-
PDF
- Split View
-
Views
-
Cite
Cite
Rupesh K. Gupta, Kimberly P. Miller, Janice K. Babus, Jodi A. Flaws, Methoxychlor Inhibits Growth and Induces Atresia of Antral Follicles through an Oxidative Stress Pathway, Toxicological Sciences, Volume 93, Issue 2, October 2006, Pages 382–389, https://doi.org/10.1093/toxsci/kfl052
Close - Share Icon Share
Abstract
The mammalian ovary contains antral follicles, which are responsible for the synthesis and secretion of hormones that regulate estrous cyclicity and fertility. The organochlorine pesticide methoxychlor (MXC) causes atresia (follicle death via apoptosis) of antral follicles, but little is known about the mechanisms by which MXC does so. Oxidative stress is known to cause apoptosis in nonreproductive and reproductive tissues. Thus, we tested the hypothesis that MXC inhibits growth and induces atresia of antral follicles through an oxidative stress pathway. To test this hypothesis, antral follicles isolated from 39-day-old CD-1 mice were cultured with vehicle control (dimethylsulfoxide [DMSO]), MXC (1–100 μg/ml), or MXC + the antioxidant N-acetyl cysteine (NAC) (0.1–10mM). During culture, growth was monitored daily. At the end of culture, follicles were processed for quantitative real-time polymerase chain reaction of Cu/Zn superoxide dismutase (SOD1), glutathione peroxidase (GPX), and catalase (CAT) mRNA expression or for histological evaluation of atresia. The results indicate that exposure to MXC (1–100 μg/ml) inhibited growth of follicles compared to DMSO controls and that NAC (1–10mM) blocked the ability of MXC to inhibit growth. MXC induced follicular atresia, whereas NAC (1–10mM) blocked the ability of MXC to induce atresia. In addition, MXC reduced the expression of SOD1, GPX, and CAT, whereas NAC reduced the effects of MXC on their expression. Collectively, these data indicate MXC causes slow growth and increased atresia by inducing oxidative stress.
Pesticides are an important part of our farming system and have an important role in maintaining crop health. In general, no pesticides are considered to be safe because their design requires that they are toxic to pests. The widespread use of pesticides is a concern because humans and wildlife species are exposed to chemicals that are designed to have some toxicity. Methoxychlor (MXC) is an organochlorine pesticide, which is widely sprayed on fruits, vegetables, forage crops, and home gardens to prevent insects from attacking them. MXC is considered to be an alternative to dichlorodiphenyltrichloroethane and to have a half-life of about 2–12 months in the environment (Wauchope et al., 1992).
Although MXC has been considered a potent environmental toxicant for several years (Cummings, 1997), it was not until recently that MXC was considered to be a reproductive toxicant (Borgeest et al., 2002, 2004; Gangadharan et al., 2001; Latchoumycandane and Mathur, 2002a; Latchoumycandane et al., 2002; Miller et al., 2005). In male sheep and rats, MXC reduces the weight of the testes, prostate, and seminal vesicles and disturbs spermatogenesis (Bal, 1984; Jackson et al., 1975; Tullner and Edgcomb, 1962). In female rodents, MXC causes ovarian atrophy (Eroschenko et al., 1995) and decreases the ability of ovarian cells to synthesize and secrete steroids (Chedrese and Feyles, 2001; Martinez and Swartz, 1992). Further, MXC specifically induces atresia (an apoptotic process) of antral follicles in vivo (Borgeest et al., 2002, 2004) and inhibits growth and increases atresia of antral follicles in vitro (Miller et al., 2005). This is of concern because antral follicles are required for fertility as they are the only follicles that are capable of releasing eggs for fertilization and producing steroid hormones that regulate menstrual/estrous cyclicity and maintain the female reproductive tract (Hirshfield, 1991). Despite the potential adverse reproductive outcomes associated with MXC exposure, little is known about the mechanisms by which MXC induces antral follicle atresia.
One of the major pathways that leads to apoptotic cell death is oxidative stress (Hirshfield, 1991; Tilly and Tilly, 1995). Oxidative stress can occur when there is an alteration in endogenous antioxidants levels. A few studies suggest that MXC may cause oxidative stress in male reproductive tissues because it alters antioxidant levels in the testes, epididymis, and sperm (Gangadharan et al., 2001; Latchoumycandane and Mathur, 2002a,b; Latchoumycandane et al., 2002). Further, a recent study suggests that MXC may cause oxidative stress in female reproductive tissues because MXC treatment impairs mitochondrial respiration, increases production of H2O2 in ovarian mitochondria, and increases oxidative damage in the ovary (Gupta et al., in press). Thus, the proposed work was designed to directly test the hypothesis that MXC inhibits growth and induces atresia of antral follicles through an oxidative stress pathway. To test this hypothesis, we tested the effect of MXC on antral follicle growth and alterations in the expression of selected antioxidant enzymes: Cu/Zn superoxide dismutase (SOD1), glutathione peroxidase (GPX), and catalase (CAT). Further, this work was designed to determine if an antioxidant, N-acetyl cysteine (NAC), protects antral follicles from MXC-induced growth inhibition and atresia. This hypothesis is supported by previous work which shows enzymes that protect against oxidative stress are important for ovarian function (Matzuk et al., 1998; Tilly and Tilly, 1995) and that MXC decreases the levels of SOD, GPX, and CAT in male reproductive tissues (Gangadharan et al., 2001; Latchoumycandane and Mathur, 2002a,b; Latchoumycandane et al., 2002). We chose these particular enzymes because SOD dismutates superoxide anion to hydrogen peroxide (H2O2), which is further detoxified to H2O by either CAT or via the glutathione pathway involving GPX. We were also particularly interested in SOD1 because it is known to be an important SOD isoform in the ovary (Matzuk et al., 1998).
MATERIALS AND METHODS
Chemicals.
MXC powder (99%) was purchased from Chemservice (West Chester, PA). Stock solutions of MXC were prepared using dimethylsulfoxide (DMSO) (Sigma, St Louis, MO) as the solvent in various concentrations (133, 13.3, and 1.33 mg/ml) that allowed an equal volume to be added to culture wells for each treatment group to control for solvent concentration. Final concentrations of MXC in culture were 1, 10, and 100 μg of MXC per milliliter. Final concentrations of NAC were 0.1, 1, 5, and 10mM. We chose NAC as opposed to other antioxidants as it is a known antioxidant, free-radical scavenger, glutathione precursor, and increases the activity of SOD and GPX enzymes (Tilly and Tilly, 1995). NAC has also been shown to protect from oxidative stress in other tissues and cell types (Hecht et al., 2002).
Animals.
Cycling female CD-1 (Charles River Laboratories, Charles River, CA) mice, 32–35 days old, were housed five animals per cage at the University of Maryland School of Medicine Central Animal Facility and provided food and water for ad libitum consumption. Temperature was maintained at 22 ± 1°C, and animals were subjected to 12-h light-dark cycles. Mice were euthanized when they were 39 days old. The University of Maryland School of Medicine Institutional Animal Use and Care Committee approved all procedures involving animal care, euthanasia, and tissue collection.
Experimental dosages.
We chose to use MXC at the doses of 1–100 μg/ml because a previous study showed that similar doses caused atresia of antral follicles (Miller et al., 2005). In addition, we chose these doses because they have been shown to affect proliferation and gene expression in ovarian cells and uterine leiomyoma cells in vitro (Chedrese and Feyles, 2001; Hodges et al., 2000). In addition, we have observed that MXC causes antral follicle atresia in vivo at concentrations of 32 and 64 mg/kg/day (32 and 64 ppm/day), 10 and 100 μg/ml MXC compromises follicle growth in culture, and 100 μg/ml MXC causes antral follicle atresia in culture (Borgeest et al., 2002, 2004; Miller et al., 2005). The selected in vitro doses are in the same range as the previously used in vivo doses. The in vitro doses are also environmentally relevant in that environmental levels of MXC range from 40–160 ppm in waters downstream of MXC-sprayed areas (Wallner et al., 1969) to 0.1–4.0 ppb/day exposures of humans via the diet (ATSDR, 2002).
NAC is a thiolic antioxidant that protects reproductive and nonreproductive tissues from oxidative stress (Hecht et al., 2002; Otala et al., 2002; Tilly and Tilly, 1995). NAC doses were based on the literature and preliminary dose-response studies conducted by us. We used NAC from 0.1 to 200mM concentrations along with DMSO vehicle control and performed in vitro follicle growth culture experiments for 96 h. Follicles treated with NAC above 10mM (i.e., 50, 100, and 200mM) did not grow compared to DMSO control, whereas follicles with NAC 0.1, 1, 5, and 10mM showed similar growth compared to DMSO control. Thus, all experiments were performed with NAC at 0.1–10mM.
In vitro follicle culture and analysis of follicle growth.
Female CD-1 mice were euthanized, and their ovaries were removed. Antral follicles were isolated mechanically from the ovary based on relative size and cleaned of interstitial tissue using fine watchmaker forceps. Sufficient numbers of antral follicles for experimental significance were isolated from unprimed mouse ovaries; follicles from two to four mice were isolated per day, providing approximately 20–40 antral follicles from each mouse. Upon isolation, follicles were placed individually in wells of a 96-well culture plate with unsupplemented α-minimal essential media (α-MEM) prior to treatment. Each experiment contained a minimum of 10–16 follicles per treatment.
For experiments examining the effects of MXC on antral follicles in culture, a dose-response regimen of MXC (1–100 μg/ml) and DMSO controls was individually prepared in supplemented α-MEM. For experiments examining the effects of NAC on antral follicles in culture, a treatment regimen of MXC 100 μg/ml, MXC 100 μg/ml + NAC (0.1–10mM), and DMSO controls was individually prepared in supplemented α-MEM. An equal volume of chemical was added for each dose to control for the amount of vehicle in each preparation. Supplemented α-MEM was prepared with 1% ITS (10 ng/ml insulin, 5.5 ng/ml transferrin, and 5.5 ng/ml selenium), 100 U/ml penicillin, 100 mg/ml streptomycin, 5 IU/ml human recombinant follicle-stimulating hormone (Dr A. F. Parlow, National Hormone and Peptide Program, Harbor–UCLA Medical Center, Torrance, CA), and 5% fetal calf serum (Atlanta Biologicals, Lawrenceville, GA).
For treatment, unsupplemented α-MEM was removed from each well and replaced with 150 μl supplemented α-MEM containing either MXC, NAC, MXC + NAC, or DMSO (vehicle). Follicles were then incubated for 0–96 h at 37°C in 95% air and 5% CO2. DMSO concentrations in all experiments were kept below 0.075%, levels that solubilized MXC in aqueous media without overt changes in growth or atresia. Nontreated (NT) controls were used in each growth experiment to control for culture conditions.
Antral follicles were cultured as described above for 96 h. Follicle growth was examined at 24-h intervals by measuring follicle diameter on two perpendicular axes with an inverted microscope equipped with a calibrated ocular micrometer. Antral follicles were considered to be those follicles with diameters of 200 μm or greater (Cortvrindt and Smitz, 2002; Miller et al., 2005), which correlates with histological appearance of these follicles. Follicle diameter measurements were averaged among treatment groups and plotted to compare the effects of chemical treatments on growth over time. Data were presented as percent change from 0 h.
Histological evaluation of atresia.
At the end of each follicle culture experiment, supplemented α-MEM was removed from each well and Dietrick's solution was immediately added to fix follicles. Follicles were fixed for at least 24 h in Dietrick's solution and transferred in histology cassettes to 70% ethanol. The tissues were dehydrated, embedded in Paraplast (VWR Scientific, West Chester, PA), serially sectioned (5 μm), mounted on glass slides, and stained with Weigert's hematoxylin and methyl blue:picric acid. Each follicle section was examined for the level of atresia as evidenced by the presence of pyknotic bodies and reported at the highest level observed throughout the tissue. Follicles were rated on a scale of 1–4 for the presence of pyknotic bodies: 1 = healthy, 2 = < 10% pyknotic bodies (early), 3 = 10–30% pyknotic bodies (mid), and 4 = > 30% pyknotic bodies (late), as previously described (Miller et al., 2005). All analyses were conducted without knowledge of treatment group. Ratings were averaged and plotted to compare the effect of chemical treatments on atresia levels.
Quantitative real-time polymerase chain reaction.
Female CD-1 mouse antral follicles were cultured as described above for 24, 48, 72, or 96 h. At each time point, follicles were collected and snap frozen at − 80°C for quantitative real-time polymerase chain reaction (qPCR) analysis. Total RNA was extracted from follicles using the RNeasy Mini Kit (Qiagen, Inc., Valencia, CA) according to the manufacturer's protocol. Reverse transcriptase generation of cDNA was performed with 0.3–1 μg of total RNA using an Omniscript RT kit (Qiagen, Inc.) with random primers according to the manufacturer's protocols. qPCR was conducted using an MJ Research Chromo4 Real-Time PCR machine (MJ Research, Inc., Waltham, MA) and accompanying software according to the manufacturer's instructions. The Chromo4 quantifies the amount of PCR product generated by measuring a dye (SYBR Green) that fluoresces when bound to double-stranded DNA. A standard curve was generated from five serial dilutions of one of the samples, thus allowing analysis of the amount of cDNA in the exponential phase. Primer sequences used were as follows: SOD1 (forward) 5′-AAG-GCC-GTG-TGC-GTG-CTG-AA-3′, (reverse) 5′-CAG-GTC-TCC-AAC-ATG-CCT-CT-3′; GPX (forward) 5′-CCT-CAA-GTA-CGT-CCG-ACC-TG-3′, (reverse) 5′-CAA-TGT-CGT-TGC-GGC-ACA-CC-3′; CAT (forward) 5′-GCA-GAT-ACC-TGT-GAA-CTG-TC-3′, (reverse) 5′-GTA-GAA-TGT-CCG-CAC-CTG-AG-3′; and β-actin (forward) 5′-GGG-CACAGT-GTG-GGT-GAC-3′, (reverse) 5′-CTG-GCA-CCA-CAC-CTT-CTAC-3′. qPCR analysis was performed using 3 μl cDNA, forward and reverse primers (5 pmol) for SOD1, GPX, CAT or β-actin, in conjunction with a DyNAmo SYBR Green qPCR kit (Finnzymes, care of Bio-Rad Laboratories, Hercules, CA). An initial incubation of 95°C for 10 min was followed by denaturing at 94°C for 10 s, annealing at 56°C (SOD1 and GPX) or 55°C (CAT and β-actin) for 10 s, and extension at 72°C for 10 s, for 50 cycles (SOD1, GPX, and CAT) or 40 cycles (β-actin), followed by final extension at 72°C for 10 min. A melting curve was generated at 55–90°C to monitor the generation of a single product. The software also generated a standard curve. β-Actin was used as an internal standard for each sample. Final values were calculated and expressed as the ratio SOD1:β-actin, GPX:β-actin, or CAT:β-actin. All experiments were performed in triplicate.
Statistical analysis.
All data were analyzed using SPSS statistical software (SPSS, Inc., Chicago, IL). For all comparisons, statistical significance was assigned at p ≤ 0.05. For multiple comparisons between controls and MXC-treated groups, we used analysis of variance, along with a Tukey's post hoc test or multiple regression analysis. At least three separate experiments were conducted prior to data analysis.
RESULTS
Effect of MXC on Follicle Growth in Culture
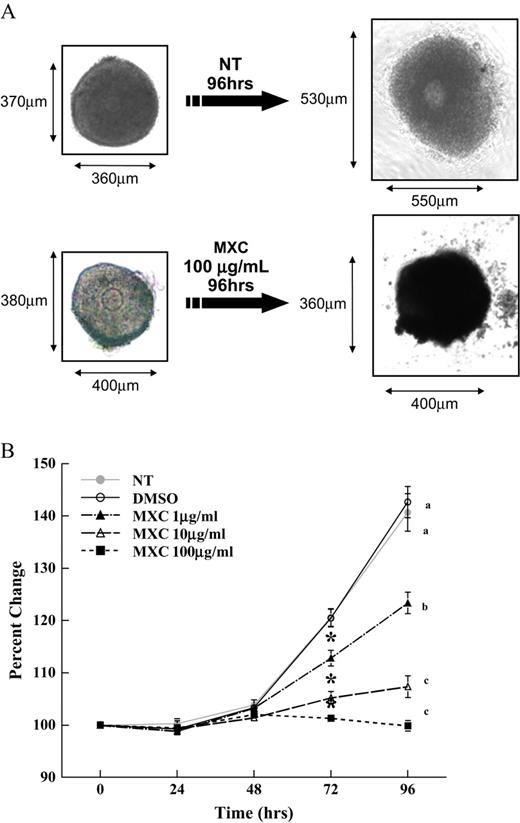
Our in vitro culture assay determined that antral follicles can be grown and maintained in culture in the absence of chemical treatment, without visible signs of cell death, and that follicle growth is similar to the published in vitro follicle growth by (Cortvrindt and Smitz, 2002; Miller et al., 2005). Representative pictures of antral follicles in culture are shown in Figure 1A. Freshly isolated antral follicles were intact and consisted of a clearly visible oocyte, multiple layers of granulosa cells, and a theca cell layer. After 96 h in culture, NT and DMSO-treated follicles grew in size, with theca cells attaching to the bottom of the culture well and granulosa cells proliferating to increase follicle diameter. After MXC treatment for 96 h, antral follicles did not grow compared to DMSO controls. While theca cells seemed to attach, the attachments were not as dense and firm as those in DMSO controls. Further, granulosa cells did not proliferate, and the follicles became dark in appearance.
Effect of MXC exposure on CD-1 mice antral follicle growth. Antral follicles isolated mechanically from CD-1 mice were exposed in vitro to MXC (1–100 μg/ml) for 96 h. Representative photographs of NT and MXC (100 μg/ml)-treated antral follicles from CD-1 mice cultured for 96 h are shown (A). Growth of follicles was monitored during culture and recorded in micrometers and reported as percent change (B). Graph represents means ± SEMs from three separate experiments. Lines with asterisk are significantly different from controls at 72 h, and lines with different letters are significantly different from each other at 96 h (n = 10–16 follicles per treatment per experiment from three separate experiments, p ≤ 0.05).
Using the follicle culture assay, the effect of MXC on follicle growth was evaluated for 96 h. Follicles treated with vehicle control showed normal growth; however, significant inhibition of antral follicle growth was observed with MXC treatment (1, 10, and 100 μg/ml) compared to DMSO control follicles at 72 and 96 h (Fig. 1B).
Effect of MXC and MXC Plus NAC Cotreatment on Follicle Growth and Atresia
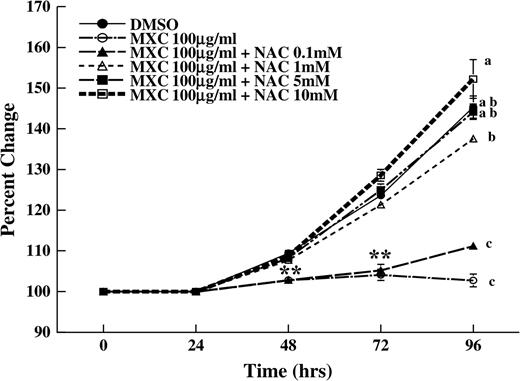
Prior to conducting experiments to test the hypothesis that NAC blocks MXC-induced growth inhibition and atresia of antral follicles, we conducted preliminary experiments to select a nontoxic level of NAC for the studies. Using the follicle culture assay, the effect of NAC on follicle growth was evaluated for 96 h. Similar growth was observed with follicles in the NT, DMSO vehicle control, and NAC (0.1–10mM) groups, but follicles cotreated with DMSO and NAC (50–200mM) did not grow (data not shown). Thus, in all subsequent experiments NAC at 0.1–10mM concentrations were used.
Inhibition of follicle growth was observed with MXC (100 μg/ml) and MXC (100 μg/ml) + NAC (0.1mM) as compared to DMSO controls (Fig. 2). In contrast, NAC (1–10mM) blocked the effect of MXC-induced growth inhibition. Specifically, MXC (100 μg/ml) + NAC (1–10mM)–treated follicles had similar growth over time compared to DMSO controls (Fig. 2).
Effect of MXC and NAC cotreatment on antral follicle growth. Antral follicles were isolated mechanically from CD-1 mice and exposed to MXC 100 μg/ml or MXC 100 μg/ml + NAC (0.1–10mM) for 96 h. Graph represents means ± SEMs from three separate experiments. Asterisks represent significant difference from controls at 48 and 72 h, and lines with different letters are significantly different from each other at 96 h (n = 12–16 follicles per treatment per experiment from three separate experiments, p ≤ 0.05).
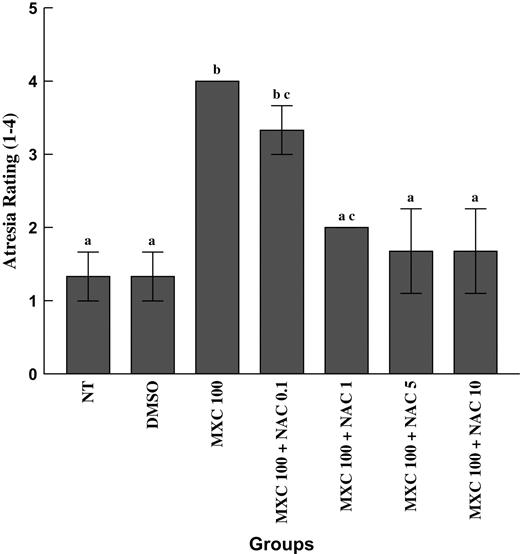
After culture, follicles were collected to determine the degree of atresia in treated and control follicles. There was little or no atresia observed in DMSO-treated follicles (Fig. 3). MXC (100 μg/ml) increased follicular atresia compared to DMSO control (Fig. 3). NAC at the 0.1mM concentration was not able to block MXC-induced atresia, but NAC at 1–10mM concentrations blocked MXC-induced atresia of antral follicles (Fig. 3). The atresia ratings were 1.33 ± 0.33 for DMSO-, 4.00 ± 0.00 for MXC (100 μg/ml)-, 3.33 ± 0.33 for MXC (100 μg/ml) + NAC (0.1mM)–, 2.00 ± 0.01 for MXC (100 μg/ml) + NAC (1mM)–, 1.67 ± 0.58 for MXC (100 μg/ml) + NAC (5mM)–, and 1.67 ± 0.58 for MXC (100 μg/ml + NAC (10mM)–treated follicles (Fig. 3).
Effect of MXC and NAC cotreatment on atresia. Antral follicles treated with DMSO, MXC 100 μg/ml, or MXC 100 μg/ml + NAC (0.1–10mM) were fixed and processed for histological evaluation of atresia and rated on a scale of 1–4, where 1 = healthy, 2 = < 10% pyknotic bodies, 3 = 10–30% pyknotic bodies, and 4 = > 30% pyknotic bodies. Graph represents means ± SEMs from three separate experiments. Bars with different letters are significantly different from each other (n = 6–9 follicles per treatment per experiment from three separate experiments, p ≤ 0.05).
Effect of MXC and MXC Plus NAC Cotreatment on mRNA Levels of SOD1, GPX, and CAT
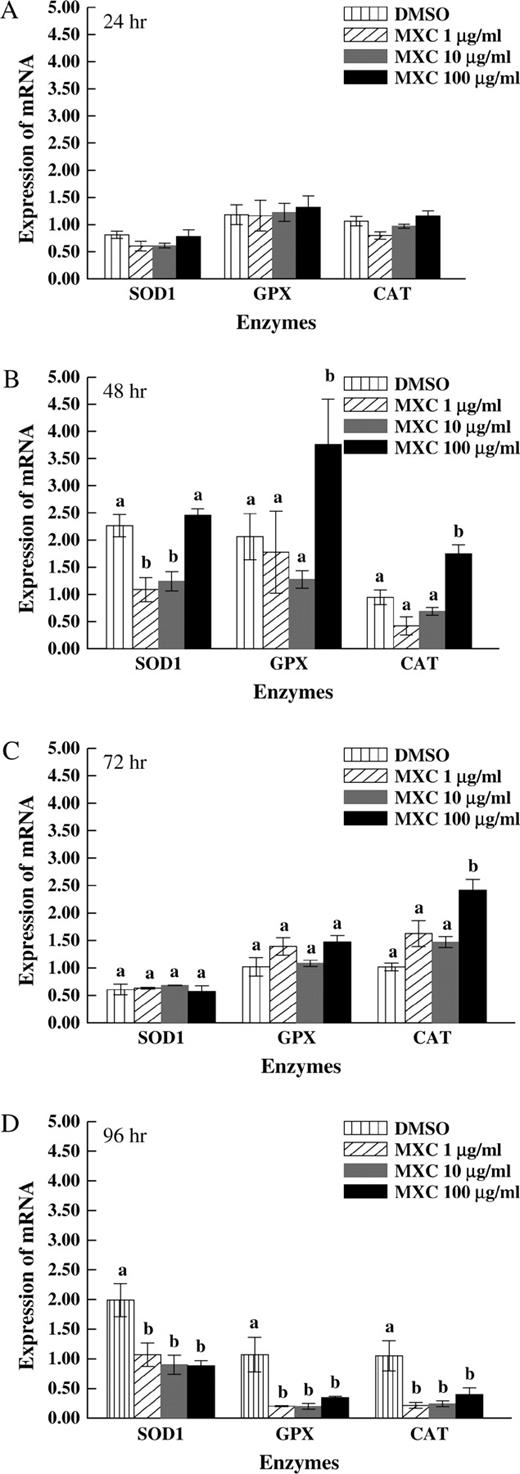
In preliminary experiments, MXC (1–100 μg/ml) did not alter the expression of SOD1, GPX, or CAT at 0–12 h. Thus, all future experiments were conducted for at least 24 h. At 24 h, MXC (1–100 μg/ml) did not significantly change the expression of SOD1, GPX, and CAT compared to DMSO control (Fig. 4A). At 48 h, MXC (1 and 10 μg/ml) significantly decreased SOD1 compared to DMSO control (Fig. 4B). MXC (100 μg/ml) did not affect SOD1 expression compared to DMSO control at this time point (Fig. 4B). MXC (100 μg/ml) significantly increased GPX and CAT expression compared to DMSO control at 48 h (Fig. 4B). At 72 h, MXC (100 μg/ml) significantly increased CAT expression compared to DMSO control (Fig. 4C). At 96 h, MXC (1–100 μg/ml) significantly decreased SOD1, GPX, and CAT expression compared to DMSO control (Fig. 4D). MXC (1–100 μg/ml) did not affect the housekeeping gene (β-actin) expression in any groups.
Effect of MXC exposure on SOD1, GPX, and CAT expression at 24, 48, 72, and 96 h in antral follicles. Antral follicles from CD-1 mice were exposed in vitro to MXC (1–100 μg/ml) for 24, 48, 72, and 96 h and subjected to qPCR for analysis of SOD1, GPX, and CAT mRNA levels. All values were normalized to β-actin as a loading control. Graph represents means ± SEMs from three separate experiments. Bars with different letters are significantly different from each other within the enzyme groups (n = 10–16 follicles per treatment per experiment from three separate experiments, p ≤ 0.05).
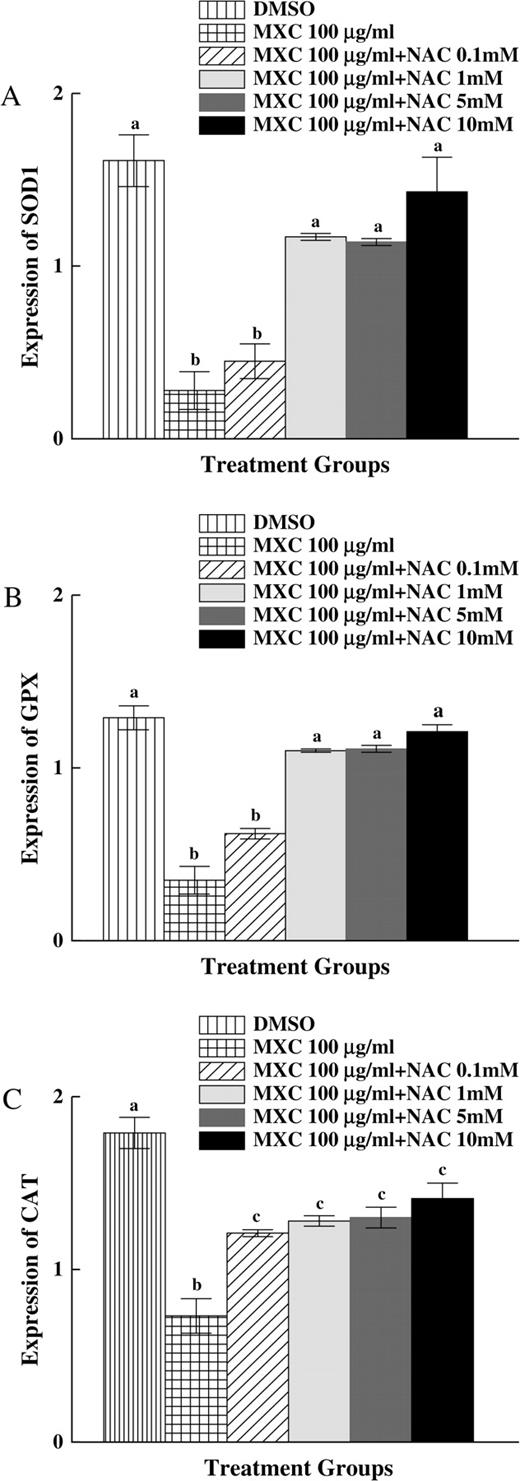
Since data in Figures 4A–4D showed that MXC decreased the expression of SOD1, GPX, and CAT, we investigated the expression of these specific enzymes after cotreatment with MXC (100 μg/ml) + NAC (0.1–10mM). Expression of SOD1 with MXC (100 μg/ml) and MXC (100 μg/ml) + NAC (0.1mM) treatment was significantly lower than DMSO control, but expression of SOD1 after MXC (100 μg/ml) + NAC (1–10mM) treatment was similar to DMSO controls (Fig. 5A). Expression of GPX after MXC (100 μg/ml) and MXC (100 μg/ml) + NAC (0.1mM) treatment was significantly lower than DMSO control, but expression of GPX after MXC (100 μg/ml) + NAC (1–10mM) treatment was similar to DMSO control (Fig. 5B). Expression of CAT after MXC (100 μg/ml) and MXC (100 μg/ml) + NAC (0.1–10mM) treatment was significantly lower than DMSO control (p ≤ 0.05), but expression of CAT after MXC (100 μg/ml) + NAC (0.1–10mM) treatment was significantly higher than MXC (100 μg/ml)-treated follicles (Fig. 5C).
Effect of MXC and NAC cotreatment on SOD1, GPX, and CAT expression in antral follicles. Antral follicles from CD-1 mice were exposed in vitro to MXC 100 μg/ml and MXC 100 μg/ml + NAC (0.1–10mM) for 96 h and subjected to qPCR for analysis of SOD1, GPX, and CAT mRNA levels (n = 10–16 follicles per treatment). All values were normalized to β-actin as a loading control. Graph represents means ± SEs from three separate experiments. Bars with different letters are significantly different from each other (n = 12–16 follicles per treatment per experiment from three separate experiments, p ≤ 0.05).
DISCUSSION
To our knowledge, this is the first study to show that MXC decreases expression of antioxidant enzymes in mouse antral follicles and that the antioxidant NAC blocks MXC-induced growth inhibition and atresia of antral follicles. Specifically, this study indicates that MXC decreases the expression of Cu/Zn SOD1, GPX, and CAT. This study also shows that cotreatment of antral follicles with NAC and MXC prevents MXC-induced growth inhibition, atresia, and decreases in the expression of SOD1, GPX, and CAT due to MXC alone.
Previous studies indicate that MXC (32 and 64 mg/kg/day) increases atresia of antral follicles in vivo (Borgeest et al., 2002, 2004) and that MXC (100 μg/ml) induces atresia of antral follicles in vitro (Miller et al., 2005). Atresia is an apoptotic process, and one of the important mechanisms leading to apoptotic cell death is oxidative stress. Oxidative stress is characterized by an inability of the cell to counteract an overwhelming production of reactive oxygen species (ROS). Cells protect themselves from ROS through various defenses including antioxidant enzymes like SOD, GPX, and CAT. In a previous study, we showed that MXC causes oxidative stress in female reproductive tissues because MXC treatment impairs mitochondrial respiration, increases production of H2O2 in ovarian mitochondria, and increases oxidative damage in the ovary (Gupta et al., in press). In the current study, we examined whether MXC affects the expression of the antioxidant enzymes SOD1, GPX, and CAT. SOD1 is important for ovarian function (Matzuk et al., 1998; Tilly and Tilly, 1995) and is responsible for dismutation of superoxide to H2O2. Further, CAT and GPX in the GSH cycle can convert H2O2 to H2O. Our study shows that MXC increases the expression of GPX at 48 h and CAT at 48 and 72 h, but dramatically reduces expression of SOD1, GPX, and CAT at 96 h. This is of interest because atresia of antral follicles due to MXC treatment is seen at 96 h of culture (Miller et al., 2005). The initial increases in enzyme expression in response to MXC may be an early attempt to try to protect the follicles from MXC-induced toxicity. It is likely, however, that the enzymes eventually become overwhelmed, and thus, their expression is reduced by 96 h.
While no studies have reported the effect of MXC on antioxidant enzymes in the ovary, there are studies showing that MXC affects antioxidant enzymes in other tissues and that other inducers of oxidative stress alter the expression of antioxidant enzymes. A study by Latchoumycandane et al. (2002) showed that MXC decreases the activity of SOD, GPX, glutathione reductase (GRX), and CAT in the epididymis and sperm of rats. In another study by Gangadharan et al. (2001), MXC decreased the activity of SOD, GRX, and GPX in goat sperm. In a study by Spolarics (1996), oxidative stress induced Cu/Zn SOD, Mn SOD, and GPX enzyme activity and gene expression. In other studies, oxidative stress decreased gene expression of Cu/Zn SOD and Mn SOD (Ookawara et al., 2002). Our study supports and expands these findings.
The pathway through which MXC decreases the expression of antioxidant enzymes is not known. Schuh et al. (2005) have shown that MXC increases the production of H2O2 in the isolated brain mitochondria from mice. Gupta et al. (in press) have shown that MXC increases H2O2 production by mitochondria isolated from the ovaries of in vivo treated mice. ROS such as H2O2 are known to induce the expression of genes, including heme oxygenase and glutathione-S-transferase (Keyse and Tyrrell, 1989; Rushmore and Pickett, 1990). Researchers have identified an antioxidant response element (ARE) in some genes, which responds to oxidative stimuli (Rushmore et al., 1991), and have investigated the mechanism by which ROS affect signal transduction pathways that regulate gene transcription through this element. These studies indicate that ROS can regulate signaling pathways involving ERK and p38 mitogen–activated protein kinases (Kang et al., 2000; Yu et al., 1999), tyrosine kinase (Dieter et al., 2001), phosphotidylinositol-3-kinase (Kang et al., 2000), protein kinase C (Huang et al., 2000), and NF-κB (Zhou et al., 2001). Regardless of the mechanism of activation of the transcription proteins, AREs are responsive to ROS and, thus, may represent part of signal transduction, allowing the cells to respond to oxidative stimuli resulting in gene modulation (Rushmore et al., 1991). Studies indicate that AREs are present in the promoter regions of Cu/Zn SOD and Mn SOD genes (Park and Rho, 2002; Scandalios, 2005), in the promoter regions of maize CAT genes (Scandalios, 2005), and in the GPX gene (Banning et al., 2005). Motifs for NF-κB and AP-1 transcription factors have been located in the promoter regions of Cu/Zn SOD, Mn SOD, GPX, and CAT genes (Jones et al., 1995; Jornot and Junod, 1997; Kim et al., 1994; Zhou et al., 2001). Thus, it is quite possible that MXC alters the expression of these enzymes through any of these pathways.
NAC is a thiolic antioxidant that protects reproductive and nonreproductive tissues from oxidative stress (Hecht et al., 2002; Otala et al., 2002; Tilly and Tilly, 1995). It acts as a free-radical scavenger and glutathione precursor and is also known to increase the activity of SOD and GPX (Tilly and Tilly, 1995). Further, a study by Tilly and Tilly (1995) showed that incubation of rat antral follicles with NAC inhibited apoptosis of follicles. Our current study shows that MXC inhibits growth, induces atresia, and decreases the expression of SOD1, GPX, and CAT and that these MXC-induced effects are blocked by the antioxidant NAC.
The mechanism by which NAC acts to prevent MXC-induced toxicity is unknown. Previous studies show that MXC increases inducible nitric oxide via the NF-κB pathway (Kim et al., 2005) and that NAC can block the stimuli that activate NF-κB (Dalton et al., 1999). Further, studies indicate that motifs for NF-κB are located in the promoter regions of SOD, GPX, and CAT (Jones et al., 1995; Jornot and Junod, 1997; Kim et al., 1994; Zhou et al., 2001). We have shown that MXC treatment decreases the expression of SOD1, GPX, and CAT and that this effect of MXC is blocked by NAC. Thus, it is possible that MXC-induced ROS alter the NF-κB pathway to further regulate the antioxidant expression causing oxidative stress and that NAC prevents these processes. In addition, previous studies have shown that MXC increases Bax levels and decreases Bcl-2 levels (Borgeest et al., 2004) and that Bax deletion and Bcl-2 overexpression offer some protection from MXC-induced antral follicle toxicity (Miller et al., 2005). Studies also indicate that Bcl-2 family members and oxidative stress pathways interact (Celli et al., 1998; Voehringer, 1999). Thus, it is possible that the MXC-induced changes in Bcl-2 family members lead to oxidative stress and/or that MXC-induced oxidative stress leads to changes in Bcl-2 family members and that these effects of MXC are blocked by NAC. Currently, it is unknown whether the MXC-induced changes in Bcl-2 family members lead to oxidative stress or whether MXC-induced oxidative stress leads to changes in Bcl-2 family members. Thus, future studies should examine this in detail.
In conclusion, MXC causes oxidative stress in antral follicles, which leads to atresia of these follicles. This study has greatly improved our understanding of the mechanisms by which MXC damages the antral follicles, and in turn, this improved understanding may lead to the development of novel targets for the treatment and/or prevention of infertility induced by chemical exposures.
The authors would like to acknowledge funding by NIH RO1 ES012893-01A2 and Colgate Palmolive. The authors would like to acknowledge Dr Dragana Tomic for her help with real-time PCR. In addition, the authors would like to thank Lynn Lewis for her assistance with formatting the manuscript.
References
Agency for Toxic Substances and Disease Registry (ATSDR) (
Bal, H. S. (
Banning, A., Deubel, S., Kluth, D., Zhou, Z., and Brigelius-Flohe, R. (
Borgeest, C., Miller, K. P., Gupta, R., Greenfeld, C., Hruska, K. S., Hoyer, P., and Flaws, J. A. (
Borgeest, C., Symonds, D., Mayer, L. P., Hoyer, P. B., and Flaws, J. A. (
Celli, A., Que, F. G., Gores, G. J., and LaRusso, N. F. (
Chedrese, P. J., and Feyles, F. (
Cortvrindt, R. G., and Smitz, J. E. (
Cummings, A. M. (
Dalton, T. P., Shertzer, H. G., and Puga, A. (
Dieter, M. Z., Freshwater, S. L., Solis, W. A., Nebert, D. W., and Dalton, T. P. (
Eroschenko, V. P., Abuel-Atta, A. A., and Grober, M. S. (
Gangadharan, B., Murugan, M. A., and Mathur, P. P. (
Gupta, R. K., Schuh, R. A., Fiskum, G., and Flaws, J. A. (
Hecht, S. S., Upadhyaya, P., Wang, M., Bliss, R. L., McIntee, E. J., and Kenney, P. M. (
Hirshfield, A. N. (
Hodges, L. C., Bergerson, J. S., Hunter, D. S., and Walker, C. L. (
Huang, H. C., Nguyen, T., and Pickett, C. B. (
Jackson, C., Jr, Lindahl, I. L., Reynolds, P., and Sidwell, G. M. (
Jones, P. L., Kucera, G., Gordon, H., and Boss, J. M. (
Jornot, L., and Junod, A. F. (
Kang, K. W., Ryu, J. H., and Kim, S. G. (
Keyse, S. M., and Tyrrell, R. M. (
Kim, H. T., Kim, Y. H., Nam, J. W., Lee, H. J., Rho, H. M., and Jung, G. (
Kim, J. Y., Oh, K. N., Han, E. H., Kim, D. H., Jeong, T. C., Lee, E. S., and Jeong, H. G. (
Latchoumycandane, C., Chitra, K. C., and Mathur, P. P. (
Latchoumycandane, C., and Mathur, P. P. (
Latchoumycandane, C., and Mathur, P. P. (
Martinez, E. M., and Swartz, W. J. (
Matzuk, M. M., Dionne, L., Guo, Q., Kumar, R., and Lebovitz, R. M. (
Miller, K. P., Gupta, R. K., Greenfeld, C. R., Babus, J. K., and Flaws, J. A. (
Ookawara, T., Suzuk, K., Haga, S., Ha, S., Chung, K. S., Toshinai, K., Hamaoka, T., Katsumura, T., Takemasa, T., Mizuno, M., et al. (
Otala, M., Erkkila, K., Tuuri, T., Sjoberg, J., Suomalainen, L., Suikkari, A. M., Pentikainen, V., and Dunkel, L. (
Park, E. Y., and Rho, H. M. (
Rushmore, T. H., Morton, M. R., and Pickett, C. B. (
Rushmore, T. H., and Pickett, C. B. (
Scandalios, J. G. (
Schuh, R. A., Kristian, T., Gupta, R. K., Flaws, J. A., and Fiskum, G. (
Spolarics, Z. (
Tilly, J. L., and Tilly, K. I. (
Tullner, W. W., and Edgcomb, J. H. (
Voehringer, D. W. (
Wallner, W. E., Leeling, N. C., and Zabik, M. J. (
Wauchope, R. D., Buttler, T. M., Hornsby, A. G., Augustijn-Beckers, P. W., and Burt, J. P. (
Yu, R., Lei, W., Mandlekar, S., Weber, M. J., Der, C. J., Wu, J. K., and Kong, A. N. (




Comments