|
| . | TT (42) . | TC (124) . | CC (62) . | . | ||
| Age (years) | 57 ± 14 | 56 ± 11 | 56 ± 12 | 0.57 | ||
| Height (cm) | 177 ± 6.6 | 177 ± 6 | 176 ± 7.8 | 0.65 | ||
| Weight (kg) | 85.6 ± 13.1 | 82.8 ± 11.3 | 81.7 ± 12.2 | 0.25 | ||
| BMI(kg/m2) | 27.2 ± 3.5 | 26.6 ± 3.5 | 26.3 ± 3.5 | 0.49 | ||
| Self-reported lactose intolerance | 1 (2.4%) | 4 (3.6%) | 1 (1.6%) | 0.81 | ||
| Milk tolerance | 39 (95%) | 107 (87%) | 48 (79%) | 0.058 | ||
| Pathological breath test | 1 (4%) | – | 27 (90%) | 0.001 (χ2) | ||
| Pathological lactose tolerance test | 0 (0%) | – | 20 (66.7%) | 0.001 (χ2) | ||
| Total calcium intake (mg/day) | 710 ± 200 | 670 ± 240 | 700 ± 240 | 0.64 | ||
| Calcium from fresh milk (mg/day) | 23 ± 55 | 26 ± 70 | 18 ± 40 | 0.95 | ||
| Calcium from yoghurt (mg/day) | 56 ± 66 | 62 ± 60 | 6057 | 0.84 | ||
| CrossLaps (pmol/l) | 0.15 ± 0.1 | 0.15 ± 0.1 | 0.17 ± 0.1 | 0.88 | ||
| Osteocalcin (ng/ml) | 18.8 ± 7.3 | 21.4 ± 7.3 | 21.4 ± 7.3 | 0.16 | ||
| 25(OH) vitamin D3 (ng/ml) | 33.53 ± 10.2 | 34.4 ± 11.8 | 31.6 ± 12.6 | 0.34 | ||
| Spinal bone density(L1–L4) Z score | 0.119 ± 1.52 | −0.288 ± 1.32 | −0.170 ± 1.18 | 0.26 | ||
| Femoral bone density (total) Z score | 0.329 ± 0.90 | 0.294 ± 0.80 | 0.325 ± 0.78 | 0.99 | ||
| . | LCT genotype . | . | . | p . | ||
|---|---|---|---|---|---|---|
| . | TT (42) . | TC (124) . | CC (62) . | . | ||
| Age (years) | 57 ± 14 | 56 ± 11 | 56 ± 12 | 0.57 | ||
| Height (cm) | 177 ± 6.6 | 177 ± 6 | 176 ± 7.8 | 0.65 | ||
| Weight (kg) | 85.6 ± 13.1 | 82.8 ± 11.3 | 81.7 ± 12.2 | 0.25 | ||
| BMI(kg/m2) | 27.2 ± 3.5 | 26.6 ± 3.5 | 26.3 ± 3.5 | 0.49 | ||
| Self-reported lactose intolerance | 1 (2.4%) | 4 (3.6%) | 1 (1.6%) | 0.81 | ||
| Milk tolerance | 39 (95%) | 107 (87%) | 48 (79%) | 0.058 | ||
| Pathological breath test | 1 (4%) | – | 27 (90%) | 0.001 (χ2) | ||
| Pathological lactose tolerance test | 0 (0%) | – | 20 (66.7%) | 0.001 (χ2) | ||
| Total calcium intake (mg/day) | 710 ± 200 | 670 ± 240 | 700 ± 240 | 0.64 | ||
| Calcium from fresh milk (mg/day) | 23 ± 55 | 26 ± 70 | 18 ± 40 | 0.95 | ||
| Calcium from yoghurt (mg/day) | 56 ± 66 | 62 ± 60 | 6057 | 0.84 | ||
| CrossLaps (pmol/l) | 0.15 ± 0.1 | 0.15 ± 0.1 | 0.17 ± 0.1 | 0.88 | ||
| Osteocalcin (ng/ml) | 18.8 ± 7.3 | 21.4 ± 7.3 | 21.4 ± 7.3 | 0.16 | ||
| 25(OH) vitamin D3 (ng/ml) | 33.53 ± 10.2 | 34.4 ± 11.8 | 31.6 ± 12.6 | 0.34 | ||
| Spinal bone density(L1–L4) Z score | 0.119 ± 1.52 | −0.288 ± 1.32 | −0.170 ± 1.18 | 0.26 | ||
| Femoral bone density (total) Z score | 0.329 ± 0.90 | 0.294 ± 0.80 | 0.325 ± 0.78 | 0.99 | ||
Data are means ± SD, or numbers (%), as appropriate.
Hydrogen breath test and lactose tolerance test
On the basis of their LCT genotype, 51 men (30 LCT CC, 21 LCT TT) underwent a conventional H2 breath test and a lactose tolerance test. The correlation between LCT genotype and a conventional hydrogen breath test was highly significant (χ2 analysis: r = 0.82; p = 0.001). Ninety percent of all LCT CC individuals had a positive breath test; 87% hadabdominal symptoms (p = 0.001). In contrast, only 4.8% of LCT TT individuals had a positive breath test, and none showed abdominal symptoms (p = 0.001). Out of all individuals with a positive hydrogen test, 96.4% were homozygous for LCT CC (Table 1).
LCT genotyping correlated highly significantly with changes in serum blood glucose concentrations (p = 0.001). Twenty-two patients (85%) carrying LCT CC did not demonstrate a significant rise in serum glucose levels, indicating pathology, while 16 LCT TT individuals (89%) showed a significant non-pathological increase of >18 mg/dl.
When both tests were taken together, in 29/30 (97%) patients homozygous for the C allele, LM was diagnosed with at least one of the tests. The same procedure applied to LCT TT individuals, detected 100% lactose-tolerant-subjects.
Milk drinking habits, anthropometric data and calcium intake
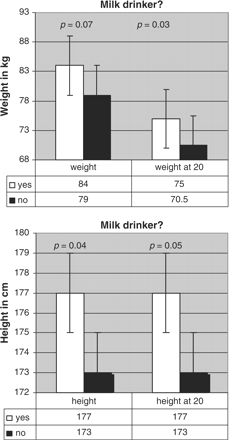
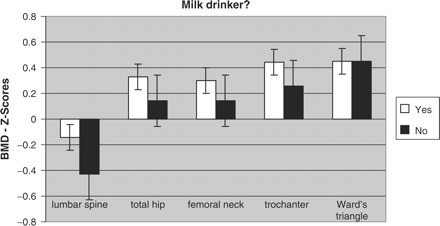
Anthropometric data, results of DXA measurements and figures of calcium consumption according to the question ‘Do you tolerate milk?’ (milk-drinker/non-milk-drinker) are shown in Table 2. Individuals who denied drinking milk (n = 31) were significantly smaller (4 cm; p = 0.04) and tended to be lighter (5 kg; p = 0.07; NS) than the other group (n = 197) (Figure 1). They also tended to have lower absolute and relative BMD values (−0.2 SD) at most sites measured (Figure 2). This was also confirmed for age 20: milk-intolerant men were 4 cm smaller (p = 0.05) and 4.5 kg lighter (p = 0.03) at that time than milk-drinkers.
General, anthropometric and nutritional data according to milk consumption
| . | Milk consumption . | . | p . | |
|---|---|---|---|---|
| . | Yes (n = 197) . | No (n = 31) . | . | |
| Age (years) | 56 ± 12 | 56 ± 11 | 0.80 | |
| Height (cm) | 177 ± 6.5 | 173 ± 6.8 | 0.04 | |
| Weight (kg) | 84 ± 11.6 | 79 ± 12.8 | 0.07 | |
| Height at age 20 (cm) | 177 ± 6.5 | 173 ± 6.7 | 0.05 | |
| Weight at age 20 (kg) | 75 ± 11 | 70.5 ± 8.2 | 0.03 | |
| BMI (kg/m2) | 26.6 ± 3.6 | 26.2 ± 3.2 | 0.59 | |
| Dislike of milk taste | 7 (3.6%) | 16 (52%) | 0.001 | |
| Self-reported lactose intolerance | 2 (1%) | 4 (13%) | 0.004 | |
| Total calcium intake (mg/week) | 4900 ± 1660 | 4080 ± 1440 | 0.025 | |
| Calcium from fresh milk (mg/day) | 28 ± 65 | 2 ± 8 | <0.001 | |
| Calcium from yogurt (mg/day) | 65 ± 62 | 40 ± 50 | 0.03 | |
| Calcium from chocolate (mg/day) | 14 ± 23 | 13 ± 21 | 0.98 | |
| Calcium from all other sources (mg/day) | 600 ± 230 | 530 ± 200 | 0.15 | |
| Spinal bone density (L1-L4) Z score | −0.145 ± 1.30 | −0.432 ± 1.20 | 0.27 | |
| Femoral bone density (total) Z score | 0.331 ± 0.83 | 0.12 ± 0.70 | 0.18 | |
| . | Milk consumption . | . | p . | |
|---|---|---|---|---|
| . | Yes (n = 197) . | No (n = 31) . | . | |
| Age (years) | 56 ± 12 | 56 ± 11 | 0.80 | |
| Height (cm) | 177 ± 6.5 | 173 ± 6.8 | 0.04 | |
| Weight (kg) | 84 ± 11.6 | 79 ± 12.8 | 0.07 | |
| Height at age 20 (cm) | 177 ± 6.5 | 173 ± 6.7 | 0.05 | |
| Weight at age 20 (kg) | 75 ± 11 | 70.5 ± 8.2 | 0.03 | |
| BMI (kg/m2) | 26.6 ± 3.6 | 26.2 ± 3.2 | 0.59 | |
| Dislike of milk taste | 7 (3.6%) | 16 (52%) | 0.001 | |
| Self-reported lactose intolerance | 2 (1%) | 4 (13%) | 0.004 | |
| Total calcium intake (mg/week) | 4900 ± 1660 | 4080 ± 1440 | 0.025 | |
| Calcium from fresh milk (mg/day) | 28 ± 65 | 2 ± 8 | <0.001 | |
| Calcium from yogurt (mg/day) | 65 ± 62 | 40 ± 50 | 0.03 | |
| Calcium from chocolate (mg/day) | 14 ± 23 | 13 ± 21 | 0.98 | |
| Calcium from all other sources (mg/day) | 600 ± 230 | 530 ± 200 | 0.15 | |
| Spinal bone density (L1-L4) Z score | −0.145 ± 1.30 | −0.432 ± 1.20 | 0.27 | |
| Femoral bone density (total) Z score | 0.331 ± 0.83 | 0.12 ± 0.70 | 0.18 | |
Data are means ± SD, or numbers (%), as appropriate.
General, anthropometric and nutritional data according to milk consumption
| . | Milk consumption . | . | p . | |
|---|---|---|---|---|
| . | Yes (n = 197) . | No (n = 31) . | . | |
| Age (years) | 56 ± 12 | 56 ± 11 | 0.80 | |
| Height (cm) | 177 ± 6.5 | 173 ± 6.8 | 0.04 | |
| Weight (kg) | 84 ± 11.6 | 79 ± 12.8 | 0.07 | |
| Height at age 20 (cm) | 177 ± 6.5 | 173 ± 6.7 | 0.05 | |
| Weight at age 20 (kg) | 75 ± 11 | 70.5 ± 8.2 | 0.03 | |
| BMI (kg/m2) | 26.6 ± 3.6 | 26.2 ± 3.2 | 0.59 | |
| Dislike of milk taste | 7 (3.6%) | 16 (52%) | 0.001 | |
| Self-reported lactose intolerance | 2 (1%) | 4 (13%) | 0.004 | |
| Total calcium intake (mg/week) | 4900 ± 1660 | 4080 ± 1440 | 0.025 | |
| Calcium from fresh milk (mg/day) | 28 ± 65 | 2 ± 8 | <0.001 | |
| Calcium from yogurt (mg/day) | 65 ± 62 | 40 ± 50 | 0.03 | |
| Calcium from chocolate (mg/day) | 14 ± 23 | 13 ± 21 | 0.98 | |
| Calcium from all other sources (mg/day) | 600 ± 230 | 530 ± 200 | 0.15 | |
| Spinal bone density (L1-L4) Z score | −0.145 ± 1.30 | −0.432 ± 1.20 | 0.27 | |
| Femoral bone density (total) Z score | 0.331 ± 0.83 | 0.12 ± 0.70 | 0.18 | |
| . | Milk consumption . | . | p . | |
|---|---|---|---|---|
| . | Yes (n = 197) . | No (n = 31) . | . | |
| Age (years) | 56 ± 12 | 56 ± 11 | 0.80 | |
| Height (cm) | 177 ± 6.5 | 173 ± 6.8 | 0.04 | |
| Weight (kg) | 84 ± 11.6 | 79 ± 12.8 | 0.07 | |
| Height at age 20 (cm) | 177 ± 6.5 | 173 ± 6.7 | 0.05 | |
| Weight at age 20 (kg) | 75 ± 11 | 70.5 ± 8.2 | 0.03 | |
| BMI (kg/m2) | 26.6 ± 3.6 | 26.2 ± 3.2 | 0.59 | |
| Dislike of milk taste | 7 (3.6%) | 16 (52%) | 0.001 | |
| Self-reported lactose intolerance | 2 (1%) | 4 (13%) | 0.004 | |
| Total calcium intake (mg/week) | 4900 ± 1660 | 4080 ± 1440 | 0.025 | |
| Calcium from fresh milk (mg/day) | 28 ± 65 | 2 ± 8 | <0.001 | |
| Calcium from yogurt (mg/day) | 65 ± 62 | 40 ± 50 | 0.03 | |
| Calcium from chocolate (mg/day) | 14 ± 23 | 13 ± 21 | 0.98 | |
| Calcium from all other sources (mg/day) | 600 ± 230 | 530 ± 200 | 0.15 | |
| Spinal bone density (L1-L4) Z score | −0.145 ± 1.30 | −0.432 ± 1.20 | 0.27 | |
| Femoral bone density (total) Z score | 0.331 ± 0.83 | 0.12 ± 0.70 | 0.18 | |
Data are means ± SD, or numbers (%), as appropriate.
Body weight and height at time of study and at age 20, according to milk-drinking habit.
Age-adjusted BMD (Z scores) at all sites measured, according to milk-drinking habit.
Distribution of calcium intake differed significantly between the groups (Table 2). We found decreases in consumption of fresh milk of −92% (p<0.001), of yogurt of −41% (p = 0.03) and of total calcium consumption of −18% (700 vs. 580 mg/week; p = 0.025) among non-milk-drinkers.
LCT genotype, bone density and calcium intake
Carriers of LCT CC tended to have lower BMD at most sites measured than individuals of the other LCT genotypes, but this did not reach statistical significance. Intake of calcium from fresh milk was 23% lower in the LCT CC genotype group (Table 1). There was no significant difference in total calcium consumption (−2%). Amounts of calcium consumed in the form of yogurt were higher in the LCT CC subgroup. There was no difference in the frequency of self-reported LM among the genotype groups. Only 1.6% of individuals with the CC genotype reported lactose intolerance. Interestingly, the percentage of TT individuals who reported lactose intolerance was about the same.
Discussion
Our main finding is a significant correlation between LCT genotypes, hydrogen breath test and lactose tolerance test. Furthermore, to our knowledge, this is the first work to compare a molecularly-defined lactose malabsorption test to a combination of both indirect tests. In fact, both indirect methods are very seldom used in daily practice, due to considerable efforts in time, material and patient compliance. In 97% of all cases with genetically determined LM, conventional tests showed positive results. On the other hand, lactose malabsorption was excluded in all cases of LCT TT, pointing out the high specificity of LCT genotyping. According to this high rate of congruent results, we suggest that genotyping of the LCT polymorphism may complement, or even replace both methods, as it is cheap (the H2 breath test is about twice as expensive) and convenient (once-in-a-lifetime examination).
In contrast, individual knowledge of personal status on LM was very poor, and correlation between LCT genotypes, self-reported LM and milk drinking habits was limited in our study population. Only a minor percentage of LCT CC individuals (1.6%) reported symptoms of lactose intolerance, despite the fact that 90% had a positive hydrogen breath test, 87% showed abdominal symptoms, and 85% demonstrated a pathological lactose tolerance test. Many men carrying LCT CC were surprised when they realized that their symptoms represented LM. We conclude that many men ignore or misinterpret symptoms of LM. Amounts of ingested lactose may often be too small to provoke symptoms, as even LM sufferers may tolerate small amounts of milk without abdominal symptoms.7 The average amount of milk consumed per day by all of the study subjects was surprisingly low: ∼20 ml, which is no more than a dash of milk in a cup of coffee. In addition, consumption of pure fresh milk often had ceased decades earlier, so that symptoms of LM might have been forgotten. Interestingly, we found very different figures concerning milk consumption in a comparable cohort of post-menopausal women, where exactly the same calcium questionnaire was used: rates of milk consumption were about five times higher in the female cohort. This may be explained partly by cultural and social gender differences.
We found remarkable differences in calcium intake rates according to milk-drinking habits: Individuals who claimed to be lactose-intolerant consumed about 92% less milk and 42% less yogurt and cheese, with a total calcium intake of 18% less than milk drinkers; this was mainly due to a decrease in the amounts of cheese and yogurt consumed, and not in the amounts of fresh milk in the person's diet. Many kinds of cheese and yogurt contain very small amounts of lactose, and can thus be tolerated by LM patients.
Milk-drinking habits significantly influenced differences in body weight and height: non-milk-drinkers were smaller and slimmer at the time of our examination. Interestingly, those individuals showed the same significant differences at the age of 20. This may relate to the peak bone mass reached in these patients, and was not influenced by physical activity or profession. Height and weight as strong skeletal parameters were paralleled by age-matched BMD. Bone density was lower in non-milk-drinkers at almost all sites measured. Differences reached up to −67% at total spine, but this was not statistically significant, perhaps due to other sources of calcium and exogenous influences in these patients (Figure 2). The fact that LCT genotypes in our study only showed only borderline correlation with BMD might either be due to different degrees of degenerative diseases, or physical activity throughout life. In turn, our findings may also indicate that alternative calcium sources could overcome the problem of hypolactasia in LCT CC men, and should be further investigated.
The effect of calcium on bone density and fractures is widely, but not entirely accepted.8–11 However, there is little information on men in this area. In a cohort-study of institutionalized elderly men (mean age 75 years), reduced calcium intake at about the same level as in our study group (about −20%) correlated with an increase in hip fracture incidence.12 The lower age in our study population might have protected our patients from osteoporotic fractures. Recently published papers demonstrated very clearly the importance of calcium-rich nutrition, when in a short-term trial in young men, milk was replaced by carbonated beverages such as cola: bone resorption markers were significantly increased even after a very short period of 10 days.13 Chevalley et al. found significant increases in aBMD in a group of pre-pubertal boys who received calcium-enriched foods.14
The largest meta-analysis on milk intake and fractures has recently been published, summarizing six studies from European countries, the UK, Canada and Australia.15 The intake of milk correlated significantly with femoral BMD, but there was no significant influence of low milk intake on osteoporotic fractures, except a small effect from the age of 80 years on in both men and women. However, there was no information on LM in this study, and many participants came from countries with a low incidence of LM, such as Sweden with 2%. This may have caused an underestimation of the problem. Further confounding factors might include different cut-offs for milk intake in these populations, or other sources of calcium or other dairy products that were not documented. The uncertainties around milk intake and LM by questionnaires are well-known problems that might be controlled by testing of genetic conditions for lactose digestion. In our opinion, a significant reduction of calcium intake is of high clinical relevance, and should be corrected by better knowledge of lactose content in foods on the part of the general public. A full declaration of ingredients in food might be helpful.
Testing of genetic predisposition for LM by LCT polymorphism is clinically useful in assessing ability to digest dairy food. Calcium supply also depends on additional cultural and behavioural factors. We recommend LCT genotyping as an economical and quick method on which to base dietary recommendations to assure adequate calcium supply.
References
Ennatah NS, Sahi T, Savilahti E, Terwilliger JD, Peltonen L, Järvela I. Identification of a variant associated with adult type hypolactasia.
Ledochowski M, Sperner-Unterweger B, Fuchs D. Lactose malsabsorption is associated with early signs of mental depression in females. A preliminary report.
Swallow DM. Genetics of lactase persistence and lactose intolerance.
Hammer HF, Petritsch W, Pristautz H, Krejs GJ. Assessment of the influence of hydrogen nonexcretion on the usefulness of the hydrogen breath test and lactose tolerance test.
Kudlacek S, Schneider B, Peterlik M, Leb G, Klaushofer K, Weber K, et al. Normative data of bone mineral density in an unselected adult Austrian population.
WHO.
Savaiano D. Lactose intolerance: a self-fulfilling prophecy leading to osteoporosis?
Shea B, Wells G, Cranney A, Zytaruk N, Guyatt G for the Osteoporosis methodology group and the Osteoporosis research advisory group. Meta-analysis of calcium supplementation for the prevention of postmenopausal osteoporosis.
Michaelsson K, Melhus H, Belloco R, Wolk A. Dietary calcium and vitamin D intake in relation to osteoporotic fracture risk.
Obermayer-Pietsch BM, Bonelli CM, Walter DE, Kuhn R, Fahrleitner-Pammer A, Berghold A, Gössler W, Stepan V, Dobnig H, Leb G, Renner W. Genetic predisposition for adult lactose intolerance and relation to diet, bone density, and bone fractures.
Grant AM, Avenell A, Campell K, McDonald AM, Wallace WA. Oral vitamin D3 and calcium for secondary prevention of low-trauma fractures in elderly people (Randomised Evaluation of Calcium Or vitamin D, RECORD): a randomised placebo-controlled trial.
Holbrook TL, Barett Connor I, Wingard D. Dietary calcium and risk of hip fracture: 14 year prospective population study.
Kristensen M, Jensen M, Kudsk J, Henriksen M, Molgaard C. Short-term effects on bone turnover of replacing milk with cola beverages: a 10-day interventional study in young men.
Chevalley T, Bonjour JP, Ferrari S, Hans D, Rizzoli R. Skeletal site selectivity in the effects of calcium supplementation on areal bone mineral density gain: a randomized double-blind, placebo controlled trial in prepubertal boys.
Author notes
From the 1Division of Endocrinology and Nuclear Medicine, Department of Internal Medicine, Medical University, Graz, 2Division of Pathophysiology, Medical University, Vienna, 3Hospital of the Brothers of Charity, Department of Internal Medicine, Vienna, and 4Center for Medical Research, Unit of Biostatistics, Medical University, Graz, Austria