-
PDF
- Split View
-
Views
-
Cite
Cite
E. Solau-Gervais, M. Soubrier, I. Gerot, L. Grange, X. Puechal, C. Sordet, J. Sibilia, B. Duquesnoy, The usefulness of bone remodelling markers in predicting the efficacy of pamidronate treatment in SAPHO syndrome, Rheumatology, Volume 45, Issue 3, March 2006, Pages 339–342, https://doi.org/10.1093/rheumatology/kei160
Close - Share Icon Share
Abstract
Objectives. Pamidronate has recently been used in SAPHO syndrome due to its anti-osteoclastic effect. The aim of this study is to determine the usefulness of bone remodelling markers for determining the efficacy of pamidronate treatment.
Methods. Thirteen patients with SAPHO syndrome were treated with pamidronate. The treatment evaluation was done using a visual analogue scale (VAS) and also erythrocyte sedimentation rate, C-reactive protein, serum crosslaps (sCTX) and osteocalcin initially and after 3 months. A relevant clinical response was defined as an improvement in VAS of at least 40%.
Results. At 3 months, 7 of 13 patients had a good clinical response, as previously defined. Five of the seven patients maintained the good response over 6 months. Before the first perfusion 6 of the 13 patients had increased sCTX (upper 3250 pmol/l). In this small cohort we tried to analyse whether the increase in bone remodelling markers was associated with a good clinical response. In the responders group the mean levels of sCTX and osteocalcin at baseline were 6783.17 and 24.66, respectively, and in the non-responders group the levels were 2152 and 11.8, respectively. There was a significant difference in sCTX between the responders and the non-responders (P = 0.0044).
Conclusion. Infusion of pamidronate is effective in SAPHO in some patients. Increased sCTX might be a prognostic marker for a good clinical response but results have to be confirmed in a larger cohort.
SAPHO syndrome (synovitis, acne, pustulosis, hyperostosis and osteitis), classified with the spondyloarthropathies, has particular characteristics. As in the spondyloarthropathies there is synovitis and the presence of HLA-B27, but the major feature of this disease is the association of hyperostosis and osteitis. This bone remodelling has led several authors to use biphosphonate as a treatment, as no therapy has been shown to be effective when non-steroidal anti-inflammatory drugs (NSAIDs) have failed.
After a few case reports [1–4], a recent open-label study on 10 patients with SAPHO syndrome treated with one infusion of 60 mg of pamidronate showed a good response but with relapses requiring further infusion [5]. In our open-label study, we reported observations of 13 patients receiving three pamidronate infusions who were followed for 6 months.
As biphosphonate is effective in SAPHO syndrome we have become interested in bone remodelling markers in this pathology. Courtney et al. [3] have reported a fall in the urinary hydroxyproline/creatinine ratio after pamidronate therapy in one patient with SAPHO syndrome. In this study we present results for bone remodelling markers such as serum crosslaps (sCTX) and osteocalcin before and after pamidronate infusion.
Materials and methods
Patients
Patients were recruited between 2001 and 2005 from the rheumatology departments at Lille, Clermont Ferrand, Strasbourg, Grenoble and Le Mans. All the patients complied with the inclusion criteria proposed by Benhamou et al. [6], had a visual analogue scale (VAS) of >40 mm and were refractory to NSAIDs. Patients had stopped second-line drugs and steroids for more than 3 months and were taking NSAIDs and/or analgesics at a stable dose.
At the initial visit clinical assessment with gender, age, date of onset, cutaneous involvement and localization of hyperostosis was done for each patient. The evaluation was done clinically on a 100 mm VAS. Biologically erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), sCTX and osteocalcin were evaluated initially and after 3 and 6 months. Patients were all in a fasting state.
Serum CTX was evaluated using a Serum CrossLaps® enzyme-linked immunosorbent assay (ELISA) (Nordic Bioscience Diagnostics) and the normal range was from 270 to 3250 pmol/l. Osteocalcin was analysed with radioimmunoassay (OSTEO-RIACT from Cisbio International) and the normal range was from 10 to 36 ng/ml.
Treatment
All patients in the study were given 60 mg of pamidronate infusion over 3 days. A good clinical response, evaluate at 3 and 6 months, was defined as an improvement in VAS of at least 40%.
Statistical analysis
Data collected from the 13 patients were analysed using the Staview program. Owing to the small size of the samples Wilcoxon, Mann–Whitney and linear regression were used for the intergroup comparisons. Statistical significance was defined as P<0.05.
Results
Clinical and biological description of the patients
Of the 13 patients (1 male and 12 females), 6 had cutaneous involvement, 10 had sternoclavicular localization and 3 had spine involvement. The mean duration of the disease was 3 yr (from 1 to 10 yr).
At the initial visit, pain VAS was on average 64.46 mm (40–89). At 3 months pain VAS decreased to 42.15 mm on average (0–100) and this decrease was maintained at 6 months with a value of 47.64 mm (5–100).
ESR was increased in 4 of the 13 patients but with a normal mean at 20.88 mm (s.d. 17.79); the CRP was slightly increased with a mean of 10.86 mg/ml (s.d. 12.80). After pamidronate infusion in three of the four patients with an inflammatory process, ESR and CRP decreased at 3 months.
The level of sCTX was increased in patients with SAPHO at 4288 pmol/l in average, compared with a normal range between 270 and 3250 pmol/l. This increase occurred in six patients. For 12 patients, sCTX was available at 3 or 6 months after pamidronate treatment. As expected, the level decreased after pamidronate infusion at 3 and 6 months in all patients, except for two who had had no initial increase in sCTX (Fig. 1). However, osteocalcin was not increased in this cohort as its average was 18.54 ng/ml. The level of osteocalcin went up to 36 ng/ml in only one case.
Evolution of sCTX (serum crosslaps) after 3 and/or 6 months after three 60 mg perfusions of pamidronate.
Two types of clinical response
At 3 months patients were either responders (improvement of at least 40% in the pain VAS) or non-responders. Seven patients were responders at 3 months. The mean pain VAS in this group was at 20.29 mm. At 6 months, five of the seven patients had maintained a good response and two had relapsed. Two patients who were non-responders at 3 months were responder at 6 months. Age and initial VAS were no different between the two groups, but mean disease duration was much more longer in the non-responders group (97 months vs 21.43 months in the responders group) and this result is significant with Mann–Whitney (P = 0.0184) and linear regression (P = 0.04) (Table 1).
Clinical data at baseline: age, gender, disease duration and visual analogue scale (VAS) in responders and non-responders
| . | Responders (n = 7) . | Non-responders (n = 6) . | P . |
|---|---|---|---|
| Age (yr) | 43.71 (16, 58) | 35 (11, 63) | 0.43 |
| Gender | 6F/1M | 6F | 0.59 |
| Disease duration (months) | 21.43 (24.89) | 97 (82.3) | 0.0184 |
| VAS | 66.43 (18.98) | 62.17 (15.69) | 0.52 |
| . | Responders (n = 7) . | Non-responders (n = 6) . | P . |
|---|---|---|---|
| Age (yr) | 43.71 (16, 58) | 35 (11, 63) | 0.43 |
| Gender | 6F/1M | 6F | 0.59 |
| Disease duration (months) | 21.43 (24.89) | 97 (82.3) | 0.0184 |
| VAS | 66.43 (18.98) | 62.17 (15.69) | 0.52 |
Parenthesis indicates standard deviation.
Clinical data at baseline: age, gender, disease duration and visual analogue scale (VAS) in responders and non-responders
| . | Responders (n = 7) . | Non-responders (n = 6) . | P . |
|---|---|---|---|
| Age (yr) | 43.71 (16, 58) | 35 (11, 63) | 0.43 |
| Gender | 6F/1M | 6F | 0.59 |
| Disease duration (months) | 21.43 (24.89) | 97 (82.3) | 0.0184 |
| VAS | 66.43 (18.98) | 62.17 (15.69) | 0.52 |
| . | Responders (n = 7) . | Non-responders (n = 6) . | P . |
|---|---|---|---|
| Age (yr) | 43.71 (16, 58) | 35 (11, 63) | 0.43 |
| Gender | 6F/1M | 6F | 0.59 |
| Disease duration (months) | 21.43 (24.89) | 97 (82.3) | 0.0184 |
| VAS | 66.43 (18.98) | 62.17 (15.69) | 0.52 |
Parenthesis indicates standard deviation.
Biological markers in the two groups
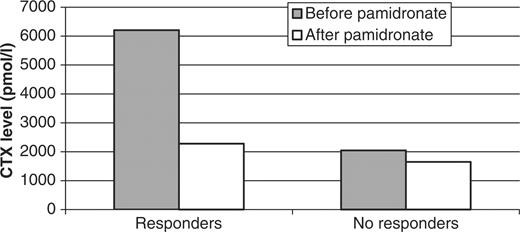
As shown in Table 2, inflammatory parameters such as ESR and CRP increased more in the responders (levels of 23.79 mm and 16 mg/l, respectively) than in the non-responders (17.5 mm and 3.66 mg/l, respectively). But a Mann–Whitney analysis showed the difference between these two groups not to be significant. However, in the good responders, before pamidronate infusion, the level of sCTX was much higher (6209.14 pmol/l) than in the non-responders (2046.67 pmol/l) and this result was statistically significant when using either Mann–Whitney (P = 0.045) or linear regression (P = 0.042) tests. Also, the level of osteocalcin increased more in the responders (24.86 ng/ml) than in the non-responders (11.17 ng/ml). These results were also statistically significant (P = 0.02).
Biological data at baseline: ESR (erythrocyte sedimentation rate), CRP (C-reactive protein), sCTX (carboxy-terminal collagen crosslinks), OC (osteocalcin) in responders and non-responders
| . | Responders (n = 7) . | Non-responders (n = 6) . | P . |
|---|---|---|---|
| ESR | 23.78 | 17.5 | 0.66 |
| CRP (mg/ml) | 16 | 6.97 | 0.19 |
| sCTX (pmol/l) | 6209.14 | 2046.67 | 0.045 |
| OC (ng/ml) | 24.86 | 11.17 | 0.02 |
| . | Responders (n = 7) . | Non-responders (n = 6) . | P . |
|---|---|---|---|
| ESR | 23.78 | 17.5 | 0.66 |
| CRP (mg/ml) | 16 | 6.97 | 0.19 |
| sCTX (pmol/l) | 6209.14 | 2046.67 | 0.045 |
| OC (ng/ml) | 24.86 | 11.17 | 0.02 |
Biological data at baseline: ESR (erythrocyte sedimentation rate), CRP (C-reactive protein), sCTX (carboxy-terminal collagen crosslinks), OC (osteocalcin) in responders and non-responders
| . | Responders (n = 7) . | Non-responders (n = 6) . | P . |
|---|---|---|---|
| ESR | 23.78 | 17.5 | 0.66 |
| CRP (mg/ml) | 16 | 6.97 | 0.19 |
| sCTX (pmol/l) | 6209.14 | 2046.67 | 0.045 |
| OC (ng/ml) | 24.86 | 11.17 | 0.02 |
| . | Responders (n = 7) . | Non-responders (n = 6) . | P . |
|---|---|---|---|
| ESR | 23.78 | 17.5 | 0.66 |
| CRP (mg/ml) | 16 | 6.97 | 0.19 |
| sCTX (pmol/l) | 6209.14 | 2046.67 | 0.045 |
| OC (ng/ml) | 24.86 | 11.17 | 0.02 |
After pamidronate infusion, at 3 months the level of sCTX decreased by up to 70% in the responders group, reaching a normal range (2276.20 pmol/l). In the non-responders the decrease was less important (Fig. 2). At 6 months, the level of sCTX increased slightly in the responders (3438.75 pmol/l) but stayed low in the non-responders (1219.25 pmol/l).
Decrease rate of sCTX (serum crosslaps) before and after pamidronate perfusion in responders and non-responders.
Discussion
SAPHO syndrome belongs to the family of spondyloarthropathies. As in spondyloarthropathy, NSAIDs are effective in treatment of the articular symptom in most patients [7]. However, the efficacy of disease-modifying anti-rheumatic drugs (DMARDs) such as sulphasalazine or methotrexate in patients who have persistent disease has not been demonstrated. More recently, antibiotic therapy has been proposed in SAPHO syndrome as Propionibacterium acnes could be responsible for the cutaneous and bone lesions. Although this alternative seems interesting case reports on only two patients have been published [8].
Pamidronate has been successfully used in several diseases with high bone remodelling, such as spondyloarthritis [9], fibrous dysplasia [10] and Paget's disease [11]. As SAPHO syndrome is a spondyloarthropathy with high bone remodelling several authors have used biphosphonate [1–5, 12]. Recently, zoledronic acid has been used in SAPHO with good results [13]. Like these authors, we confirmed the efficacy of pamidronate and have shown that most patients maintained a good response over 6 months. In our work, patients received three infusions of 60 mg pamidronate as in Paget's disease [11] and in the first study of Sayag-Boukris et al. [14]. Amital also improved the osteoarticular symptoms in 10 patients after pamidronate. Patients were treated initially with just 60 mg of pamidronate, but all patients except one had had between two and four infusions by the end of the study. This shows that more than one infusion of 60 mg pamidronate is required.
In this study we show for the first time that there is an important increase in sCTX in SAPHO. The level of osteocalcin was increased (up to 36 ng/ml) in only one patient. This probably highlights the greater importance of bone resorption compared with bone formation. However, we cannot exclude the weak of sensitivity of the osteocalcin test. These changes in bone remodelling markers in SAPHO have never been described. In spondyloarthropathy, however, Acebes et al. [15] found a normal and a significant increase in bone resorption and Toussirot et al. [16] showed that some of the bone resorption markers were enhanced in subgroups of spondyloarthropathy, mainly in patients with raised ESR. These studies were done with urinary markers. Since the introduction of serum crosslaps [17] no study has been done in spondyloarthropathy.
Serum CTX decreases dramatically after pamidronate infusion. We know that biphosphonate and pamidronate in particular decrease bone remodelling markers such sCTX [17] and alkaline phosphatase. In the literature only Courtney et al. [3] have shown the fall in the urinary hydroxyproline:creatinine ratio after pamidronate therapy in one patient.
Because not all of the patients responded to the treatment, and as some of them had a large increase in sCTX, we wanted to know if this increase was related to the response to pamidronate. We showed that the initial level of sCTX was significantly higher in responders than in non-responders. This has never been described in SAPHO. However, in osteoporosis the decrease in bone resorption in patients taking biphosphonate correlated reasonably well with changes in spine bone mineral density [17] and could be predictive of the reduction in fracture risk [18]. Disease duration has also been found to be related to the clinical response. It is possible that in the first years of the disease bone resorption is more important and biphosphonates more effective.
In conclusion, infusion of pamidronate is effective in SAPHO patients who do not respond to NSAIDs, and increased sCTX might be a prognostic marker for a good clinical response. Controlled studies are required, however.
We thank the French ‘Réseau de rhumatologie’ for its support.
The authors declare no conflicts of interest.
References
Guignard S, Job-Deslandre C, Sayag-Boukris V, Kahan A. Pamidronate treatment in SAPHO syndrome.
Courtney PA, Hosking DJ, Fairbairn KJ, Deighton CM. Treatment of SAPHO with pamidronate.
Kerrison C, Davidson JE, Cleary AG, Beresford MW. Pamidronate in the treatment of childhood SAPHO syndrome.
Amital H, Applbaum YH, Aamar S, Daniel N, Rubinow A. SAPHO syndrome treated with pamidronate: an open-label study of 10 patients.
Benhamou CL, Chamot AM, Kahn MF. Synovitis-acne-pustulosis hyperostosis-osteomyelitis syndrome (SAPHO). A new syndrome among the spondyloarthropathies?
Hayem G, Bouchaud-Chabot A, Benali K et al. SAPHO syndrome: a long-term follow-up study of 120 cases.
Ballara SC, Siraj QH, Maini RN, Venables PJ. Sustained response to doxycycline therapy in two patients with SAPHO syndrome.
Maksymowych WP, Jhangri GS, Fitzgerald AA et al. A six-month randomized, controlled, double-blind, dose-response comparison of intravenous pamidronate (60 mg versus 10 mg) in the treatment of nonsteroidal antiinflammatory drug-refractory ankylosing spondylitis.
Chapurlat RD, Hugueny P, Delmas PD, Meunier PJ. Treatment of fibrous dysplasia of bone with intravenous pamidronate: long-term effectiveness and evaluation of predictors of response to treatment.
Tucci JR, Bontha S. Intravenously administered pamidronate in the treatment of Paget's disease of bone.
Marshall H, Bromilow J, Thomas AL, Arden NK. Pamidronate: a novel treatment for the SAPHO syndrome?
Kopterides P, Pikazis D, Koufos C. Successful treatment of SAPHO syndrome with zoledronic acid.
Sayag-Boukris V, Loussadi I, Cormier C, Laroche F, Menkes CJ, Kahan A. Efficacy of pamidronate in the treatment of SAPHO syndrome.
Acebes C, de la Piedra C, Traba ML et al. Biochemical markers of bone remodeling and bone sialoprotein in ankylosing spondylitis.
Toussirot E, Ricard-Blum S, Dumoulin G, Cedoz JP, Wendling D. Relationship between urinary pyridinium cross-links, disease activity and disease subsets of ankylosing spondylitis.
Rosen HN, Moses AC, Garber J et al. Serum CTX: a new marker of bone resorption that shows treatment effect more often than other markers because of low coefficient of variability and large changes with bisphosphonate therapy.
Author notes
Departments of Rheumatology, Lille University Hospital, Lille, 1Clermont Ferrand University Hospital, Clermont Ferrand, 2Grenoble University Hospital, Grenoble, 3Le Mans Hospital and 4Strasbourg University Hospital, Strasbourg, France.




Comments