-
PDF
- Split View
-
Views
-
Cite
Cite
D. Laroche, T. Pozzo, P. Ornetti, C. Tavernier, J. F. Maillefert, Effects of loss of metatarsophalangeal joint mobility on gait in rheumatoid arthritis patients, Rheumatology, Volume 45, Issue 4, April 2006, Pages 435–440, https://doi.org/10.1093/rheumatology/kei168
Close - Share Icon Share
Abstract
Objective. To evaluate the effects of loss of range of motion (ROM) of the metatarsophalangeal (MTP) joint on the kinematic parameters of walking in rheumatoid arthritis (RA) patients.
Methods. Inclusion of RA patients with inactive disease, no synovitis of the inferior limb and reduced ROM of the MTP joints. Evaluation of the ROM of the MTP dorsal and plantar flexion, and gait analysis using a three-dimensional computerized movement analysis. Calculation of gait parameters and maximal flexion and extension of the hips and knees during walking. Analysis 1 compared the ROM of dorsal and plantar flexion in patients with or without walking pain; 2 compared the gait parameters between patients and controls; 3 investigated a relationship between gait parameters and (i) the ROM of the MTP dorsal and plantar flexion and (ii) the pain at walking; 4 investigated the relationship between the ROM of the MTP dorsal and plantar flexion and maximal flexion and extension of the hip and knee joints during walking.
Results. Nine patients and seven controls were included. The MTP ROM was no different in patients presenting with or without pain at walking. The walking velocity was lower and the stride length shorter in patients than in controls. The walking velocity and the stride length were positively related to the MTP dorsal flexion ROM (r2=0.75 and 0.67). There was a negative relationship between maximal flexion of the knee and hips during walking and the underlying MTP dorsal flexion ROM (r2=0.67 and 0.54).
Conclusion. In RA patients, reduced MTP dorsal flexion mobility induces changes in the walking parameters, including the kinematics of the overlying lower limb joints. Treatment of an RA-impaired forefoot should focus on MTP mobility as well as on pain.
Despite the frequent involvement of the inferior limb joints in rheumatoid arthritis (RA), the effects of such involvement on walking behaviour are not fully understood. Studies on the walking pattern in RA patients have demonstrated a slowing in the walking velocity, shortened stride length, a lowering in the heel-strike, push-off impacts and increased double-stance period [1, 2]. Abnormalities in the foot pressures during walking, such as a shift of the loads from the medial side of the foot towards the centre, and a delayed and reduced forefoot loading have been demonstrated [1–3]. The heel strike, foot flat and toe-off sequence have been shown to be replaced by a flat-footed antalgic type of gait [4–6]. The three rocker functions of the feet are impaired: the first is minimally affected, the second is prolonged and the third, which is the most severely affected, is reduced [7]. Although other factors are involved, such changes appear to be mainly due to pain, the weight being moved away from the most painful parts of the foot [5].
However, most studies have investigated the static and dynamic pressures under the forefoot, or the walking pattern, with regard to pain [8], rather than to the loss of range of motion (ROM) of the joints. In addition, kinematic studies have usually evaluated the motions of foot segment and ankle joints, or developed multisegment foot models for measurement and description of intrasegmental foot or ankle motion in patients or normal subjects [9, 10], without obtaining relevant data on the consequences of such joint abnormalities on lower limb kinematics. Moreover, most studies have been characterized by a heterogeneous population, and the results might have been influenced by numerous factors. In particular, the respective effects on gait of synovitis, pain and loss of joint mobility, and those of hip, knee or foot abnormalities could not be determined.
In particular, data on the effects of a loss of the ROM of the metatarsophalangeal (MTP) joints are lacking. However, synovitis and erosions of the MTP joints are the most frequent and earliest manifestations of RA, and frequently lead to loss of the ROM of the MTP, deformity and subluxation in MTP joints. This joint is involved in the walking process, specifically at the end of the stance phase, during the period of forefoot support, when ankle dorsal flexors produce force to accelerate the body forward [11]. Thus, the loss of MTP ROM in RA patients might induce important changes in the patient's gait, such as modifications in the coordination between legs to correct for the loss of ROM and produce a smooth stride movement. Analysis of this point might be of great interest since it might influence the choice of rehabilitation or surgical techniques [12, 13]. If the loss of MTP ROM in RA patients induces important changes in walking kinematics, including knee and hip movement, local treatments should include the improvement or preservation of MTP ROM as a major objective.
The aim of this study was to evaluate the effects of loss of MTP ROM on the walking kinematics, including hip and knee movements, in RA patients.
Methods
Participants
Out-patients aged 18 to 75 yr, with RA defined using the American College of Rheumatology (ACR) 1987 criteria, were included. The patients were assessed by an experienced rheumatologist. All presented with non-active disease [defined as a disease activity score 28 (DAS 28) of <3.2], no synovitis of the inferior limb on physical examination, a reduced range of motion of the MTP joints, no marked limitation of the ankle joint movement and no movement limitation or pain in the knee and hips joints, according to the opinion of the examiner. In addition, control patients with no musculoskeletal disorder were included. Exclusion criteria were other musculoskeletal disorders, Alzheimer's disease, Parkinson's disease, motoneuronal disorders, non-stabilized diabetes mellitus, hypertension or respiratory insufficiency, pregnancy and lactation. Written informed consent for participation in the study was obtained from all patients according to the Declaration of Helsinki. The study was approved by the local ethics committee.
RA evaluation
In all patients, the following data were obtained: patient's assessment of global pain (100 mm visual analogue scale), patient's assessment of pain during walking (yes/no and 100 mm visual analogue scale), patient's overall evaluation of disease activity (100 mm visual analogue scale), 28 tender joint count, 28 swelling joint count, erythrocyte sedimentation rate (ESR), DAS 28, and Heath Assessment Questionnaire (HAQ).
Evaluation of the MTP ROM
The MTP dorsal and plantar flexion ROM were calculated using the ELITE system (see below). The intraobserver reliability of this method was assessed on 30 subjects evaluated twice, and was found to be good, with intraclass coefficients of correlation of 0.78 [95% confidence interval (CI) = 0.59–0.89] and 0.74 (95% CI = 0.53–0.87) for dorsal and plantar flexion ROM measurements, respectively. The patients sat on a chair and put their feet on another chair, positioned in front of the first one. The hip and the malleolus were at the same height with respect to the floor. In this manner, the knee is in maximal extension. The patients were asked to lock their ankle joint to about 90°, then to perform maximal dorsal flexion and plantar flexion of the MTP joints only, without ankle flexion or extension. Reflective targets were attached to the skin overlying the following body landmarks: laterally on the fifth MTP joints, lateral malleolus and distally on the big toe. The patients were asked to actively perform maximal plantar flexion, then maximal dorsal flexion, of the MTP joints. The spatial coordinates of each marker were recorded using the ELITE system, to enable calculation of joint motion. For each participant, the dorsal and plantar flexion ROM of the MTP were obtained for both feet.
Gait analysis
Body kinematics were recorded during barefoot walking along a 4-m long straight path indicated by a line drawn on the floor, under self-selected speed. Reflective targets (15 mm diameter) were attached to the skin overlying the following body landmarks: laterally on the fifth MTP joints, lateral malleolus, lateral tibial tables, greater trochanters, and anterior inferior iliac spines. The body movements were recorded using the three-dimensional computerized movement analysis ELITE® system (BTS, Italy) at a rate of 100 Hz, using eight video-based cameras with infrared strobes located around a 4 × 4 × 2 m acquisition volume. Subjects started walking 1 m before entering the acquisition volume in order to eliminate the initiation steps. Ten successive trials were performed.
Data processing has been described elsewhere [14–17]. The spatial coordinates of each marker were recorded, the body being represented as an interconnected chain of rigid segments. Each trial was separated to gait cycles, one cycle corresponding to the sequence heel-strike to heel-strike. Each limb was analysed independently. Then, a custom-made program using Matlab™ software (The Mathworks, USA) was used to calculate the following variables: walking frequency, walking velocity, stride length, duration of stance and double support phase, maximal extension and flexion of the knee and hip joints, and knee and hip joints mobility during walking.
The walking frequency, walking velocity, stride length, duration of stance and double support phase are classically used simple describers of gait. The walking frequency, walking velocity and stride length were calculated using the time-fluctuations of the body mid-point coordinates, as previously described [14], normalized by body height, and were expressed in Hz, m/s and m, respectively. The body mid-point corresponds to the average of the coordinates of the right and left anterior inferior iliac spines and the right and left greater trochanters. Its displacement can be considered as a good appreciation of the movement of the centre of mass [14]. The duration of stance and double support phase were calculated using the low inferior limb axis, defined as the virtual lines joining the greater trochanters and the malleolus [14, 15], and were expressed as a percentage of total gait cycle duration. The knee and hip joint mobility during walking were calculated using their orientations in the saggital plane in function of vertical line. Four right and four left segments were taken into account: the feet (defined as the virtual lines joining the MTP and lateral malleolus targets), the shanks (defined as the virtual lines joining the lateral malleolus and the lateral tibial table targets), the thighs (defined as the virtual lines joining the lateral tibial tables and the greater trochanters), and the hips (defined as the virtual lines joining the greater trochanters and the anterior inferior iliac spines).
All these parameters were obtained for each gait cycle, then averaged in order to obtain one value per patient, which was entered into the analysis. A gait cycle corresponds to the time between two maxima of the elevation angle of the virtual line joining the greater trochanter and the malleolus.
Statistical analysis
Analysis was conducted in several steps.
In the first step, the characteristics of the population were described.
In the second step, the range of motion of the MTP, defined as the angular amplitude between the maximal plantar flexion and dorsal flexion, was compared between patients and controls.
In the third step, the dorsal and plantar flexion MTP ROM obtained in patients complaining of walking pain were compared with those obtained in the other patients using the Mann–Whitney test. The dorsal and plantar flexion MTP ROM were defined as the mean of the measurements for the right and left feet.
In the fourth step, the usual gait describers (walking frequency, walking velocity, stride length, stance duration and double support phase duration) obtained in patients were compared with those obtained in controls using the Mann–Whitney test.
The fifth step of the analysis used only the patients’ data, and the parameters found to be significantly different between patients and controls in the third step. Spearman's r correlation was used to investigate the relationship between these parameters and (i) the dorsal and plantar flexion MTP ROM and (ii) the 100 mm VAS for pain at walking.
Finally, the effects of the loss of dorsal and plantar MTP flexion ROM on the walking kinematics of the knee and hips were investigated. The relationship between the maximal flexion and extension of these joints during walking and the dorsal, then the plantar flexion ROM of the underlying same limb MTP were evaluated using the Spearman's r correlation test. Since the right and the left inferior limb of a single patient cannot be considered as independent, only one inferior limb per patient was randomly entered to the analysis.
Data were analysed using the Statistical Package for the Social Sciences (SPSS 10.1. 2000). Statistical significance was defined as P<0.05.
Results
Nine patients (three males and six females), mean age 60.6 ± 6.8 yr (range 51–72 yr), mean disease duration 10 ± 6 yr (range 1.5–19 yr), and seven controls (two males, five females), mean age 58.5 ± 7.4 yr (range 50–74 yr) were included. The disease characteristics are shown in Table 1.
Patients' characteristics
| Disease duration (yr) (mean ± s.d.) | 10.1 ± 6 |
| Patients’ assessment of global pain (mm) (mean ± s.d.) | 27.3 ± 19 |
| Patients’ assessment of pain at walking (mm) (mean ± s.d.) | 23 ± 24 |
| Patients’ with pain at walking (%) | 55.6 |
| Patients’ overall assessment of disease activity (mm) (mean ± s.d.) | 26.7 ± 20 |
| 28 tender joint count (mean ± s.d.) | 1 ± 1 |
| 28 swelling joint count (mean ± s.d.) | 1.1 ± 3.3 |
| ESR (mm/h) (mean) | 13.1 ± 7 |
| DAS 28 (mean ± s.d.) | 2.7 ± 0.5 |
| HAQ (mean ± s.d.) | 0.82 ± 0.6 |
| Disease duration (yr) (mean ± s.d.) | 10.1 ± 6 |
| Patients’ assessment of global pain (mm) (mean ± s.d.) | 27.3 ± 19 |
| Patients’ assessment of pain at walking (mm) (mean ± s.d.) | 23 ± 24 |
| Patients’ with pain at walking (%) | 55.6 |
| Patients’ overall assessment of disease activity (mm) (mean ± s.d.) | 26.7 ± 20 |
| 28 tender joint count (mean ± s.d.) | 1 ± 1 |
| 28 swelling joint count (mean ± s.d.) | 1.1 ± 3.3 |
| ESR (mm/h) (mean) | 13.1 ± 7 |
| DAS 28 (mean ± s.d.) | 2.7 ± 0.5 |
| HAQ (mean ± s.d.) | 0.82 ± 0.6 |
Patients' characteristics
| Disease duration (yr) (mean ± s.d.) | 10.1 ± 6 |
| Patients’ assessment of global pain (mm) (mean ± s.d.) | 27.3 ± 19 |
| Patients’ assessment of pain at walking (mm) (mean ± s.d.) | 23 ± 24 |
| Patients’ with pain at walking (%) | 55.6 |
| Patients’ overall assessment of disease activity (mm) (mean ± s.d.) | 26.7 ± 20 |
| 28 tender joint count (mean ± s.d.) | 1 ± 1 |
| 28 swelling joint count (mean ± s.d.) | 1.1 ± 3.3 |
| ESR (mm/h) (mean) | 13.1 ± 7 |
| DAS 28 (mean ± s.d.) | 2.7 ± 0.5 |
| HAQ (mean ± s.d.) | 0.82 ± 0.6 |
| Disease duration (yr) (mean ± s.d.) | 10.1 ± 6 |
| Patients’ assessment of global pain (mm) (mean ± s.d.) | 27.3 ± 19 |
| Patients’ assessment of pain at walking (mm) (mean ± s.d.) | 23 ± 24 |
| Patients’ with pain at walking (%) | 55.6 |
| Patients’ overall assessment of disease activity (mm) (mean ± s.d.) | 26.7 ± 20 |
| 28 tender joint count (mean ± s.d.) | 1 ± 1 |
| 28 swelling joint count (mean ± s.d.) | 1.1 ± 3.3 |
| ESR (mm/h) (mean) | 13.1 ± 7 |
| DAS 28 (mean ± s.d.) | 2.7 ± 0.5 |
| HAQ (mean ± s.d.) | 0.82 ± 0.6 |
On clinical examination, all patients presented with a subluxation of MTP 2 to 5. A bilateral hallux valgus was observed in two patients. The MTP 1 mobility was reduced in all.
In patients, the mean angular amplitudes of the right and left MTP were 44 ± 15° (range 12–63°) and 39 ± 11° (range 18.9–58.3°), respectively, and were significantly different from what observed in the controls (mean 68.5 ± 15° and 69.9 ± 18° for the right and left feet, respectively, P<0.014).
The mean plantar flexion ROM of patients was 20 ± 8° (range 4.7–30.6°) (right feet), and 12.1 ± 15° (range –14.2 to 29.8°) (left feet). The mean dorsal flexion ROM was 24.6 ± 21° (range −9.3 to 59.1°) (right feet), and 27.9 ± 9° (range 14.1–49.4°) (left feet).
Five out of nine patients suffered from pain during walking. There was no difference between patients presenting with pain at walking and other patients in MTP plantar flexion ROM, while the dorsal flexion ROM tended to be decreased in patients with pain at walking compared with other patients, but without reaching statistical significance (mean 17.2 ± 11° vs 37.5 ± 13°, respectively, P = 0.063).
The usual gait describers in patients and controls are shown in Table 2. There was no difference between patients and controls in walking frequency, stance duration and double support phase duration. However, walking velocity and stride length were lower in patients than in controls.
Gait describers in patients and controls
| . | Patients . | Controls . |
|---|---|---|
| Walking frequency (Hz) (mean ± s.d.) | 1.12 ± 0.12 | 1.09 ± 0.13 |
| Walking velocity (m/s) (mean ± s.d.) | 1.21 ± 0.17* | 1.34 ± 0.07 |
| Stride length (m) (mean ± s.d.) | 1.4 ± 0.14** | 1.58 ± 0.11 |
| Stance duration (% of total gait cycle duration) (mean ± s.d.) | 65.4 ± 1.3 | 65.3 ± 1.2 |
| Double-support phase duration (% of total gait cycle duration) (mean ± s.d.) | 15.6 ± 1.3 | 15.4 ± 1.3 |
| . | Patients . | Controls . |
|---|---|---|
| Walking frequency (Hz) (mean ± s.d.) | 1.12 ± 0.12 | 1.09 ± 0.13 |
| Walking velocity (m/s) (mean ± s.d.) | 1.21 ± 0.17* | 1.34 ± 0.07 |
| Stride length (m) (mean ± s.d.) | 1.4 ± 0.14** | 1.58 ± 0.11 |
| Stance duration (% of total gait cycle duration) (mean ± s.d.) | 65.4 ± 1.3 | 65.3 ± 1.2 |
| Double-support phase duration (% of total gait cycle duration) (mean ± s.d.) | 15.6 ± 1.3 | 15.4 ± 1.3 |
*P = 0.04 compared with controls, Mann–Whitney test. **P = 0.02 compared with controls, Mann–Whitney test.
Gait describers in patients and controls
| . | Patients . | Controls . |
|---|---|---|
| Walking frequency (Hz) (mean ± s.d.) | 1.12 ± 0.12 | 1.09 ± 0.13 |
| Walking velocity (m/s) (mean ± s.d.) | 1.21 ± 0.17* | 1.34 ± 0.07 |
| Stride length (m) (mean ± s.d.) | 1.4 ± 0.14** | 1.58 ± 0.11 |
| Stance duration (% of total gait cycle duration) (mean ± s.d.) | 65.4 ± 1.3 | 65.3 ± 1.2 |
| Double-support phase duration (% of total gait cycle duration) (mean ± s.d.) | 15.6 ± 1.3 | 15.4 ± 1.3 |
| . | Patients . | Controls . |
|---|---|---|
| Walking frequency (Hz) (mean ± s.d.) | 1.12 ± 0.12 | 1.09 ± 0.13 |
| Walking velocity (m/s) (mean ± s.d.) | 1.21 ± 0.17* | 1.34 ± 0.07 |
| Stride length (m) (mean ± s.d.) | 1.4 ± 0.14** | 1.58 ± 0.11 |
| Stance duration (% of total gait cycle duration) (mean ± s.d.) | 65.4 ± 1.3 | 65.3 ± 1.2 |
| Double-support phase duration (% of total gait cycle duration) (mean ± s.d.) | 15.6 ± 1.3 | 15.4 ± 1.3 |
*P = 0.04 compared with controls, Mann–Whitney test. **P = 0.02 compared with controls, Mann–Whitney test.
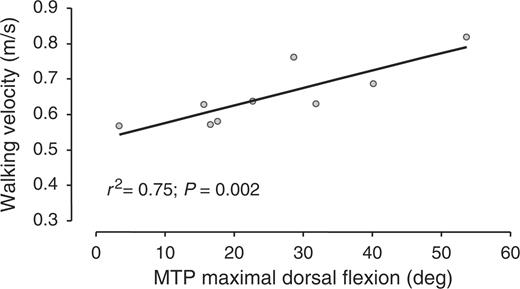
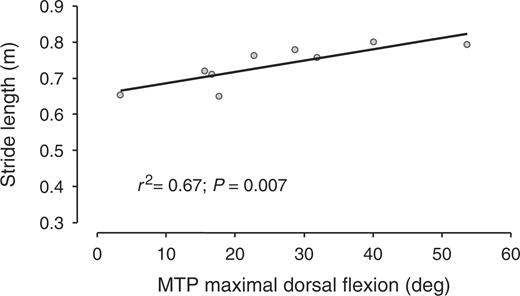
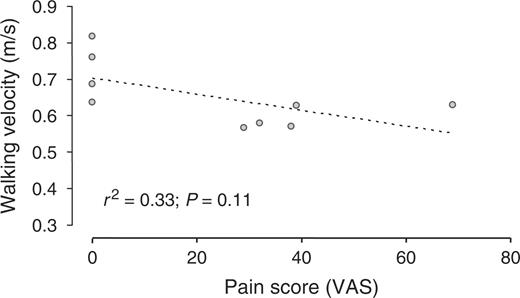
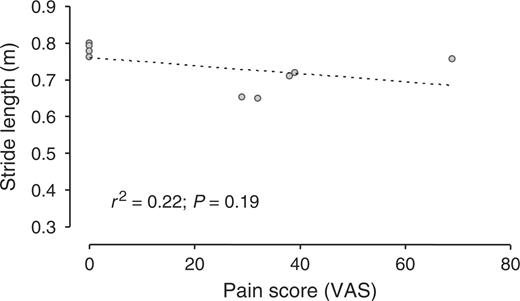
In patients, walking velocity was positively related to the dorsal flexion ROM of the MTP (r2 = 0.75, P = 0.002; Fig. 1), and was not related to the plantar flexion ROM of the MTP. Stride length was positively related to the dorsal flexion ROM of the MTP (r2 = 0.67, P = 0.007; Fig. 2), and was not related to the plantar flexion ROM of the MTP. Walking velocity and stride length did not correlate to the 100 mm VAS for pain at walking (Figs 3 and 4).
Walking velocity as a function of MTP dorsal flexion range of motion.
Stride length as a function of MTP dorsal flexion range of motion.
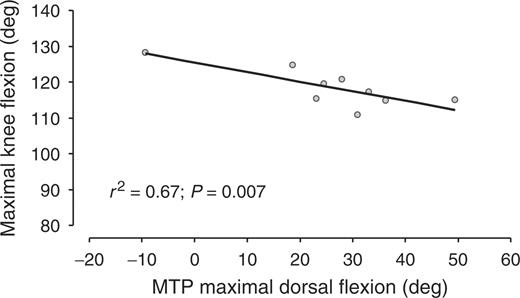
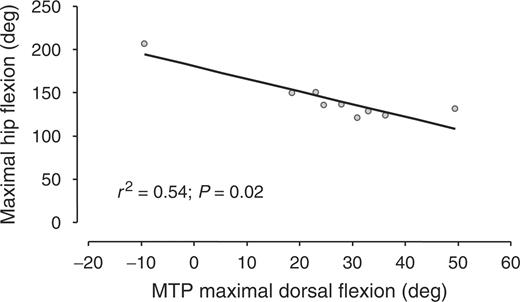
There was a strong negative relationship between the maximal flexion of knees and hips during walking and the underlying dorsal flexion ROM of the MTP (r2 = 0.67, P = 0.007, and r2 = 0.54, P = 0.02, respectively) (Figs 5 and 6). On the contrary, there was no relationship between the maximal extension of knees and hips during walking and the maximal dorsal flexion ROM of the MTP (r2 = 0.15, P = 0.31, and r2 = 0.24, P = 0.19, respectively), or between all of these parameters and the maximal plantar flexion ROM of the MTP. Finally, since hip and knee kinematics during walking are related to the walking velocity in normal subjects, and since velocity was related to the MTP dorsal flexion ROM in the patients of the present study, a new analysis was added a posteriori, aimed at evaluating whether the observed changes in knee and hip flexion during walking were directly due to the loss of dorsal flexion ROM of the MTP or were due to the consequences of such ROM loss on walking velocity. In this new analysis, the relationship between walking velocity and knee and hip maximal flexion during walking in RA patients was investigated using the Spearman's r correlation test, but no relationship was found.
Maximal knee flexion during walking as a function of the dorsal flexion range of motion of underlying MTP.
Hip maximal flexion during walking in function of the dorsal flexion range of motion of underlying MTP.
Discussion
In this study, walking velocity and stride length were decreased in patients with inactive RA and decreased MTP ROM, compared with controls. Such a decrease was strongly related to the dorsal flexion ROM of the MTP and not to the plantar flexion ROM of the MTP or to the pain at walking. Otherwise, maximal flexion of knees and hips during walking were related to the loss of dorsal flexion ROM of the MTP, suggesting that such disability might induce changes in overlying joint kinematics.
A limitation of this study is the low number of patients, which induced a lack of statistical power and, possibly, the absence of demonstration of some true relationships. In particular, the absence of difference in MTP dorsal flexion ROM between patients with and without pain at walking might be due to such a lack of power. On the other hand, the advantage inherent in this limitation is that only very strong relationships could be demonstrated. Another limitation is the hallux valgus presented by two out of the nine patients. Such an abnormality might have influenced the results. Unfortunately, due to the small number of patients such a hypothesis could not be investigated.
At the present time, the effects of a loss of ROM in MTP joints on locomotor apparatus, and on the kinematics of walking, have not been well documented in RA. One interest of the present study is that potential bias or confounding factors were avoided; the hip and knee ROM were within normal limits, and the patients had inactive disease with no inferior limb synovitis, whereas it has been shown that inferior limb synovitis induces gait abnormalities [18]. It was not possible to avoid all confounding factors. In particular, more than half of the included patients suffered from pain at walking. However, the level of pain during walking was not found to be related to gait parameters. It must be pointed out that due to the lack of statistical power, and to the particular subgroup of selected patients, one should not conclude from the present results that pain does not influence gait in RA patients.
In a previous study, no correlation was found between gait parameters and forefoot structural deformity [19], but the structural deformity was assessed using a global index, which did not provide the same information as the measurement of MTP mobility. The present results suggest that the loss of MTP dorsal flexion ROM does induce relevant gait changes. The mechanisms of such changes probably include a direct effect of the reduced mobility, since MTP dorsal flexion contributes or facilitates the body progression at the end of the stance phase. Another potential complementary mechanism might be impairment of plantar tactile sensation induced by loss of MTP movement and MTP deformity. It has recently been shown that such a loss induces a modification of joint kinematics, ground contact forces and push off [15, 20].
The lack of any observed relationship between reduced plantar flexion mobility and gait parameters might be considered as surprising since MTP plantar flexors ensure support and propulsion of the body by facilitating impulsion, swing initiation and forward acceleration of the trunk [11, 21]. However, the main contribution of the plantar flexors occurs at the end of the stance phase in the movement from the maximum dorsiflexion to the neutral position. Consequently, the effect of loss in plantar flexion should have less consequence on gait behaviour.
In a previous study, the ROM in hip and knee flexion and extension during gait were no different in RA patients with preserved hip and knee ROM compared with normal subjects [22]. Such results and the present study cannot, however, be considered as contradictory since in the previous study only 8 out of 26 feet presented with MTP subluxation, and the correlation between hip and knee ROM, and the MTP ROM, was not evaluated. In the present study, the observed negative relationship between MTP dorsal flexion ROM and maximal knee and hip flexion during walking is of great interest. They might correspond to an adaptation of the angular variation of limbs segment to the reduced MTP mobility. Several recent studies have demonstrated that temporal angular variation of the thigh, shank and foot are remarkably constant. Inferior limb coordination is governed by a common principle of multisegment coordination [23, 24] which simplifies the spatiotemporal control of locomotion, and is controlled by the nervous system [16, 25]. In RA patients with reduced MTP mobility, the central nervous system might change lower limb segment coordination in order to reduce forefoot pressure and pain, and/or to reduce energy consumption, and/or to allow or facilitate walking. From a clinical point of view, whatever the goals and mechanisms of this adjustment, the mechanical aspect of these changes is of particular interest, since the modifications in forces exerted at the hip and knee might result in a secondary structural degradation of these joints. Such a hypothesis needs to be evaluated in further studies.
The treatment of impaired feet in RA includes the use of orthoses and surgery [26]. Orthotic devices reduce pain, and have been shown to result in changes in three-dimensional kinematics in patients with painful rearfoot deformity [27]. In patients with forefoot pain and/or deformity, orthoses have been shown to reduce pain, and to alter the pressure distribution beneath the plantar surface. However, conflicting results have been published on gait describers [8, 28–31]. Different types of surgical procedures have been proposed in RA patients with forefoot pain and deformity, particularly resection arthroplasty of the MTP joints, with or without fusion of the first MTP joint [26]. The present results emphasize the importance of the treatment of impaired MTP in RA patients, and suggest that the goal of such treatment should be to prevent a reduction in mobility and to improve the MTP ROM if already reduced, as well as to relieve pain. In particular, they suggest that fusion of the first MTP joint might be proposed very cautiously. Particular attention should be given to dorsal, rather than plantar, flexion mobility. The three-dimensional kinematics of surgery in RA patients with forefoot involvement should be assessed in order to evaluate the results in terms of MTP mobility.

This study was supported by the Dijon University Hospital, by the Institut National de Santé et de la Recherche Médicale (INSERM) and by the Conseil Régional de Bourgogne.
The authors have declared no conflicts of interest.
References
Simkin A. The dynamic vertical force distribution during level walking under normal and rheumatic feet.
O’Connell PG, Lohmann Siegel K, Kepple TM, Stanhope SJ, Gerber LH. Forefoot deformity, pain, and mobility in rheumatoid and nonarthritic subjects.
Sharma M, Dhanendran M, Hutton WC, Corbett M. Changes in load bearing in the rheumatoid foot.
Thompson C. Surgical treatment of disorders of the fore part of the foot.
Minns RJ, Craxford AD. Pressure under the forefoot in rheumatoid arthritis. A comparison of static and dynamic methods of assessment.
Craxford AD, Stevens J, Park C. Management of the deformed rheumatoid forefoot. A comparison of conservative and surgical methods.
Siegel KL, Kepple TM, O’Connell PG, Gerber LH, Stanhope SJ. A technique to evaluate foot function during the stance phase of gait.
Hodge MC, Bach TM, Carter GM. Orthotic management of plantar pressure and pain in rheumatoid arthritis.
Hunt AE, Smith RM, Torode M, Keenan AM. Inter-segment foot motion and ground reaction forces over the stance phase of walking.
Woodburn J, Nelson KM, Siegel KL, Kepple TM, Gerber LH. Multisegment foot motion during gait: proof of concept in rheumatoid arthritis.
Neptune RR, Kautz SA, Zajac FE. Contributions of the individual ankle plantar flexors to support, forward progression and swing initiation during walking.
Beauchamp CG, Kirby T, Rudge SR, Worthington BS, Nelson J. Fusion of the first metatarsophalangeal joint in forefoot arthroplasty.
Andersen JA, Klaborg KE. Forefoot amputation in rheumatoid arthritis.
Courtine G, Schieppati M. Human walking along a curved path. I. Body trajectory, segment orientation and the effect of vision.
Ivanenko YP, Grasso R, Macellari V, Lacquaniti F. Control of foot trajectory in human locomotion: role of ground contact forces in simulated reduced gravity.
Borghese NA, Bianchi L, Lacquaniti F. Kinematic determinants of human locomotion.
Bianchi L, Angelini D, Orani GP, Lacquaniti F. Kinematic coordination in human gait: relation to mechanical energy cost.
Hamilton J, Brydson G, Fraser S, Grant M. Walking ability as a measure of treatment effect in early rheumatoid arthritis.
Platto MJ, O’Connell PG, Hicks JE, Gerber LH. The relationship of pain and deformity of the rheumatoid foot to gait and an index of functional ambulation.
Eils E, Behrens S, Mers O, Thorwesten L, Volker K, Rosenbaum D. Reduced plantar sensation causes a cautious walking pattern.
Anderson FC, Pandy MG. Individual muscle contributions to support in normal walking.
Isacson J, Brostrom LA. Gait in rheumatoid arthritis: an electrogoniometric investigation.
Bianchi L, Angelini D, Lacquaniti F. Individual characteristics of human walking mechanics.
Mann RA, Horton GA. Management of the foot and ankle in rheumatoid arthritis.
Woodburn J, Helliwell PS, Barker S. Changes in 3D joint kinematics support the continuous use of orthoses in the management of painful rearfoot deformity in rheumatoid arthritis.
Mejjad O, Vittecoq O, Pouplin S, Grassin-Delyle L, Weber J, Le Loet X. Foot orthotics decrease pain but do not improve gait in rheumatoid arthritis patients.
Li CY, Imaishi K, Shiba N et al. Biomechanical evaluation of foot pressure and loading force during gait in rheumatoid arthritic patients with and without foot orthosis.
Kavlak Y, Uygur F, Korkmaz C, Bek N. Outcome of orthoses intervention in the rheumatoid foot.
Author notes
1UFR STAPS, INSERM/ERM 0207, University of Burgundy and 2Department of Rheumatology, Dijon University Hospital, Dijon, France.
- rheumatoid arthritis
- gait
- limb
- forefoot
- hip region
- knee region
- knee joint
- metatarsophalangeal joint
- pain
- range of motion
- synovitis
- leg
- gait analysis
- kinematics
- mobility
- limitation of joint movement
- walking speed
- length of stride during ambulation
- characteristics of gait
- knee flexion
- plantar flexion
- range of thoracic spine flexion
- disease remission




Comments