-
PDF
- Split View
-
Views
-
Cite
Cite
D. R. Blake, P. Robson, M. Ho, R. W. Jubb, C. S. McCabe, Preliminary assessment of the efficacy, tolerability and safety of a cannabis-based medicine (Sativex) in the treatment of pain caused by rheumatoid arthritis, Rheumatology, Volume 45, Issue 1, January 2006, Pages 50–52, https://doi.org/10.1093/rheumatology/kei183
Close - Share Icon Share
Abstract
Objectives. To assess the efficacy of a cannabis-based medicine (CBM) in the treatment of pain due to rheumatoid arthritis (RA).
Methods. We compared a CBM (Sativex) with placebo in a randomized, double-blind, parallel group study in 58 patients over 5 weeks of treatment. The CBM was administered by oromucosal spray in the evening and assessments were made the following morning. Efficacy outcomes assessed were pain on movement, pain at rest, morning stiffness and sleep quality measured by a numerical rating scale, the Short-Form McGill Pain Questionnaire (SF-MPQ) and the DAS28 measure of disease activity.
Results. Seventy-five patients were screened and 58 met the eligibility criteria. Thirty-one were randomized to the CBM and 27 to placebo. Mean (s.d.) daily dose achieved in the final treatment week was 5.4 (0.84) actuations for the CBM and 5.3 (1.18) for placebo. In comparison with placebo, the CBM produced statistically significant improvements in pain on movement, pain at rest, quality of sleep, DAS28 and the SF-MPQ pain at present component. There was no effect on morning stiffness but baseline scores were low. The large majority of adverse effects were mild or moderate, and there were no adverse effect-related withdrawals or serious adverse effects in the active treatment group.
Conclusions. In the first ever controlled trial of a CBM in RA, a significant analgesic effect was observed and disease activity was significantly suppressed following Sativex treatment. Whilst the differences are small and variable across the population, they represent benefits of clinical relevance and show the need for more detailed investigation in this indication.
Evidence from basic science and human trials suggests that cannabis-based medicines (CBM) may have therapeutic potential in a range of medical conditions, particularly in the treatment of intractable pain [1, 2]. Cannabis has been used historically in the treatment of pain due to rheumatoid arthritis (RA), but this has never been formally evaluated in a clinical trial. Δ-9-Tetrahydrocannabinol (THC) and cannabidiol (CBD) are recognized as key therapeutic constituents that act synergistically together and with other plant constituents [3]. THC has analgesic activity in both nociceptive and neuropathic pain [1, 2]. Both THC and CBD have anti-inflammatory effects [4], and CBD was found to block progression of disease and produce clinical improvement in a murine model of RA [5]. In a recent survey [6] of 2969 people who agreed to fill in a questionnaire about medicinal cannabis, 947 (32%) stated that they had obtained the drug from the black market for symptom relief. Of these, 155 (16%) gave symptom relief for arthritis (type not specified) as the reason for smoking cannabis. This was the fifth-commonest indication after multiple sclerosis, neuropathy, chronic pain and depression.
We present the results of the first controlled trial of a CBM in the symptomatic treatment of RA in humans.
Patients and methods
This was a preliminary multicentre, double-blind, randomized, parallel-group comparison of a CBM (Sativex) and placebo administered for 5 weeks in the treatment of pain caused by RA. Sativex consists of a blend of whole plant extracts which delivers approximately equal amounts of THC and CBD. This ratio was selected to reflect the proportions found in cannabis used historically for medicinal purposes, and to maximize the potential for synergism [7]. Minor cannabinoids, including cannabinol, cannabichromene and cannabigerol, are also present in trace quantities. All three of these have been found to have anti-inflammatory properties in laboratory studies, as have other plant components, such as terpenoids and flavonoids [3]. Sativex was administered by oromucosal spray, each activation delivering 2.7 mg THC and 2.5 mg CBD. Eligible patients had a diagnosis of RA meeting ACR criteria, with active arthritis not adequately controlled by standard medication. NSAID and prednisolone regimes had to have been stabilized for 1 month and DMARDs for 3 months prior to enrolment, and were maintained constant throughout the study. Exclusion criteria included a history of psychiatric disorders or substance misuse, severe cardiovascular, renal or hepatic disorder, or a history of epilepsy. Dosing was restricted to the evening to minimize possible intoxication-type reactions, with randomized treatment allocation using permuted blocks of four. Starting dose was one actuation within 0.5 h of retiring, and this was increased by one actuation every 2 days to a maximum of six actuations according to individual response. Stable dosing was then maintained for a further 3 weeks. Patients gave written informed consent to participate, and the study was approved by each local research ethics committee.
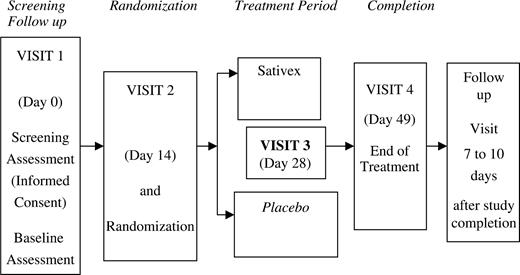
Primary efficacy variable was pain on movement measured by a 0–10 numerical rating scale (NRS) each morning. Baseline score (obtained as an average of the last 4 days of the 14-day baseline period) was compared with the average of the last 14 days of treatment. Secondary outcomes included NRS measures of pain at rest, sleep quality and morning stiffness, the Short-Form McGill Pain Questionnaire (SF-MPQ) and the 28-joint disease activity score (DAS28). The study plan is shown in Fig. 1. Based on previous results for pain on movement, it was calculated that 23 patients/group would be required to detect 2 units difference with 90% power. A minimum of 54 patients were to be recruited to allow for dropouts. Normally distributed data were to be analysed using one-way analysis of covariance. Change from baseline to endpoint was to be compared between the two treatment groups with the baseline score considered as a covariate. Non-parametric analysis was to be used if there was considerable departure from normality.
Results
The protocol and all study documentation was approved by the Independent Research Ethics Committee representing each of the eight participating centres. Seventy-five patients were screened and 58 met the eligibility criteria. Written informed consent, as specified by the Declaration of Helsinki (2000), was obtained from all patients prior to screening. Thirty-one of the eligible patients were randomized to CBM and 27 to placebo. One patient withdrew from the active treatment group (unrelated surgery) and three from placebo (adverse events). There were no significant differences in demographics between groups (Table 1). Mean (s.d.) daily dose achieved in the final treatment week was 5.4 (0.84) actuations for CBM and 5.3 (1.18) for placebo.
Summary of demography and patient baseline characteristics (intention-to-treat population)
| . | . | Treatment group . | . | . | ||
|---|---|---|---|---|---|---|
| . | . | Sativex (n = 31) . | Placebo (n = 27) . | Total (n = 58) . | ||
| Age (yr) | Mean | 60.9 | 64.9 | 62.8 | ||
| s.d. | 10.6 | 8.5 | 9.8 | |||
| Sex | Male | 8 (26%) | 4 (15%) | 12 (21%) | ||
| Female | 23 (74%) | 23 (85%) | 46 (79%) | |||
| Height (cm) | Mean | 163.67 | 158.92 | 161.46 | ||
| s.d. | 9.00 | 8.10 | 8.85 | |||
| Weight (kg) | Mean | 76.96 | 70.60 | 74.00 | ||
| s.d. | 17.57 | 20.71 | 19.20 | |||
| Alcohol (U/week) | Mean | 2.3 | 3.4 | 2.8 | ||
| s.d. | 3.6 | 5.7 | 4.7 | |||
| Smoker | Yes | 4 (13%) | 3 (11%) | 7 (12%) | ||
| No | 27 (87%) | 24 (89%) | 51 (88%) | |||
| Recreational use | Yes | 1 (3%) | 1 (4%) | 2 (3%) | ||
| of cannabis | No | 30 (97%) | 26 (96%) | 56 (97%) | ||
| Medicinal use | Yes | 1 (3%) | 0 | 1 (2%) | ||
| of cannabis | No | 30 (97%) | 27 (100%) | 57 (98%) | ||
| . | . | Treatment group . | . | . | ||
|---|---|---|---|---|---|---|
| . | . | Sativex (n = 31) . | Placebo (n = 27) . | Total (n = 58) . | ||
| Age (yr) | Mean | 60.9 | 64.9 | 62.8 | ||
| s.d. | 10.6 | 8.5 | 9.8 | |||
| Sex | Male | 8 (26%) | 4 (15%) | 12 (21%) | ||
| Female | 23 (74%) | 23 (85%) | 46 (79%) | |||
| Height (cm) | Mean | 163.67 | 158.92 | 161.46 | ||
| s.d. | 9.00 | 8.10 | 8.85 | |||
| Weight (kg) | Mean | 76.96 | 70.60 | 74.00 | ||
| s.d. | 17.57 | 20.71 | 19.20 | |||
| Alcohol (U/week) | Mean | 2.3 | 3.4 | 2.8 | ||
| s.d. | 3.6 | 5.7 | 4.7 | |||
| Smoker | Yes | 4 (13%) | 3 (11%) | 7 (12%) | ||
| No | 27 (87%) | 24 (89%) | 51 (88%) | |||
| Recreational use | Yes | 1 (3%) | 1 (4%) | 2 (3%) | ||
| of cannabis | No | 30 (97%) | 26 (96%) | 56 (97%) | ||
| Medicinal use | Yes | 1 (3%) | 0 | 1 (2%) | ||
| of cannabis | No | 30 (97%) | 27 (100%) | 57 (98%) | ||
Summary of demography and patient baseline characteristics (intention-to-treat population)
| . | . | Treatment group . | . | . | ||
|---|---|---|---|---|---|---|
| . | . | Sativex (n = 31) . | Placebo (n = 27) . | Total (n = 58) . | ||
| Age (yr) | Mean | 60.9 | 64.9 | 62.8 | ||
| s.d. | 10.6 | 8.5 | 9.8 | |||
| Sex | Male | 8 (26%) | 4 (15%) | 12 (21%) | ||
| Female | 23 (74%) | 23 (85%) | 46 (79%) | |||
| Height (cm) | Mean | 163.67 | 158.92 | 161.46 | ||
| s.d. | 9.00 | 8.10 | 8.85 | |||
| Weight (kg) | Mean | 76.96 | 70.60 | 74.00 | ||
| s.d. | 17.57 | 20.71 | 19.20 | |||
| Alcohol (U/week) | Mean | 2.3 | 3.4 | 2.8 | ||
| s.d. | 3.6 | 5.7 | 4.7 | |||
| Smoker | Yes | 4 (13%) | 3 (11%) | 7 (12%) | ||
| No | 27 (87%) | 24 (89%) | 51 (88%) | |||
| Recreational use | Yes | 1 (3%) | 1 (4%) | 2 (3%) | ||
| of cannabis | No | 30 (97%) | 26 (96%) | 56 (97%) | ||
| Medicinal use | Yes | 1 (3%) | 0 | 1 (2%) | ||
| of cannabis | No | 30 (97%) | 27 (100%) | 57 (98%) | ||
| . | . | Treatment group . | . | . | ||
|---|---|---|---|---|---|---|
| . | . | Sativex (n = 31) . | Placebo (n = 27) . | Total (n = 58) . | ||
| Age (yr) | Mean | 60.9 | 64.9 | 62.8 | ||
| s.d. | 10.6 | 8.5 | 9.8 | |||
| Sex | Male | 8 (26%) | 4 (15%) | 12 (21%) | ||
| Female | 23 (74%) | 23 (85%) | 46 (79%) | |||
| Height (cm) | Mean | 163.67 | 158.92 | 161.46 | ||
| s.d. | 9.00 | 8.10 | 8.85 | |||
| Weight (kg) | Mean | 76.96 | 70.60 | 74.00 | ||
| s.d. | 17.57 | 20.71 | 19.20 | |||
| Alcohol (U/week) | Mean | 2.3 | 3.4 | 2.8 | ||
| s.d. | 3.6 | 5.7 | 4.7 | |||
| Smoker | Yes | 4 (13%) | 3 (11%) | 7 (12%) | ||
| No | 27 (87%) | 24 (89%) | 51 (88%) | |||
| Recreational use | Yes | 1 (3%) | 1 (4%) | 2 (3%) | ||
| of cannabis | No | 30 (97%) | 26 (96%) | 56 (97%) | ||
| Medicinal use | Yes | 1 (3%) | 0 | 1 (2%) | ||
| of cannabis | No | 30 (97%) | 27 (100%) | 57 (98%) | ||
Efficacy endpoints are shown in Table 2. Statistically significant improvements in pain on movement, pain at rest, quality of sleep, DAS28 and the SF-MPQ pain at present component were seen following CBM in comparison with placebo.
Efficacy endpoints: difference between change from baseline between CBM and placebo after 5 weeks of treatment
| . | Baseline (mean/median)a . | . | Endpoint (mean/median)a . | . | . | . | . | ||
|---|---|---|---|---|---|---|---|---|---|
| Efficacy endpoint . | CBM . | Placebo . | CBM . | Placebo . | Difference (mean/mediana) . | 95% confidence interval . | P . | ||
| Morning pain on movementa | 7.0 | 6.7 | 4.8 | 5.3 | −0.95 | −1.83, −0.02 | 0.044 | ||
| Morning pain at resta | 5.3 | 5.3 | 3.1 | 4.1 | −1.04 | −1.90, −0.18 | 0.018 | ||
| Morning stiffnessa | 3.5 | 3.8 | 3.0 | 3.2 | −0.09 | −0.58, 0.23 | 0.454 | ||
| Quality of sleep | 5.7 | 5.8 | 3.4 | 4.6 | −1.17 | −2.20, −0.14 | 0.027 | ||
| DAS 28 | 5.9 | 6.0 | 5.0 | 5.9 | −0.76 | −1.23, −0.28 | 0.002 | ||
| SF-MPQ, total intensity of paina (a) | 15.0 | 20.0 | 10.5 | 13.0 | 3.00 | −3.00, 9.00 | 0.302 | ||
| SF-MPQ, intensity of pain at presenta (b) | 48.0 | 50.0 | 33.0 | 50.0 | −3.00 | −18.0, 9.00 | 0.574 | ||
| SF-MPQ, pain at present (c) | 3.2 | 3.2 | 2.6 | 3.3 | −0.72 | −1.30, −0.14 | 0.016 | ||
| . | Baseline (mean/median)a . | . | Endpoint (mean/median)a . | . | . | . | . | ||
|---|---|---|---|---|---|---|---|---|---|
| Efficacy endpoint . | CBM . | Placebo . | CBM . | Placebo . | Difference (mean/mediana) . | 95% confidence interval . | P . | ||
| Morning pain on movementa | 7.0 | 6.7 | 4.8 | 5.3 | −0.95 | −1.83, −0.02 | 0.044 | ||
| Morning pain at resta | 5.3 | 5.3 | 3.1 | 4.1 | −1.04 | −1.90, −0.18 | 0.018 | ||
| Morning stiffnessa | 3.5 | 3.8 | 3.0 | 3.2 | −0.09 | −0.58, 0.23 | 0.454 | ||
| Quality of sleep | 5.7 | 5.8 | 3.4 | 4.6 | −1.17 | −2.20, −0.14 | 0.027 | ||
| DAS 28 | 5.9 | 6.0 | 5.0 | 5.9 | −0.76 | −1.23, −0.28 | 0.002 | ||
| SF-MPQ, total intensity of paina (a) | 15.0 | 20.0 | 10.5 | 13.0 | 3.00 | −3.00, 9.00 | 0.302 | ||
| SF-MPQ, intensity of pain at presenta (b) | 48.0 | 50.0 | 33.0 | 50.0 | −3.00 | −18.0, 9.00 | 0.574 | ||
| SF-MPQ, pain at present (c) | 3.2 | 3.2 | 2.6 | 3.3 | −0.72 | −1.30, −0.14 | 0.016 | ||
aThese scores were not normally distributed and were therefore analysed non-parametrically (Wilcoxon rank-sum test, Hodges–Lehmann median difference and 95% CI). Other outcomes were subjected to analysis of covariance. SF-MPQ was developed to assess three components of pain: the sensation of pain, its emotional effect and the patient's cognitive assessment of the pain. Component (a) is a score derived from 15 adjectives describing pain, (b) is a single VAS score and (c) is a verbal rating scale extending from ‘none’ to ‘excruciating’ [10].
Efficacy endpoints: difference between change from baseline between CBM and placebo after 5 weeks of treatment
| . | Baseline (mean/median)a . | . | Endpoint (mean/median)a . | . | . | . | . | ||
|---|---|---|---|---|---|---|---|---|---|
| Efficacy endpoint . | CBM . | Placebo . | CBM . | Placebo . | Difference (mean/mediana) . | 95% confidence interval . | P . | ||
| Morning pain on movementa | 7.0 | 6.7 | 4.8 | 5.3 | −0.95 | −1.83, −0.02 | 0.044 | ||
| Morning pain at resta | 5.3 | 5.3 | 3.1 | 4.1 | −1.04 | −1.90, −0.18 | 0.018 | ||
| Morning stiffnessa | 3.5 | 3.8 | 3.0 | 3.2 | −0.09 | −0.58, 0.23 | 0.454 | ||
| Quality of sleep | 5.7 | 5.8 | 3.4 | 4.6 | −1.17 | −2.20, −0.14 | 0.027 | ||
| DAS 28 | 5.9 | 6.0 | 5.0 | 5.9 | −0.76 | −1.23, −0.28 | 0.002 | ||
| SF-MPQ, total intensity of paina (a) | 15.0 | 20.0 | 10.5 | 13.0 | 3.00 | −3.00, 9.00 | 0.302 | ||
| SF-MPQ, intensity of pain at presenta (b) | 48.0 | 50.0 | 33.0 | 50.0 | −3.00 | −18.0, 9.00 | 0.574 | ||
| SF-MPQ, pain at present (c) | 3.2 | 3.2 | 2.6 | 3.3 | −0.72 | −1.30, −0.14 | 0.016 | ||
| . | Baseline (mean/median)a . | . | Endpoint (mean/median)a . | . | . | . | . | ||
|---|---|---|---|---|---|---|---|---|---|
| Efficacy endpoint . | CBM . | Placebo . | CBM . | Placebo . | Difference (mean/mediana) . | 95% confidence interval . | P . | ||
| Morning pain on movementa | 7.0 | 6.7 | 4.8 | 5.3 | −0.95 | −1.83, −0.02 | 0.044 | ||
| Morning pain at resta | 5.3 | 5.3 | 3.1 | 4.1 | −1.04 | −1.90, −0.18 | 0.018 | ||
| Morning stiffnessa | 3.5 | 3.8 | 3.0 | 3.2 | −0.09 | −0.58, 0.23 | 0.454 | ||
| Quality of sleep | 5.7 | 5.8 | 3.4 | 4.6 | −1.17 | −2.20, −0.14 | 0.027 | ||
| DAS 28 | 5.9 | 6.0 | 5.0 | 5.9 | −0.76 | −1.23, −0.28 | 0.002 | ||
| SF-MPQ, total intensity of paina (a) | 15.0 | 20.0 | 10.5 | 13.0 | 3.00 | −3.00, 9.00 | 0.302 | ||
| SF-MPQ, intensity of pain at presenta (b) | 48.0 | 50.0 | 33.0 | 50.0 | −3.00 | −18.0, 9.00 | 0.574 | ||
| SF-MPQ, pain at present (c) | 3.2 | 3.2 | 2.6 | 3.3 | −0.72 | −1.30, −0.14 | 0.016 | ||
aThese scores were not normally distributed and were therefore analysed non-parametrically (Wilcoxon rank-sum test, Hodges–Lehmann median difference and 95% CI). Other outcomes were subjected to analysis of covariance. SF-MPQ was developed to assess three components of pain: the sensation of pain, its emotional effect and the patient's cognitive assessment of the pain. Component (a) is a score derived from 15 adjectives describing pain, (b) is a single VAS score and (c) is a verbal rating scale extending from ‘none’ to ‘excruciating’ [10].
Adverse effects (AE) occurring in two or more patients are shown in Table 3. AE in CBM group were all of mild or moderate intensity except for two (6%) rated severe (constipation; ‘malaise’) compared with six (22%) in the placebo group. Eight patients (26%) receiving CBM experienced transient dizziness at some point, though in all cases this was rated as mild. The exact timing of these episodes was recorded in six of these patients: four occurred during the initial 2-week titration period, the other two at 16 days. There were no withdrawals due to AE in the CBM group compared with three (11%) for placebo, and no serious AE following the active treatment compared with two (7%) in the placebo group.
Adverse events recorded as ‘possibly’, ‘probably’ or ‘definitely’ related to study drug occurring in more than one patient
| Adverse event . | CBM (n = 31) . | Placebo (n = 27) . | All patients (n = 58) . |
|---|---|---|---|
| Dizziness (all mild) | 8 (26%) | 1 (4%) | 9 (16%) |
| Light-headedness | 3 (10%) | 1 (4%) | 4 (7%) |
| Dry mouth | 4 (13%) | 0 | 4 (7%) |
| Nausea | 2 (6%) | 1 (4%) | 3 (5%) |
| Arthritic pains | 1 (3%) | 1 (4%) | 2 (4%) |
| Constipation | 1 (3%) | 1 (4%) | 2 (4%) |
| Drowsiness | 1 (3%) | 1 (4%) | 2 (4%) |
| Fall | 2 (6%) | 0 | 2 (4%) |
| Headache | 1 (3%) | 1 (4%) | 2 (4%) |
| Palpitations | 0 | 2 (7%) | 2 (4%) |
| Vomiting | 0 | 2 (7%) | 2 (4%) |
| Serious adverse events | 0 | 2 (7%) | 2 (4%) |
| Adverse events leading to withdrawal | 0 | 3 (11%) | 3 (5%) |
| Adverse event . | CBM (n = 31) . | Placebo (n = 27) . | All patients (n = 58) . |
|---|---|---|---|
| Dizziness (all mild) | 8 (26%) | 1 (4%) | 9 (16%) |
| Light-headedness | 3 (10%) | 1 (4%) | 4 (7%) |
| Dry mouth | 4 (13%) | 0 | 4 (7%) |
| Nausea | 2 (6%) | 1 (4%) | 3 (5%) |
| Arthritic pains | 1 (3%) | 1 (4%) | 2 (4%) |
| Constipation | 1 (3%) | 1 (4%) | 2 (4%) |
| Drowsiness | 1 (3%) | 1 (4%) | 2 (4%) |
| Fall | 2 (6%) | 0 | 2 (4%) |
| Headache | 1 (3%) | 1 (4%) | 2 (4%) |
| Palpitations | 0 | 2 (7%) | 2 (4%) |
| Vomiting | 0 | 2 (7%) | 2 (4%) |
| Serious adverse events | 0 | 2 (7%) | 2 (4%) |
| Adverse events leading to withdrawal | 0 | 3 (11%) | 3 (5%) |
Adverse events recorded as ‘possibly’, ‘probably’ or ‘definitely’ related to study drug occurring in more than one patient
| Adverse event . | CBM (n = 31) . | Placebo (n = 27) . | All patients (n = 58) . |
|---|---|---|---|
| Dizziness (all mild) | 8 (26%) | 1 (4%) | 9 (16%) |
| Light-headedness | 3 (10%) | 1 (4%) | 4 (7%) |
| Dry mouth | 4 (13%) | 0 | 4 (7%) |
| Nausea | 2 (6%) | 1 (4%) | 3 (5%) |
| Arthritic pains | 1 (3%) | 1 (4%) | 2 (4%) |
| Constipation | 1 (3%) | 1 (4%) | 2 (4%) |
| Drowsiness | 1 (3%) | 1 (4%) | 2 (4%) |
| Fall | 2 (6%) | 0 | 2 (4%) |
| Headache | 1 (3%) | 1 (4%) | 2 (4%) |
| Palpitations | 0 | 2 (7%) | 2 (4%) |
| Vomiting | 0 | 2 (7%) | 2 (4%) |
| Serious adverse events | 0 | 2 (7%) | 2 (4%) |
| Adverse events leading to withdrawal | 0 | 3 (11%) | 3 (5%) |
| Adverse event . | CBM (n = 31) . | Placebo (n = 27) . | All patients (n = 58) . |
|---|---|---|---|
| Dizziness (all mild) | 8 (26%) | 1 (4%) | 9 (16%) |
| Light-headedness | 3 (10%) | 1 (4%) | 4 (7%) |
| Dry mouth | 4 (13%) | 0 | 4 (7%) |
| Nausea | 2 (6%) | 1 (4%) | 3 (5%) |
| Arthritic pains | 1 (3%) | 1 (4%) | 2 (4%) |
| Constipation | 1 (3%) | 1 (4%) | 2 (4%) |
| Drowsiness | 1 (3%) | 1 (4%) | 2 (4%) |
| Fall | 2 (6%) | 0 | 2 (4%) |
| Headache | 1 (3%) | 1 (4%) | 2 (4%) |
| Palpitations | 0 | 2 (7%) | 2 (4%) |
| Vomiting | 0 | 2 (7%) | 2 (4%) |
| Serious adverse events | 0 | 2 (7%) | 2 (4%) |
| Adverse events leading to withdrawal | 0 | 3 (11%) | 3 (5%) |
Discussion
Cannabis was first proposed as a useful analgesic for a spectrum of rheumatic diseases in 2800 BC. By 1997 the British Medical Association had concluded that herbal cannabis was unsuitable for medical use. Whilst there is extensive data—though often anecdotal—supporting an analgesic effect of cannabis, many trials have produced equivocal results. There are hundreds of different compounds in herbal cannabis, more than 60 of which are unique to the plant (cannabinoids), and many of these may interact, with additional synergistic or antagonistic effects [3]. There are at least two and probably three cannabinoid receptors. These are found in high concentration in areas of nociceptive transmission within the CNS and on nociceptive peripheral nerves. CB1 receptors are potentially important targets for pharmacological modification. CB2 receptors are located primarily within the immune system.
We have assessed the analgesic and anti-inflammatory activity of a standardized whole-plant CBM with defined ratios and dosages of THC and CBD in a cohort of rheumatoid patients, with disease of extended duration and with poor analgesic control. A significant analgesic effect was observed and disease activity was significantly suppressed. Whilst the differences are small and variable across the population, they represent benefits of clinical relevance and indicate the need for more detailed study of dosage, formulation and ideal patient subgroup. The suppression of pain on movement, the primary endpoint, suggests a peripheral analgesic action. The suppression of pain at rest may suggest a more central effect. The modest suppression of the present gold standard inflammation activity measure, the DAS28, might indicate an influence on the immune effector system. This is consistent with the observation that cannabidiol suppressed a murine model of chronic arthritis, suppressing lymphocyte proliferation, the granulocytic cell reactive oxygen burst and lipopolysaccharide induced cytokine (TNF) production [5]. The improvement in sleep, a relevant clinical bonus, was probably due mainly to nocturnal symptom relief rather than a specific hypnotic effect since this was not observed in a sleep laboratory study of the compound at this dosage [8]. There was no effect on morning stiffness, but baseline scores were surprisingly low. The trial did not demonstrate significant toxicity and CBM was generally well tolerated.
We believe this to be the first controlled study of a CBM in rheumatoid arthritis, and the results are encouraging. The beneficial effects occurred in the context of a dosing regime restricted to evening dosing in order to minimize any possible intoxication-type reactions. However, 24-h dosing with this CBM (Sativex) using a self-titration regime in the context of multiple sclerosis resulted in only minimal intoxication scores [9]. Larger, more prolonged studies of CBM in rheumatoid arthritis are indicated.

The study was funded by GW Pharmaceuticals. We are grateful to Dr A. B. Hassell, Dr D. Tull, Dr M. J. B. Duckworth, Dr E. Montague and Dr T. M. Johnson for their collaboration in the study, to Mr Peter Clark for statistical advice and analysis, and to the clinical research team at the Cannabinoid Research Institute for their invaluable practical support.
The protocol was developed with a company that sent a donation to the Royal National Hospital for Rheumatic Diseases charity. P.J.R. is Medical Director of GW Pharmaceuticals. D.R.B. and C.S.M received honoraria from GW Pharmaceuticals for their work on the study design and protocol development.
References
Robson P. Human studies of cannabinoids and medicinal cannabis. In: Pertwee RG, ed.
McPartland J, Russo E. Cannabis and cannabis extracts: greater than the sum of their parts?
Formukong EA, Evans AT, Evans FJ. Analgesic and anti-inflammatory activity of constituents of Cannabis sativa L.
Malfait AM, Gallily R, Sumariwalla PF et al. The non-psychoactive cannabis-constituent cannabidiol is an oral anti-arthritic therapeutic in murine collagen-induced arthritis.
Ware MA, Adams H, Guy GW. The medicinal use of cannabis in the UK: results of a nationwide survey.
Whittle BA, Guy GW. Development of cannabis-based medicines: risks, benefit and serendipity. In: Guy GW, Whittle BA, Robson PJ, eds.
Nicholson AN, Turner C, Stone BM, Robson PJ. Effect of delta-9-THC and cannabidiol on nocturnal sleep and early morning behaviour in young adults.
Wade DT, Makela P, Robson P, House H, Bateman C. Do cannabis-based medicinal extracts have general or specific effects on symptoms in multiple sclerosis? A double-blind, randomized, placebo-controlled study on 160 patients.
Author notes
Royal National Hospital for Rheumatic Diseases, Bath, 1Cannabinoid Research Institute, Oxford Science Park, Oxford, 2Department of Rheumatology, Northampton General Hospital, Northampton and 3Department of Rheumatology, Selly Oak Hospital, Birmingham, UK.




Comments