-
PDF
- Split View
-
Views
-
Cite
Cite
E. Reefman, H. Kuiper, M. F. Jonkman, P. C. Limburg, C. G. M. Kallenberg, M. Bijl, Skin sensitivity to UVB irradiation in systemic lupus erythematosus is not related to the level of apoptosis induction in keratinocytes, Rheumatology, Volume 45, Issue 5, May 2006, Pages 538–544, https://doi.org/10.1093/rheumatology/kei249
Close - Share Icon Share
Abstract
Objectives. Accumulation of apoptotic cells has been suggested to be involved in the pathogenesis of systemic lupus erythematosus (SLE). As sunlight exposure is one of the factors that can trigger disease activity, we hypothesized that UV light may induce increased numbers of apoptotic cells in SLE.
Methods. Fourteen SLE patients and 16 controls were irradiated with UVB to determine their minimal erythemal dose (MED). Subsequently, skin was irradiated with 1 MED and 2 MED, respectively, and after 24 h skin biopsies were analysed immunohistologically for the number of apoptotic cells and presence of pyknotic nuclear debris.
Results. MED was significantly decreased in SLE patients and the presence of decreased MED was associated with a history of butterfly rash. Decreased MED was not related to other skin-related ACR criteria or to autoantibody specificities. No differences were detected in the numbers of apoptotic keratinocytes between patients and controls or in the amount of pyknotic nuclear debris following 1 and 2 MED irradiation, respectively. Absolute UVB doses were correlated with the number of apoptotic keratinocytes; dose-responses did not differ significantly between patients and controls.
Conclusions. Increased sensitivity of SLE patients to UVB, although associated with a history of malar rash, is not related to increased induction of apoptosis or increased levels of secondary necrosis in the skin. Thus, compared with controls, UVB-induced apoptosis is not increased in SLE patients under physiological conditions.
Systemic lupus erythematosus (SLE) is a systemic autoimmune disease characterized by the presence of autoantibodies to nuclear and cytoplasmic antigens in conjunction with a wide range of clinical manifestations. Photosensitivity is one of the characteristics of SLE, affecting 10–50% of patients [1]. Most, but not all, cutaneous lupus lesions occur in light-exposed areas and can be triggered by sunlight exposure. Sunlight exposure itself can induce systemic disease activity. The processes that induce cutaneous and systemic inflammatory lesions have not been elucidated, but in recent years apoptotic cells have been suggested to be one of the major factors involved.
The precise role of apoptotic cells in the development and progression of autoimmunity is unknown. Increased production and/or inefficient clearance of apoptotic cells by phagocytes could result in the accumulation of apoptotic cells, as seen in SLE patients [2–4]. Furthermore, increased numbers of apoptotic keratinocytes have been detected in cutaneous lupus erythematosus (LE) lesions [5]. During the apoptotic process, intracellular constituents are presented in excess to the immune system as a result of the cell-surface expression of intracellular constituents and/or the post-translational alteration of cellular proteins during apoptosis [6–9]. Binding of autoantibodies specific for these antigens may change the clearance of apoptotic cells. In the skin of SLE patients, deposition of immunoglobulin (Ig) G is often found at the dermal–epidermal junction, the so-called lupus band, or in the epidermis [10, 11]. In addition, autoantibodies specific for SSA/Ro and SSB/La are associated with cutaneous LE [12, 13].
Ultraviolet (UV) irradiation, especially UVB, is a potent inducer of apoptosis. The increased sensitivity to sunlight seen in a subpopulation of SLE patients may reflect increased susceptibility of keratinocytes to becoming apoptotic after exposure to UV light. During apoptosis, cells first shrink and their nuclei condense; subsequently they disintegrate into well-enclosed apoptotic bodies. Apoptotic keratinocytes may be detected in skin biopsies after exposure to UV by haematoxylin–eosin (H&E) staining as cells characterized by eosinophilic cytoplasm and pyknotic nuclei, also known as sunburn cells (SBC) [15]. In vitro, apoptosis finally leads to plasma membrane permeabilization, known as secondary necrosis. However, it is believed that this will not occur under physiological circumstances in vivo because of the rapid clearance of apoptotic cells by macrophages or surrounding cells [16].
In the present study we tested the hypothesis that UV light induces increased numbers of apoptotic cells in SLE. First, we assessed the sensitivity of SLE skin to UVB by determining the minimal erythemal dose (MED). Secondly, we analysed whether photosensitivity is related to the induction of apoptosis in the skin of SLE patients.
Materials and methods
Patients and controls
Consecutive patients were asked to participate in the study. Patients eligible for the study had to fulfil at least four American College of Rheumatology (ACR) criteria for SLE and to be in an inactive phase of the disease, defined as SLEDAI (Systemic Lupus Erythematosus Disease Activity Index) ≤4 [17] and no active cutaneous disease. Fourteen patients [age 45.1 ± 13.1 yr (mean ± s.d.); 1 male, 13 females] were included. Table 1 shows patient characteristics and the immunosuppressive medication used at the time of the study. Skin-related ACR criteria were based on patient history. Autoantibodies to double-stranded DNA (dsDNA) were determined using the Farr assay and reactivity to SSA/Ro, SSB/La, non-histone ribonuclear protein (nRNP) and Sm was determined by counter-immunoelectrophoresis. Sixteen healthy volunteers [age 37.6 ± 17.0 yr (mean ± s.d.), 3 males, 13 females] were included as controls. Patients and controls in whom the buttock skin had been exposed to sunlight or other sources of UVB over the past 6 months were excluded from the study. Skin types were determined according to the Fitzpatrick skin typing chart [18]. Skin type distribution (apart from two type 5 or higher scores in non-Caucasian patients) were similar in patients (two type 2, 10 type 3, one type 5, one type 6) and controls (two type 2, 14 type 3).
Patient characteristics, use of medication and autoantibody specificity at the time of the study
| Patient no. . | Sex . | Age (yr) . | Disease duration (yr) . | Butterfly rash . | Discoid lesions . | Photosensitivity . | Prednisone (mg/day) . | Hydroxychloroquine (mg/day) . | Azathioprine (mg/day) . | Autoantibody specificities . |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | F | 62 | 22 | – | + | – | 10 | – | 100 | dsDNA/SSA |
| 2 | F | 31 | 8 | + | – | – | 5 | 400 | 100 | dsDNA |
| 3 | F | 50 | 11 | + | – | + | 5 | – | 75 | dsDNA |
| 4 | F | 48 | 18 | + | – | – | 8 | 200 | – | dsDNA/nRNP |
| 5 | F | 61 | 11 | – | – | – | – | – | 150 | dsDNA |
| 6 | F | 54 | 13 | – | – | – | 5 | – | 100 | dsDNA/Sm |
| 7 | F | 51 | 31 | + | + | + | – | 200 | – | dsDNA/nRNP |
| 8 | F | 64 | 26 | – | – | + | 3.5 | – | 25 | SSA/SSB/dsDNA/Sm |
| 9 | F | 47 | 4 | – | – | – | 10 | – | 100 | dsDNA/SSA |
| 10 | F | 23 | 2 | + | – | – | 2.5 | – | 100 | dsDNA |
| 11 | F | 27 | 1 | – | – | + | 5 | 800 | – | nRNP/Sm |
| 12 | F | 40 | 14 | – | – | + | 5 | 400 | 75 | dsDNA/SSA/nRNP/Sm |
| 13 | M | 39 | 8 | + | – | – | 5 | – | 75 | SSA/SSB/dsDNA |
| 14 | F | 35 | 2 | + | – | – | 3.75 | 400 | – | SSA/SSB/dsDNA/Sm |
| Patient no. . | Sex . | Age (yr) . | Disease duration (yr) . | Butterfly rash . | Discoid lesions . | Photosensitivity . | Prednisone (mg/day) . | Hydroxychloroquine (mg/day) . | Azathioprine (mg/day) . | Autoantibody specificities . |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | F | 62 | 22 | – | + | – | 10 | – | 100 | dsDNA/SSA |
| 2 | F | 31 | 8 | + | – | – | 5 | 400 | 100 | dsDNA |
| 3 | F | 50 | 11 | + | – | + | 5 | – | 75 | dsDNA |
| 4 | F | 48 | 18 | + | – | – | 8 | 200 | – | dsDNA/nRNP |
| 5 | F | 61 | 11 | – | – | – | – | – | 150 | dsDNA |
| 6 | F | 54 | 13 | – | – | – | 5 | – | 100 | dsDNA/Sm |
| 7 | F | 51 | 31 | + | + | + | – | 200 | – | dsDNA/nRNP |
| 8 | F | 64 | 26 | – | – | + | 3.5 | – | 25 | SSA/SSB/dsDNA/Sm |
| 9 | F | 47 | 4 | – | – | – | 10 | – | 100 | dsDNA/SSA |
| 10 | F | 23 | 2 | + | – | – | 2.5 | – | 100 | dsDNA |
| 11 | F | 27 | 1 | – | – | + | 5 | 800 | – | nRNP/Sm |
| 12 | F | 40 | 14 | – | – | + | 5 | 400 | 75 | dsDNA/SSA/nRNP/Sm |
| 13 | M | 39 | 8 | + | – | – | 5 | – | 75 | SSA/SSB/dsDNA |
| 14 | F | 35 | 2 | + | – | – | 3.75 | 400 | – | SSA/SSB/dsDNA/Sm |
Positive score for skin-related ACR criteria was based on patient history. Autoantibody specificities directed against dsDNA, SS-A/Ro (SSA), SS-B/La (SSB), non-histone ribonuclear protein (nRNP) and/or Sm complex (Sm). F, female, M, male.
Patient characteristics, use of medication and autoantibody specificity at the time of the study
| Patient no. . | Sex . | Age (yr) . | Disease duration (yr) . | Butterfly rash . | Discoid lesions . | Photosensitivity . | Prednisone (mg/day) . | Hydroxychloroquine (mg/day) . | Azathioprine (mg/day) . | Autoantibody specificities . |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | F | 62 | 22 | – | + | – | 10 | – | 100 | dsDNA/SSA |
| 2 | F | 31 | 8 | + | – | – | 5 | 400 | 100 | dsDNA |
| 3 | F | 50 | 11 | + | – | + | 5 | – | 75 | dsDNA |
| 4 | F | 48 | 18 | + | – | – | 8 | 200 | – | dsDNA/nRNP |
| 5 | F | 61 | 11 | – | – | – | – | – | 150 | dsDNA |
| 6 | F | 54 | 13 | – | – | – | 5 | – | 100 | dsDNA/Sm |
| 7 | F | 51 | 31 | + | + | + | – | 200 | – | dsDNA/nRNP |
| 8 | F | 64 | 26 | – | – | + | 3.5 | – | 25 | SSA/SSB/dsDNA/Sm |
| 9 | F | 47 | 4 | – | – | – | 10 | – | 100 | dsDNA/SSA |
| 10 | F | 23 | 2 | + | – | – | 2.5 | – | 100 | dsDNA |
| 11 | F | 27 | 1 | – | – | + | 5 | 800 | – | nRNP/Sm |
| 12 | F | 40 | 14 | – | – | + | 5 | 400 | 75 | dsDNA/SSA/nRNP/Sm |
| 13 | M | 39 | 8 | + | – | – | 5 | – | 75 | SSA/SSB/dsDNA |
| 14 | F | 35 | 2 | + | – | – | 3.75 | 400 | – | SSA/SSB/dsDNA/Sm |
| Patient no. . | Sex . | Age (yr) . | Disease duration (yr) . | Butterfly rash . | Discoid lesions . | Photosensitivity . | Prednisone (mg/day) . | Hydroxychloroquine (mg/day) . | Azathioprine (mg/day) . | Autoantibody specificities . |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | F | 62 | 22 | – | + | – | 10 | – | 100 | dsDNA/SSA |
| 2 | F | 31 | 8 | + | – | – | 5 | 400 | 100 | dsDNA |
| 3 | F | 50 | 11 | + | – | + | 5 | – | 75 | dsDNA |
| 4 | F | 48 | 18 | + | – | – | 8 | 200 | – | dsDNA/nRNP |
| 5 | F | 61 | 11 | – | – | – | – | – | 150 | dsDNA |
| 6 | F | 54 | 13 | – | – | – | 5 | – | 100 | dsDNA/Sm |
| 7 | F | 51 | 31 | + | + | + | – | 200 | – | dsDNA/nRNP |
| 8 | F | 64 | 26 | – | – | + | 3.5 | – | 25 | SSA/SSB/dsDNA/Sm |
| 9 | F | 47 | 4 | – | – | – | 10 | – | 100 | dsDNA/SSA |
| 10 | F | 23 | 2 | + | – | – | 2.5 | – | 100 | dsDNA |
| 11 | F | 27 | 1 | – | – | + | 5 | 800 | – | nRNP/Sm |
| 12 | F | 40 | 14 | – | – | + | 5 | 400 | 75 | dsDNA/SSA/nRNP/Sm |
| 13 | M | 39 | 8 | + | – | – | 5 | – | 75 | SSA/SSB/dsDNA |
| 14 | F | 35 | 2 | + | – | – | 3.75 | 400 | – | SSA/SSB/dsDNA/Sm |
Positive score for skin-related ACR criteria was based on patient history. Autoantibody specificities directed against dsDNA, SS-A/Ro (SSA), SS-B/La (SSB), non-histone ribonuclear protein (nRNP) and/or Sm complex (Sm). F, female, M, male.
The subjects all gave written consent according to the Declaration of Helsinki. The study design was approved by the Medical Ethical committee of the UMCG, Groningen.
Irradiation protocol
UVB irradiation was performed using Waldman 800 ‘sky’ lights with TL-12 lamps (Philips, Eindhoven, The Netherlands) at a distance of 15 cm from an unexposed area of the buttock skin. A Diffey grid [19] was used to irradiate the skin with 10 different doses (0.026–0.200 J/cm2) during one exposure. After 24 h the MED was determined by two independent observers, with more than 90% agreement. In case of disagreement, the mean of the two separate values was taken. The reproducibility of MED assessment was determined by a second irradiation in 10 subjects: five healthy controls and five patients. After determination of the MED, subjects were irradiated with 1 MED or 2 MED on the other buttock. After 24 h, 4 mm skin biopsies were taken from non-irradiated skin and from the two areas of skin irradiated with 1 MED and 2 MED. Biopsies were fixed in formaldehyde.
Chemicals and antibodies
Diaminobenzidine (DAB) solution contains 25 mg DAB, 50 mg imidazole in 50 ml phosphate-buffered saline (PBS) and was filtered before use; 50 μl of 30% H2O2 was added just before incubation. For haematoxylin staining, we used Mayer's haemalum solution (Merck, Darmstadt, Germany), and Kaiser's glycerol gelatin (Merck) was used as a mounting medium.
Rabbit antibody to cleaved caspase 3 antibodies (#9661S) was purchased from Signaling Technology (Beverly, MA, USA). Secondary antibodies [goat anti-rabbit IgG–horseradish peroxidase (HRP) and rabbit anti-goat IgG–HRP] were obtained from DakoCytomation (Glostrup, Denmark).
Immunohistochemistry
Skin sections (4 μm) on glass slides coated with 3-amino-propyltriethoxysilane were used for all experiments. Sections were deparaffinized by subsequent incubations in xylene (10 min), 100% ethanol (5 min) and 96% ethanol (2 min), twice, followed by ethanol 70% (2 min) and distilled water. H&E staining was performed according to a standard protocol using the linear stainer from Medite (Burgdorf, Germany). For cleaved caspase 3 staining, slides were boiled for 10 min in 1 mmol/l EDTA (ethylenediamine tetraacetate) buffer (pH 8.0) and rinsed with PBS. Endogenous peroxidase was blocked by incubation in 0.37% H2O2 in PBS for 30 min. Slides were incubated with anti-cleaved caspase 3 antibodies diluted 1 : 75 in 1% bovine serum albumin (BSA)/PBS for 1 h at room temperature. Subsequently, slides were washed in PBS (three times) and incubated for 30 min at room temperature with goat anti-rabbit IgG labelled with HRP (1 : 50 in 1% BSA/PBS), then washed again (three times) in PBS followed by incubation with rabbit anti-goat IgG–HRP for another 30 min at room temperature. After washing in PBS (three times), slides were incubated in DAB solution for 15–20 min, and washed subsequently with distilled water (five times). Slides were then counterstained with haematoxylin for 1 min, washed in distilled water (five times), dehydrated in 96% ethanol and subsequently in 100% ethanol, then mounted.
Scoring procedure
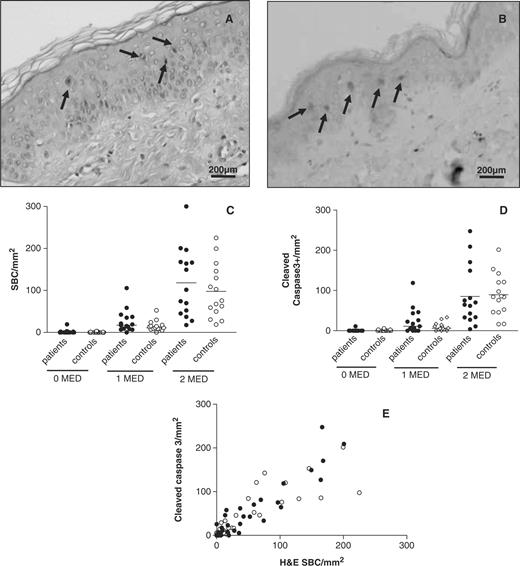
Using Olympus Soft Pro software (Tokyo, Japan), the surface area of the epidermis was determined by manually drawing a line around this area and calculating the total surface (mm2). Subsequently, the numbers of SBC and pyknotic nuclei were scored by calculating their numbers in three sequential H&E-stained sections. A pyknotic nucleus was defined as one whole pyknotic nucleus or a group of pyknotic nuclear fragments (as indicated in Fig. 3A by black arrows and white arrows, respectively). The numbers of SBC or pyknotic nuclei per mm2 were determined by dividing the numbers counted by epidermal surface area and calculating the mean value for the three sections. Cleaved caspase 3-positive cells were scored accordingly.
Statistics
Differences in MED, numbers of apoptotic cells and debris between groups were determined using the Mann–Whitney test. Correlations between MED values and medication, and between numbers of SBC and amount of pyknotic nuclear debris were analysed with the Spearman rank correlation test. To study associations between increased sensitivity and ACR criteria, medication use or autoantibodies present in serum, the χ2 test was performed. To analyse differences in correlation between patients and controls, slopes of linear regression lines were compared (GraphPad Software, San Diego, CA, USA). To analyse the relationship between absolute doses of UVB and numbers of apoptotic cells, two tests were performed. First, we used the Spearman rank correlation test to determine whether a correlation existed; secondly, a curve fit was made using data from patients and controls, followed by a sign test on rank differences, in order to analyse whether patients and controls were equally distributed around the calculated dose–response curve (SPSS 12.0.1., Chicago, IL, USA).
Results
Sensitivity of SLE patients to a single dose of UVB
The MEDs of patients and controls were determined as an indicator of sensitivity to a single dose of UVB. To determine the MED, 10 different doses of UVB were applied within one exposure using a Diffey grid. This device has been reported to be comparable in accuracy to conventional multiexposure testing [20]. To assess the reproducibility of this method, MED was determined on two consecutive days in 10 subjects. Reproducibility ranged from 0 to 22% (median 3%).
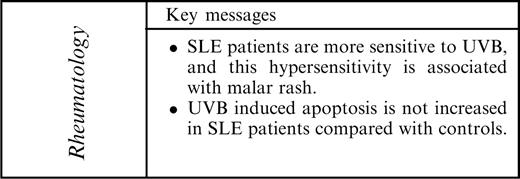
The MED of SLE patients (0.090 ± 0.036 J/cm2; mean ± s.d.) was lower compared with controls (0.112 ± 0.036 J/cm2) (P = 0.012). MED values ranged from 0.05 to 0.18 J/cm2 in patients and from 0.085 to 0.20 J/cm2 in controls (Fig. 1). Six out of the 14 patients (43%) had MED values lower than any of the controls and were defined as sensitive patients. We assessed whether age, disease duration, use of medication, particular autoantibodies or a history of skin-related ACR criteria (Table 1) was associated with the presence of increased sensitivity. No correlations could be found between age and MED values in patients (r = 0.03, P = 0.92) or between MED values and disease duration in the patients (r = − 0.16, P = 0.61). As shown in Table 2, no association was found between decreased MED and use of medication. Also, medication doses did not correlate with MED values (corticosteroids, r = 0.20, P = 0.50; hydroxychloroquine, r = 0.30; P = 0.30; azathioprine, r = 0.23, P = 0.45). Furthermore, no association was found with antibody specificities. Butterfly (malar) rash was associated with increased sensitivity to UVB but not discoid lupus or photosensitivity by history (Table 2).
Presence of autoantibodies and history of skin-related ACR criteria in patients with and without increased sensitivity to UVB
| Autoantibodies . | Sensitive patients (n = 6) . | Non-sensitive patients (n = 8) . | P . |
|---|---|---|---|
| DsDNA | 6 | 6 | 0.19 |
| SSA/Ro | 1 | 5 | 0.09 |
| SSB/La | 1 | 1 | 0.83 |
| nRNP | 1 | 3 | 0.39 |
| Sm | 0 | 2 | 0.19 |
| ACR criterion | |||
| Butterfly rash | 5 | 2 | 0.03* |
| Discoid LE | 0 | 2 | 0.19 |
| Photosensitivity | 2 | 4 | 0.53 |
| Medication | |||
| Corticosteroids | 5 | 6 | 0.91 |
| Hydroxychloroquine | 3 | 3 | 0.80 |
| Azathioprine | 4 | 5 | 1.00 |
| Autoantibodies . | Sensitive patients (n = 6) . | Non-sensitive patients (n = 8) . | P . |
|---|---|---|---|
| DsDNA | 6 | 6 | 0.19 |
| SSA/Ro | 1 | 5 | 0.09 |
| SSB/La | 1 | 1 | 0.83 |
| nRNP | 1 | 3 | 0.39 |
| Sm | 0 | 2 | 0.19 |
| ACR criterion | |||
| Butterfly rash | 5 | 2 | 0.03* |
| Discoid LE | 0 | 2 | 0.19 |
| Photosensitivity | 2 | 4 | 0.53 |
| Medication | |||
| Corticosteroids | 5 | 6 | 0.91 |
| Hydroxychloroquine | 3 | 3 | 0.80 |
| Azathioprine | 4 | 5 | 1.00 |
Sensitive patients had a lower MED than any of the controls (MED<0.085 J/cm2). Numbers of patients are given that were positive for the respective autoantibody specificities, the ACR criteria and the medications indicated. *Significant P-value (P<0.05 by χ2 test).
Presence of autoantibodies and history of skin-related ACR criteria in patients with and without increased sensitivity to UVB
| Autoantibodies . | Sensitive patients (n = 6) . | Non-sensitive patients (n = 8) . | P . |
|---|---|---|---|
| DsDNA | 6 | 6 | 0.19 |
| SSA/Ro | 1 | 5 | 0.09 |
| SSB/La | 1 | 1 | 0.83 |
| nRNP | 1 | 3 | 0.39 |
| Sm | 0 | 2 | 0.19 |
| ACR criterion | |||
| Butterfly rash | 5 | 2 | 0.03* |
| Discoid LE | 0 | 2 | 0.19 |
| Photosensitivity | 2 | 4 | 0.53 |
| Medication | |||
| Corticosteroids | 5 | 6 | 0.91 |
| Hydroxychloroquine | 3 | 3 | 0.80 |
| Azathioprine | 4 | 5 | 1.00 |
| Autoantibodies . | Sensitive patients (n = 6) . | Non-sensitive patients (n = 8) . | P . |
|---|---|---|---|
| DsDNA | 6 | 6 | 0.19 |
| SSA/Ro | 1 | 5 | 0.09 |
| SSB/La | 1 | 1 | 0.83 |
| nRNP | 1 | 3 | 0.39 |
| Sm | 0 | 2 | 0.19 |
| ACR criterion | |||
| Butterfly rash | 5 | 2 | 0.03* |
| Discoid LE | 0 | 2 | 0.19 |
| Photosensitivity | 2 | 4 | 0.53 |
| Medication | |||
| Corticosteroids | 5 | 6 | 0.91 |
| Hydroxychloroquine | 3 | 3 | 0.80 |
| Azathioprine | 4 | 5 | 1.00 |
Sensitive patients had a lower MED than any of the controls (MED<0.085 J/cm2). Numbers of patients are given that were positive for the respective autoantibody specificities, the ACR criteria and the medications indicated. *Significant P-value (P<0.05 by χ2 test).
Determination of minimal erythemal dose (MED) in 14 SLE patients and 16 controls. The MED of SLE patients was significantly lower than that in the control group. MED is expressed as J/cm2. The P-value was determined with the two-tailed Mann–Whitney test. The horizontal line denotes the mean.
Apoptosis induction in skin after two different UVB doses
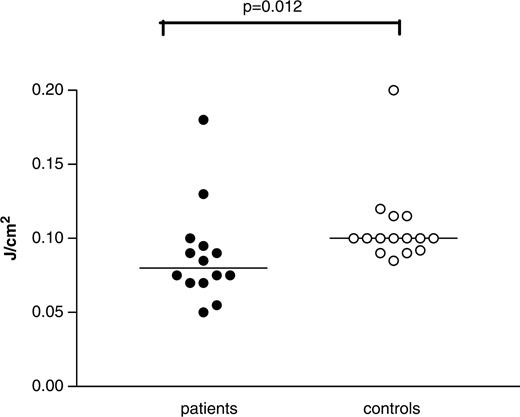
Apoptosis induction was studied in previously sun-protected buttock skin 24 h after exposure to 1 and 2 MED of UVB. Apoptotic keratinocytes could be detected in H&E-stained sections by their eosinophilic cytoplasm and pyknotic nuclei (Fig. 2A), as well as by staining for cleaved caspase 3 (Fig. 2B). As expected, hardly any apoptotic cells were detected in unexposed skin in patients and controls, both defined as SBC by H&E staining or by the presence of cleaved caspase 3-positive cells (Fig. 2C and D). After exposure to 1 MED, numbers of apoptotic cells increased significantly in patients and controls [26.9 ± 28.1 (mean ± s.d.) vs 15.3 ± 12.7 SBC/mm2]. Exposure to 2 MED led to a 3- to 9-fold increase in apoptotic cells compared with 1 MED in both groups, resulting in 116.1 ± 79.4 SBC/mm2 in the skin of patients and 97.8 ± 63.6 SBC/mm2 in controls. Significant differences in the number of apoptotic cells could not be detected between patients and controls by either H&E staining or by staining for cleaved caspase 3 at any of the UVB doses used. The two methods for the detection of apoptotic keratinocytes were highly correlated (r = 0.89, P<0.0001), indicating that the data reliably represent the number of apoptotic cells induced (Fig. 2E).
Apoptosis induction in SLE patients and controls 24 h after UVB irradiation. (A) Representative H&E staining of skin irradiated with 2 MED at 100× magnification. (B) Representative immunohistochemical staining for cleaved caspase 3, visualized using DAB. Arrows indicate apoptotic cells. (C) Numbers of sunburn cells (SBC) detected in the epidermis of H&E-stained sections (SBC/mm2). (D) Numbers of cleaved caspase 3-positive cells detected in the epidermis (cleaved Casp3+/mm2). (E) Relationship between number of SBC and cleaved caspase 3 positive cells per mm2 of skin in SLE patients and controls (• patients; ○ controls).
Pyknotic nuclear debris present in skin of SLE patients and controls 24 h after UVB irradiation. (A) Two representative H&E-stained sections showing SBC (triangle arrows) and nuclear debris (black arrow indicates one intact pyknotic nucleus; white arrow indicates pyknotic fragmented nuclei) in skin biopsies after irradiation with UVB. Magnification 400×. (B) Numbers of pyknotic nuclei found in unexposed skin and in skin exposed to 1 MED and 2 MED. (C) Relationship between formation of SBC and numbers of pyknotic nuclei found (• patients; ○ controls). Patients, r = 0.88, P<0.0001; controls, r = 0.89, P<0.0001; not significant.
Induction of pyknotic nuclear debris after two different UVB doses
Besides the formation of SBC, pyknotic nuclear debris could be seen in the epidermal layer by H&E staining (Fig. 3A). We determined the amount of pyknotic nuclear debris in unexposed and exposed skin by counting the numbers of these whole or fragmented pyknotic nuclei present between the keratinocytes. Hardly any of these nuclei could be detected in unexposed skin. Numbers increased after exposure to increasing doses of UVB (Fig. 3B). No differences in numbers of these nuclei could be detected at any dose of UVB between SLE patients and controls. A high correlation was found between the formation of SBC and the occurrence of nuclear debris (patients, r = 0.88, P<0.0001; controls, r = 0.89, P<0.0001). The correlation was linear and did not differ between patients and controls (Fig. 3C).
Relationship between absolute UVB dose and induction of apoptosis
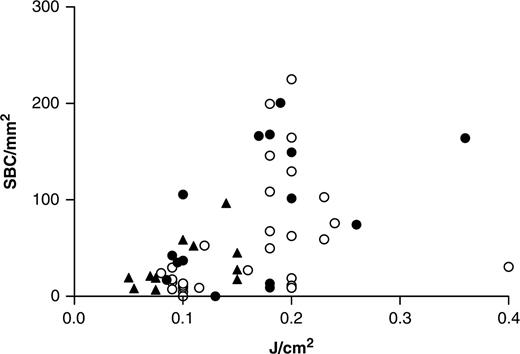
Absolute UVB values have been shown to correlate more strongly with DNA damage and apoptosis than standardized MED [21]. As shown before, SLE patients had significantly lower MED values. As a consequence, absolute doses of UVB received by the patient group were lower compared with controls. However, the same numbers of apoptotic cells were induced in patients and controls after exposure to 1 and 2 MED doses, respectively. Next, we investigated the correlation between absolute doses of UVB and numbers of apoptotic cells induced, comparing patients and controls. A significant correlation was found both for patients and controls (r = 0.56, P = 0.002 and r = 0.60, P<0.001, respectively) (Fig. 4). The relationship between numbers of apoptotic cells and absolute doses of UVB was not linear. To compare the dose–response in patients vs controls, we performed a curve fit after which the distribution of the patient and control values was compared in a sign test on rank differences. This test showed that patients and controls are distributed equally along this curve (χ2 = 1.45, P = 0.23), indicating that the dose–response did not differ significantly between patients and controls. Furthermore, we compared the number of SBCs in sensitive patients (as defined earlier) with that in less sensitive patients or controls. Sensitive patients had significantly lower numbers of SBC in their skin after 2 MED UVB compared with less sensitive patients (P = 0.01, data not shown). Taken together, these results indicate that apoptosis is not increased in lupus patients when they receive the same absolute UVB dose compared with controls.
Relationship between SBC induction and absolute UVB doses. Number of SBC/mm2 in the skin after 24 h is shown on the y-axis while absolute UVB dose (J/cm2) received is shown on the x-axis. Spearman's correlation test shows a significant correlation between numbers of SBC and absolute doses of UVB (r = 0.56, P = 0.002 for patients and r = 0.60, P<0.001 for controls). (▴ sensitive patients; • other patients; ○ controls).
Discussion
In this study we observed that SLE patients have a lower MED for UVB compared with controls. This was associated with a history of butterfly rash but not with discoid lesions or photosensitivity.
Because inter-individual variation in skin types leads to differences in sensitivity to UV light between individuals, the MED has been introduced to standardize the sensitivity of subjects to UV. Discrepancies between previous studies investigating the sensitivity of SLE patients to UV light might reflect differences in patient characteristics or the use of medication [22–24]. Erythema can be influenced by topical administration of medications such as corticosteroids [25]; however, oral corticosteroids up to 80 mg daily were reported not to decrease erythema [26]. None of the patients in this study was using topical corticosteroids or high doses of corticosteroids (Table 1). Also, the use of other (immunosuppressive) drugs was not associated with decreased MED. Therefore, the lower MED detected in our patient group reflects, in all likelihood, increased sensitivity to a single dose of UVB.
Single or multiple exposures with moderate doses of UVB have been reported to induce skin lesions in SLE patients [24, 27, 28]. Although UVA exposure can also induce skin lesions in SLE patients, considerably higher doses are needed [29]. Furthermore, UVA is weakly absorbed by biomolecules, such as DNA or proteins and is therefore a weaker inducer of apoptosis. In skin hardly any apoptotic keratinocytes can be detected after UVA exposure [31, 32]. In contrast, UVB is known to be a potent inducer of apoptosis [14, 30]. In this study we wanted to investigate the induction of apoptotic cells in relation to the increased UV sensitivity seen in SLE patients. Therefore, UVB was chosen in this study as the UV source for irradiation.
We determined whether the increased sensitivity to UVB in some of the patients was associated with autoantibody specificities or skin-related ACR criteria. Association has been reported between autoantibody specificities for SSA/Ro and SSB/La and cutaneous LE, either induced naturally or by provocative phototesting [12, 13, 27]. Other reports, however, have not found this association [28]. Also, in our study, no association was found between increased sensitivity and any autoantibody specificity, although the presence of such an association cannot be excluded because of the limited number of patients studied. However, our data indicate an association between increased UVB sensitivity and a history of butterfly rash. Butterfly (or malar) rash is a common feature in SLE and presents as a photosensitive eruption, although sun exposure is not always required [1, 33]. The increased sensitivity to a single dose of UVB might reflect the susceptibility of these patients to the development of a butterfly rash.
Accumulation of apoptotic cells as a result of an increased rate of apoptosis, decreased elimination of apoptotic cells or a combination of both has been hypothesized to be an important factor in the development of the inflammatory lesions seen in the skin in SLE patients [34, 35]. In LE lesions, increased numbers of apoptotic cells [36], increased Fas antigen expression and decreased Bcl-2 expression [5] have been reported. We investigated the induction of apoptosis before the development of lesions, which might give a better indication of whether apoptosis is involved in the initiation of LE lesions. We showed that apoptosis induction is not increased in the skin of SLE patients after irradiation with UVB compared with controls.
Two methods were used for the detection of apoptotic cells in biopsies: morphological detection in H&E-stained sections and specific staining for the cleaved form of caspase 3. Detection of SBC by H&E staining has been used extensively for many years [30, 37]. The presence of cleaved caspase 3 is an early marker of apoptosis, as its cleavage initiates all the morphological changes [38]. Although these two methods show positive results in different, partly overlapping time periods, we demonstrated that these methods are well correlated. Terminal deoxynucleotidyl transferase-mediated in situ end-labelling (TUNEL), although historically well known to be used in the field of apoptosis detection, was not used as several reports have stated that the TUNEL technique is not specific for apoptosis and will also stain necrotic [39–42] and highly proliferating cells [43].
By H&E staining, pyknotic nuclear debris was detected 24 h after UVB irradiation. As suggested by the strong and linear correlation between SBC and nuclear debris, the progression of the apoptotic process into postapoptotic events, such as secondary necrosis, was probably responsible for the occurrence of nuclear debris. Therefore, levels of nuclear debris detected after 24 h could be a reflection of apoptosis occurring in the earlier phases after UVB irradiation. Other studies have shown that the relative contribution of apoptosis induced up to 12 h after irradiation to the total amount of apoptosis that is seen later is very low [37, 44, 45]. The contribution of early apoptotic cell induction in our study seems to be one-fifth or less of the total amount of apoptosis occurring over a 24-h period, as can be deduced from Figs 2C and 3B. In accordance with the data on apoptotic keratinocytes, the amount of nuclear debris present in the skin before and after UVB exposure did not differ between patients and controls at any dose. Neither did we find differences between patients and controls in the linear relationship between the formation of SBC and nuclear debris. This indicates that the level of apoptosis in the earlier phases and the rate at which secondary necrosis occurs is comparable between SLE patients and controls. Further studies, however, are needed to determine the number of apoptotic cells present at earlier and, especially, later time points after irradiation.
Furthermore, despite the lower absolute dose of UVB received by patients compared with controls in the present study, no differences in the relationship between the induction of apoptosis and the absolute dose of UVB were detected between patients and controls. Apoptosis induction is, therefore, comparable between SLE patients and healthy controls under physiological conditions.
In conclusion, we suggest that, rather than the number of apoptotic cells that are induced, it is the clearance of these cells that might be involved in the pathogenesis of light-induced skin lesions in SLE patients. Studies are under way to test this hypothesis.
We thank Wim Sluiter of the Department Pathology and Laboratory Medicine, University Hospital, Groningen, The Netherlands, for advice on statistical analysis.
No conflict of interest has been declared by the authors.
References
Herrmann M, Voll RE, Zoller OM, Hagenhofer M, Ponner BB, Kalden JR. Impaired phagocytosis of apoptotic cell material by monocyte-derived macrophages from patients with systemic lupus erythematosus.
Courtney PA, Crockard AD, Williamson K, Irvine AE, Kennedy RJ, Bell AL. Increased apoptotic peripheral blood neutrophils in systemic lupus erythematosus: relations with disease activity, antibodies to double stranded DNA, and neutropenia.
Perniok A, Wedekind F, Herrmann M, Specker C, Schneider M. High levels of circulating early apoptic peripheral blood mononuclear cells in systemic lupus erythematosus.
Baima B, Sticherling M. Apoptosis in different cutaneous manifestations of lupus erythematosus.
Casciola-Rosen LA, Anhalt G, Rosen A. Autoantigens targeted in systemic lupus erythematosus are clustered in two populations of surface structures on apoptotic keratinocytes.
Saegusa J, Kawano S, Koshiba M et al. Oxidative stress mediates cell surface expression of SS-A/Ro antigen on keratinocytes.
Utz PJ, Hottelet M, Schur PH, Anderson P. Proteins phosphorylated during stress-induced apoptosis are common targets for autoantibody production in patients with systemic lupus erythematosus.
Utz PJ, Anderson P. Posttranslational protein modifications, apoptosis, and the bypass of tolerance to autoantigens.
Halberg P, Ullman S, Jorgensen F. The lupus band test as a measure of disease activity in systemic lupus erythematosus.
Provost TT, Reichlin M. Immunopathologic studies of cutaneous lupus erythematosus.
Sontheimer RD, Thomas JR, Gilliam JN. Subacute cutaneous lupus erythematosus: a cutaneous marker for a distinct lupus erythematosus subset.
Sontheimer RD, Maddison PJ, Reichlin M, Jordon RE, Stastny P, Gilliam JN. Serologic and HLA associations in subacute cutaneous lupus erythematosus, a clinical subset of lupus erythematosus.
Kulms D, Zeise E, Poppelmann B, Schwarz T. DNA damage, death receptor activation and reactive oxygen species contribute to ultraviolet radiation-induced apoptosis in an essential and independent way.
Kulms D, Schwarz T. Molecular mechanisms of UV-induced apoptosis.
Proskuryakov SY, Konoplyannikov AG, Gabai VL. Necrosis: a specific form of programmed cell death?
Bombardier C, Gladman DD, Urowitz MB, Caron D, Chang CH. Derivation of the SLEDAI. A disease activity index for lupus patients. The Committee on Prognosis Studies in SLE.
Diffey BL, De Berker DA, Saunders PJ, Farr PM. A device for phototesting patients before PUVA therapy.
Diffey BL. Sun protection factor determination in vivo using a single exposure on sunscreen-protected skin.
Heenen M, Giacomoni PU, Golstein P. Individual variations in the correlation between erythemal threshold, UV-induced DNA damage and sun-burn cell formation.
Casciola-Rosen L, Rosen A. Ultraviolet light-induced keratinocyte apoptosis: a potential mechanism for the induction of skin lesions and autoantibody production in LE.
Furukawa F. Photosensitivity in cutaneous lupus erythematosus: lessons from mice and men.
Wolska H, Blaszczyk M, Jablonska S. Phototests in patients with various forms of lupus erythematosus.
Takiwaki H, Shirai S, Kohno H, Soh H, Arase S. The degrees of UVB-induced erythema and pigmentation correlate linearly and are reduced in a parallel manner by topical anti-inflammatory agents.
Greenwald JS, Parrish JA, Jaenicke KF, Anderson RR. Failure of systemically administered corticosteroids to suppress UVB-induced delayed erythema.
Hasan T, Nyberg F, Stephansson E et al. Photosensitivity in lupus erythematosus, UV photoprovocation results compared with history of photosensitivity and clinical findings.
Kind P, Lehmann P, Plewig G. Phototesting in lupus erythematosus.
Lehmann P, Holzle E, Kind P, Goerz G, Plewig G. Experimental reproduction of skin lesions in lupus erythematosus by UVA and UVB radiation.
Kumakiri M, Hashimoto K, Willis I. Biologic changes due to long-wave ultraviolet irradiation on human skin: ultrastructural study.
Fabbri P, Cardinali C, Giomi B, Caproni M. Cutaneous lupus erythematosus: diagnosis and management.
Bijl M, Limburg PC, Kallenberg CG. New insights into the pathogenesis of systemic lupus erythematosus (SLE): the role of apoptosis.
Reefman E, Dijstelbloem HM, Limburg PC, Kallenberg CG, Bijl M. Fcgamma receptors in the initiation and progression of systemic lupus erythematosus.
Chung JH, Kwon OS, Eun HC et al. Apoptosis in the pathogenesis of cutaneous lupus erythematosus.
Murphy G, Young AR, Wulf HC, Kulms D, Schwarz T. The molecular determinants of sunburn cell formation.
Kohler C, Orrenius S, Zhivotovsky B. Evaluation of caspase activity in apoptotic cells.
Charriaut-Marlangue C, Ben Ari Y. A cautionary note on the use of the TUNEL stain to determine apoptosis.
Grasl-Kraupp B, Ruttkay-Nedecky B, Koudelka H, Bukowska K, Bursch W, Schulte-Hermann R. In situ detection of fragmented DNA (TUNEL assay) fails to discriminate among apoptosis, necrosis, and autolytic cell death: a cautionary note.
Ohno M, Takemura G, Ohno A et al. ‘Apoptotic’ myocytes in infarct area in rabbit hearts may be oncotic myocytes with DNA fragmentation: analysis by immunogold electron microscopy combined with in situ nick end-labeling.
Stadelmann C, Bruck W, Bancher C, Jellinger K, Lassmann H. Alzheimer disease: DNA fragmentation indicates increased neuronal vulnerability, but not apoptosis.
Kawashima K, Doi H, Ito Y, Shibata MA, Yoshinaka R, Otsuki Y. Evaluation of cell death and proliferation in psoriatic epidermis.
Bang B, Rygaard J, Baadsgaard O, Skov L. Increased expression of Fas on human epidermal cells after in vivo exposure to single-dose ultraviolet (UV) B or long-wave UVA radiation.
Author notes
Departments of 1Rheumatology and Clinical Immunology, 2Pathology and Laboratory Medicine and 3Dermatology, University Medical Center Groningen, Groningen, The Netherlands.




Comments