-
PDF
- Split View
-
Views
-
Cite
Cite
A. Angel-Moreno Maroto, E. Martínez-Quintana, L. Suárez-Castellano, J.-L. Pérez-Arellano, Painful hypertrophic osteoarthropathy successfully treated with octreotide. The pathogenetic role of vascular endothelial growth factor (VEGF), Rheumatology, Volume 44, Issue 10, October 2005, Pages 1326–1327, https://doi.org/10.1093/rheumatology/keh720
Close - Share Icon Share
Sir, Hypertrophic pulmonary osteoarthropathy (HPOA) is a syndrome defined by digital clubbing and distal periostitis in tubular bones [1]. It is associated with a number of medical conditions, mainly lung cancer, congenital cyanotic heart disease and other systemic illnesses with pulmonary involvement. Symptoms may include bone pain, occasionally severe. The pathogenesis of this syndrome is elusive. A possible role for some unknown growth factor(s), probably platelet or tumour-derived, not cleared by the lungs, has been proposed [2]. As hyperaemia and neoangiogenesis have been identified as common pathological features, a hypothetical role for vascular endothelial growth factor (VEGF) in subperiosteal bone inducing periosteal thickening, is gaining acceptance [3, 4]. This hypothesis would be reinforced if VEGF inhibition improved HPOA-related symptoms.
We recently assisted a patient with painful HPOA, associated with Fallot's tetralogy, who dramatically responded to octreotide, a potent VEGF inhibitor.
A 34-yr-old man was admitted with bilateral leg pain. He had Fallot's tetralogy with pulmonary artery atresia. A left-to-right bypass from the ascending aorta to the right pulmonary artery, and later two Blalock–Taussig procedures to attach the left subclavian to the left pulmonary artery had been performed in the past, with only transient success. The patient had been offered no further surgical interventions and was rejected for cardiopulmonary transplantation.
He had grade III dyspnoea. In the last 2 months, progressive, severe pain along both legs and ankles developed, which had not responded to usual analgesics and impeded his sleep. The pain was alleviated partially by immersion of his legs in cool water.
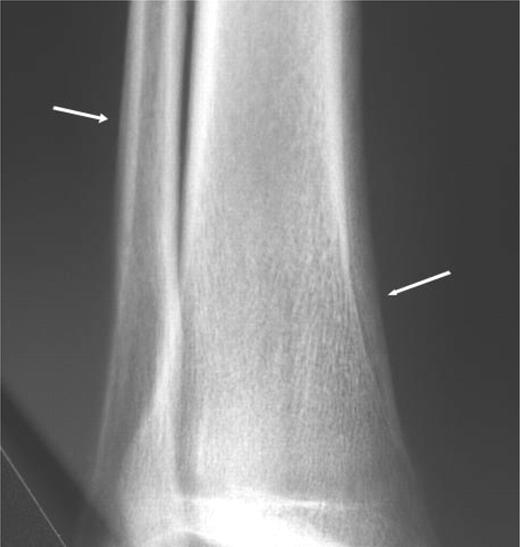
The patient was cyanotic. Prominent digital clubbing of his fingers and toes and mild ankle oedema were present. Intense tenderness was elicited along the distal two-thirds of both tibial shafts and around the ankles, without signs of cellulitis or arthritis. Polycythaemia and severe hypoxaemia were prominent. X-rays showed typical, bilateral periosteal thickening in the distal tibiae and fibulae (Fig. 1).
Bilateral periosteal thickening in the distal tibiae and fibulae.
Paracetamol, ketorolac, amitriptyline, indomethacin and dexamethasone were sequentially tried in combination, without relief. After informed consent had been obtained, subcutaneous octreotide, 100 μg twice daily, was added. After 3–4 days, complete pain relief was achieved and the other drugs could be withdrawn. No adverse events were recorded. After 2 weeks, octreotide was tapered to 100 μg daily and the patient was discharged. After about 1 week, the pain reappeared and the patient was readmitted. His poor cardiorespiratory status was then rapidly deteriorating. Octreotide was increased to 100 μg twice daily but, owing to untreatable dyspnoea, multiple other palliative measures were needed, including morphine (thus precluding the evaluation of a specific response to octreotide), and the patient finally died.
Our patient fulfilled HPOA criteria [1], in this case associated with advanced Fallot's tetralogy, with pain resistant to combined analgesia. Octreotide induced and maintained complete pain relief in monotherapy. Moreover, the pain reappeared as the dose was tapered. This case is the second reported in HPOA-related pain responding to octreotide, and the first in HPOA associated with congenital cyanotic heart disease.
Complete pain relief with octreotide was reported in a patient with lung cancer-related HPOA [5]. These and other authors [6] related this effect to some hormone-inhibiting or intrinsic analgesic actions of octreotide. As more recent data are accumulating on VEGF involvement in the pathogenesis of HPOA, the effect of octreotide could be explained by VEGF inhibition.
The pathological hallmark of HPOA is neoangiogenesis, plus oedema and osteoblast proliferation in distal tubular bones, leading to subperiosteal new bone formation. VEGF, a powerful endothelial cell-stimulating factor, is very likely to play a central role in the pathogenesis of HPOA for several reasons [3, 4]. Firstly, abnormal platelet function seems to be involved in diseases with right-to-left shunting and VEGF is a platelet-derived factor that can also be ectopically produced by some tumours or locally induced in response to hypoxia. Secondly, VEGF receptors are expressed in subperiosteal bone-forming cells. Finally, both increased VEGF plasma levels and/or tissue expression have been reported in virtually all the medical diseases associated with HPOA, correlating with disease activity [4].
Recently, octreotide has been shown to inhibit the production of VEGF and endothelial proliferation [7]. Interestingly, bisphosphonates, also effective for pain relief in HPOA (an effect previously attributed to its action on osteoclasts) [8], are also potent VEGF inhibitors [9].
Our case confirms the effectiveness of octreotide in HPOA associated with different underlying medical conditions. It also demonstrates that HPOA-related symptoms can be blunted by different VEGF inhibitors, strongly supporting the central role of VEGF in the pathogenesis of this syndrome. Perhaps the use of an anti-VEGF monoclonal antibody, such as bevacizumab, will more specifically confirm this hypothesis.
The authors have declared no conflicts of interest.
References
Martinez-Lavin M, Matucci-Cerinic M, Jajic I, Pineda C. Hypertrophic osteoarthropathy: consensus on its definition, classification, assessment and diagnostic criteria.
Martinez-Lavin M. Pathogenesis of hyperthrophic osteoarthropathy.
Silveira LH, Martinez-Lavin M, Pineda C, Fonseca MC, Navarro C. Vascular endothelial growth factor and hypertrophic osteoarthropathy.
Olan F, Portela M, Navarro C, Gaxiola M, Silveira LH, Ruiz V, Martinez-Lavin M. Circulating vascular endothelial growth factor concentration in a case of pulmonary hypertrophic osteoarthropathy. Correlation with disease activity.
Johnson SA, Spiller PA, Faull CM. Treatment of resistant pain in hypertrophic pulmonary osteoarthropathy with subcutaneous octreotide.
Dasgupta P. Somatostatin analogues: multiple roles in cellular proliferation, neoplasia and angiogenesis.
Garske LA, Bell SC. Pamidronate results in symptom control of hypertrophic pulmonary osteoarthropathy in cystic fibrosis.
Author notes
1Universidad de Las Palmas de Gran Canaria, Ciencias Médicas y Quirúrgicas and 2Hospital Universitario Insular, Las Palmas de Gran Canaria, Spain




Comments