Serotonin syndrome, variously described as serotonin toxicity or serotonin toxidrome, is often an iatrogenic adverse drug reaction. In this article serotonin syndrome refers to the broad spectrum of clinical presentations in humans secondary to a relative or absolute increase in serotonin levels in the central and peripheral nervous system.
A study based in general practice reported the incidence of serotonin syndrome at 0.5–0.9 cases per 1000 patient-months of treatment. This is likely to be an underestimate as it pertains to patients on monotherapy with selective serotonin reuptake inhibitors (SSRIs); this rises to 15% where overdose with serotonergic medication has occurred (Reference Isbister, Buckley and WhyteIsbister 2007). Serotonin syndrome can present as a spectrum of different symptoms and signs and in varying degrees of severity. Less severe presentations of the syndrome may remain undiagnosed, with symptoms such as anxiety, confusion, restlessness, headache, insomnia, agitation and hypomanic behaviour perhaps being attributed to the mental illness under treatment or misdiagnosed.
Serotonin syndrome: a brief history
One of the earliest descriptions of the syndrome emanates from 1955 and is that of a 60-year-old physician with pulmonary tuberculosis treated with iproniazid; after receiving 100 mg of pethidine he displayed symptoms of acute onset consistent with serotonin toxicity (Reference SheeShee 1960). Several other reports followed, but another 5 years elapsed before the development of the hypothesis that these symptoms resulted from excess serotonin. It was much later, in the early 1980s, that the syndrome was set in its modern day context. An animal model of serotonin syndrome, with some differences, has also been described and generally confirms the role of 5-HT2A receptors in the development of raised temperature and rigidity (Reference JacobsJacobs 1974).
Reference SternbachSternbach (1991), after closer analysis of 12 out of 38 published cases of symptoms in humans, defined this condition as ‘serotonin syndrome’. However, he concluded that:
‘Further work is needed to establish the diagnostic criteria, incidence, and predisposing factors, to identify the role of 5-HT antagonists in treatment, and to differentiate the syndrome from neuroleptic malignant syndrome’.
Sternbach’s criteria (Box 1) suggested that, in the presence of a known serotonergic agent, three or more out of ten symptoms should be present to merit the diagnosis, that alternative possible causes be excluded and that no recent increase or addition of a psychotropic agent has occurred. These criteria have not been specifically validated, and indeed controversy continues regarding the wording of the requirements, not least from the point of fulfilling even the first criterion, i.e. the precise definition of ‘a known serotonergic agent’.
BOX 1 Sternbach’s criteria for serotonin syndrome
Sternbach’s criteria stipulate that three conditions must be met:
-
1 there should have been a recent addition or increase in a ‘known’ serotonergic agent
-
2 there should not have been a recent addition or increase in a ‘neuroleptic’ (antipsychotic) agent
-
3 possible alternative causes such as infection, substance misuse or withdrawal and metabolic upset should be excluded.
In addition, three or more of the ten symptoms listed below must be present:
-
Mental status changes
-
Pyrexia
-
Myoclonus
-
Hyperreflexia
-
Incoordination
-
Agitation
-
Diaphoresis
-
Tremor
-
Shivering
-
Diarrhoea
(Adapted from Reference SternbachSternbach 1991)
Reference Hegerl, Bottlender and GallinatHegerl et al (1998) subsequently modified Sternbach’s criteria on the basis of the observed correlation between adverse effects and paroxetine dose, and developed the Serotonin Syndrome Scale, which they described as a scale ‘for the operationalized assessment of both the presence and the severity of the core symptoms of the serotonin syndrome’.
Reference Radomski, Dursun and ReveleyRadomski et al (1999), following the detailed review of a further 24 cases reported in the medical literature from 1991 to 1995, proposed a severity-based classification for serotonin syndrome into mild, full-blown and toxic states.
The Hunter Serotonin Toxicity Criteria (HSTC) (Reference Dunkley, Isbister and SibbrittDunkley 2003) were based on an Australian study of 473 patients who had taken an overdose of SSRIs and had been referred to the Hunter Area Toxicology Service between 1987 and 2002. The authors concluded that the presence of at least five symptoms was sufficient for a clinical toxicologist to diagnose serotonin toxicity. The authors’ observation of associated pyrexia and hypertonicity among the more severe cases led to an algorithm combining seven features (Box 2). The HSTC are said to be the most sensitive, specific and easy-to-use decision rules currently available for serotonin syndrome (Reference Haddad and DursunHaddad 2008; Reference Monte, Chuang and BodmerMonte 2010).
BOX 2 The Hunter Serotonin Toxicity Criteria
Requirement – in the presence of a serotonergic agent:
-
1 clonus (inducible, spontaneous or ocular) is the cardinal sign
-
2 agitation
-
3 diaphoresis
-
4 tremor
-
5 hyperreflexia
-
6 rigidity
-
7 body temperature greater than 38°C
Items 6 and 7 were derived from their frequent presence in severe and toxic serotonin syndrome
(Adapted from Reference Dunkley, Isbister and SibbrittDunkley 2003)
Serotonin
Serotonin, or 5-hydroxytryptamine (5-HT, C10H12N2O), is a monoamine neurotransmitter derived from dietary tryptophan. Serotonin was discovered in 1948 and it received its name from its apparent property of being a vasoconstriction-inducing constituent of serum. Serotonin has a role in many body processes, including the regulation of mood and other emotional behaviours as well as pain perception, aggression, vomiting, the sleep cycle, appetite, haemostasis, sexual function and body temperature control.
The serotonin receptor and its many subtypes is the most prolific of all neuroreceptors in the human body. At least seven families of serotonin receptor (5-HT1–7) have been identified, most of which have several further subtypes. Serotonin does not cross the blood–brain barrier and only about 1 mg of serotonin is produced in the central nervous system (CNS). Metabolism proceeds mainly in the liver via monoamine oxidase enzymes; the main end product, 5-hydroxyindoleacetic acid (5-HIAA), is excreted via the kidneys.
Predominantly used in the treatment of depression, SSRIs are also used to treat seemingly diverse conditions such as eating disorders, neuropathic pain, sexual behaviour, sleep, aggression and anxiety disorders (Reference Lee and ChenLee 2010). An interplay of over- and underactivity of various 5-HT receptor subtypes, including 5-HT1A, 5-HT2A and 5-HT2C, as well as other neurotransmitter systems, notably the noradrenergic and glutamatergic, are thought to be involved (Reference Monte, Chuang and BodmerMonte 2010).
Serotonin syndrome and the role of cytochrome P450 enzymes
Many drugs, including SSRIs, can precipitate serotonin syndrome either directly or indirectly via their action on the cytochrome P450 (CYP) enzyme system (Box 3). CYP is a large family of oxidative enzymes located in the endoplasmic reticulum of cells. The highest densities of these enzymes occur in the liver, but they are also found in the small intestine wall, kidneys, placenta, brain and lungs. The importance of the CYP enzyme system lies in its role not only in the production, but also in the breakdown, of steroids, cholesterol, vitamins and prostacyclins, and in the metabolism of food and medications – notably antidepressants, opioids and antipsychotics, among others.
BOX 3 Drugs associated with serotonin toxicity, by mode of action
Precursors of excess serotonin or serotonin agonists
Buspirone, L-tryptophan, L-dopa, trazodone
Reduced reuptake of serotonin
Tricyclic antidepressants, especially clomipramine, imipramine
Amphetamines, cocaine
Serotonin–noradrenaline reuptake inhibitors (SNRIs), especially venlafaxine
Selective serotonin reuptake inhibitors (SSRIs)
Trazodone
St John’s wort, tramadol
Reduced breakdown of serotonin
Monoamine oxidase inhibitors (MAOIs): reversible: moclobemide irreversible: tranylcypromine, phenelzine
Linezolid, selegiline, St John’s wort
Drugs that increase the release of serotonin
Amphetamine, cocaine, fenfluramine, methamphetamine, 3,4-methylenedioxy-methamphetamine (MDMA, ecstasy), methylphenidate, pethidine, reserpine, tramadol
Others
Buspirone, carbamazepine, lithium, methylene blue
Phenylpiperidine opioids: dextromethorphan, fentanyl, methadone, pethidine, tramadol
Novel psychoactive substances
(Adapted from Reference Haddad and DursunHaddad 2008; Reference Buckley, Dawson and IsbisterBuckley 2014)
Over 50 CYP enzymes are known. Of these, only half a dozen appear to be involved in the metabolism of 90% of drugs. Each CYP is encoded by a particular gene, one half of the gene pair originating from each of the biological parents. The pattern of inheritance and gene expression, deletion or aberration results in genetically based inter-individual and interracial variations in the effective activity of CYP isoenzymes. This genetic polymorphism is hypothesised to be one of the key mechanisms accounting for a range of drug effects.
Around one in ten ‘Caucasians’ (White/European people) is thought to have reduced activity of the CYP2D6 isoenzymes (Reference Park, Wackermah and StimmelPark 2014), which alone are thought to be involved in the metabolism of 25% of marketed drugs, including antidepressants, antipsychotics, opioids, anti-emetics and anti arrhythmics (Reference ZhouZhou 2009). The required therapeutic dose of half of the antidepressants in use is believed to be closely related to the individual’s CYP2D6 genotype; non-response has been suggested to result from possessing multiple copies of the CYP2D6 coding gene. A drug or substrate may be metabolised by more than one CYP isoenzyme (Reference Park, Wackermah and StimmelPark 2014). Different CYP enzymes may be involved in the metabolism of the prodrug, the parent compound or the (active) metabolites of the drug (Reference Lynch and PriceLynch 2007). Additional considerations include genetic polymorphisms of the neurotransmitter transporters and of their receptors.
Serotonin syndrome and foods
Grapefruit juice
Grapefruit juice is of particular relevance, as many medications may be taken with it at breakfast. Grapefruit, and at lower concentration essential citrus oils, contain bergamottin, a furanocoumarin. Furanocoumarins are a group of plant-produced chemicals that are toxic to insects and/or fungi. Bergamottin has a prominent inhibitory effect on intestinal CYP3A4 enzyme. CYP3A4 constitutes 70% of the CYP present in the epithelial cells of the small intestine and 30% of the CYP present in the liver. A single glass of grapefruit juice (200 ml), reduces the activity of intestinal CYP3A4 by up to a half within 4 h. Further, this inhibitory effect continues into the next day, with almost a third of the reduced activity still evident; it can take up to 3 days to subside.
It has been suggested that grapefruit juice also inhibits an intestinal P-glycoprotein pump which transports many of the CYP3A4 substrates, notably drugs, from the enterocytes back into the gut lumen. This results in reduced first-pass metabolism and, consequently, increased bioavailability of several drugs. Sertraline is predominantly metabolised by CYP3A4, and a 50% increase in bioavailability has been reported when it is consumed along with grapefruit juice; the degree of inhibition, however, is subject to inter-individual variability (Reference Lee, Chan and HarralsonLee 1999). Where there is liver damage, for instance in alcohol misuse, there is an added reduction in the hepatocyte CYP contribution; this effect may then become even more pronounced (Reference Kiani and ImamKiani 2007).
Caffeine
Caffeine is a ubiquitous constituent of many foods and drinks and its consumption is often higher in people with mental illnesses. Caffeine affects the release of catecholamines, and is known to improve mood, but it may worsen psychosis. Some people with depression may also be more sensitive to the effects of caffeine, and experience a worsening of anxiety and agitation (Reference Cauli and MorelliCauli 2005).
Caffeine is mainly metabolised via CYP1A2; fluvoxamine, which is a potent inhibitor of this enzyme, can reduce caffeine clearance by up to 80% and potentially precipitate caffeine toxicity. Caffeine itself can also inhibit CYP1A2; theoretically, it may increase available levels of other, serotonergic, drugs metabolised by CYP1A2, notably tricyclic antidepressants (Reference GillmanGillman 2010a). Reference Herraiz and ChapparoHerraiz & Chapparo (2006) state that the constituents of prepared coffee demonstrate (reversible) inhibitory effects on both monoamine oxidase A (MAO-A) and monoamine oxidase B (MAO-B) both of these enzymes in the mitochondrial outer membrane are crucial in the oxidative catabolism of various neurotransmitters, the former being more specifically involved in the inactivation of serotonin. Consumption of large amounts of caffeine in tandem with the ingestion of serotonergic medications, particularly antidepressants, may contribute to the development of serotonin syndrome in susceptible patients (Reference Shioda, Nisijima and NishidaShioda 2004).
Others
Other important CYP enzyme interactions with serotonergic medications include St John’s wort (Hypericum perforatum), which inhibits CYP3A4 and also contains constituents inhibiting several other enzymes (CYP1A2, 2C9, 2C19, 2D6), cigarette smoke (CYP1A2) and alcohol, especially red wine (CYP2E1). Some of these substances interact with each other; for example, cigarette smoke induces metabolism of caffeine (Reference Zhou, Chan and PanZhou 2004).
Presentation
Patients in mental health services are often treated with combinations of antidepressants and antipsychotics; the antagonistic role of olanzapine and risperidone at 5-HT2A receptors and the partial agonism of many atypical antipsychotics at 5-HT1A receptors is often disregarded. Further, it has been suggested that some people with psychiatric illness, especially those with schizophrenia, have abnormal thermoregulation, which includes an elevated baseline temperature (Reference Chang and CastleChang 2004). In susceptible individuals, serotonin syndrome may be precipitated when serotonin levels at specific synapses in the CNS are multiplied manifold.
In practice, serotonin syndrome usually results from drug interactions, classically secondary to the combined overdose of an SSRI with an (irreversible) monoamine oxidase inhibitor (MAOI), which in around 50% of cases is believed to result in severe serotonin syndrome. Moderate serotonin toxicity has been noted in 15% of people who took large quantities of SSRIs in overdoses (Reference Buckley, Dawson and IsbisterBuckley 2014). It can also occur where the drugs used have previously unrecognised serotonergic properties. Among others, anticonvulsants, anti-emetics, antimicrobials, anti-migraine and recreational drugs have been associated with serotonin syndrome (Reference GillmanGillman 2010b; Reference Monte, Chuang and BodmerMonte 2010; Reference Cooper and SejnowskiCooper 2013).
Severe serotonin toxicity can have a rapid onset and deterioration; death can ensue within 24 h. This may be alarming not only for the observer but also for the patient, who often remains alert in the early stages (Reference GillmanGillman 2005). Symptoms may be noted from 2 h and most present within 6–8 h of addition/ingestion of the relevant substance (Reference Ener, Sharon and MeglatheryEner 2003; Reference Haddad and DursunHaddad 2008). Chronic, less dramatic presentations have been described where the only symptom is anxiety, restlessness or diarrhoea, thus perhaps escaping recognition. In some cases symptoms may be misattributed to deterioration in mental state, with the risk of increasing or additional medication (Reference Ener, Sharon and MeglatheryEner 2003).
Serotonin syndrome: mechanism
Presynaptic neurons in the raphe nuclei, largely restricted to the basal plate of the pons and medulla, synthesise and release serotonin. The serotonin transporter (SERT) located on these cells plays a major role in reabsorption of much of this; some of this intrasynaptic serotonin also binds to autoreceptors, triggering a negative feedback loop, thus modulating its own release. Serotonergic medications through different mechanisms act on various steps of this process: SSRIs block the action of SERT, thereby increasing the available serotonin in the synaptic space by limiting the recycling process. Increased serotonin neurotransmission results, mainly at postsynaptic 5-HT1A and 5-HT2A receptors; currently both these subtypes, as well as peripheral and central serotonin, are implicated in the wide range of symptoms and signs in serotonin syndrome (Reference Haddad and DursunHaddad 2008).
There appears to be a sequential stimulatory effect in the presence of excess serotonin, which corresponds roughly to the spectrum of increasing toxicity. This may be observed clinically when the more abundant 5-HT1A receptors are stimulated at lower levels, accounting for some of the less severe symptoms, including, initially, hypothermia. Overstimulation of 5-HT2A receptors occurs at much higher serotonin concentrations; this subtype is now generally considered to be the mediator of the more severe, toxic aspects of serotonin syndrome, with marked pyrexia and neuromuscular hyperactivity. Not only is there a complex interplay between these two receptors, but also very high serotonin levels potentiate glutamatergic, noradrenergic and dopaminergic pathways, with progression to extreme toxic states; such toxicity may be precipitated subsequent to the ingestion of cocaine or 3,4-methylenedioxy-methamphetamine (MDMA, ecstasy) (Reference Haddad and DursunHaddad 2008).
There is support for the theory that a particular concentration of serotonin is a prerequisite for the development of serotonin syndrome. However, there is considerable inter-individual variation and this variability has its basis in genetic factors, which include polymorphisms of CYP2D6, SERT or the serotonin receptors themselves (Reference GillmanGillman 2005; Reference Fox, Jensen and GallagherFox 2007).
Diagnosis
Diagnosis is clinical and associated with a history of current or recent ingestion of a serotonergic drug(s). Measurement of serum serotonin levels does not add to the diagnosis; psychotropic drugs can accumulate in the brain in concentrations that are 10–40 times higher than in the serum. Acute medical presentations with generalised clonus (usually easier to elicit in the lower limbs) associated with rising temperature should prompt careful scrutiny of the pharmacotherapy and the consumption of certain foods and supplements, such as caffeine in its various forms, grapefruit juice and over-the-counter medicines such as St John’s wort. Other possible causes should be excluded. Serotonin syndrome should be considered when any patient presents with a combination of suggestive clinical features. In practice it can be difficult to identify clonus and hyperreflexia in severely toxic cases with progression to rigidity.
It may be simpler to consider the symptoms under three group headings:
-
neuromuscular: tremor, ocular clonus,Footnote a myoclonus, hyperreflexia, akathisia and muscular rigidity
-
autonomic: fever, flushed skin, tachycardia, diaphoresis, diarrhoea, abdominal cramps and tachypnoea
-
mental state/CNS: agitation, insomnia, hypomania,Footnote b hallucinations (visual), confusion and seizures.
Differential diagnosis
Before diagnosing serotonin toxicity it is important to consider neuroleptic (antipsychotic) malignant syndrome (Table 1) and other alternative or even comorbid possible causes, such as infection, metabolic upset and substance misuse/withdrawal states. Additional factors that may contribute to serotonin syndrome include dietary supplements, lithium ingestion, electroconvulsive therapy (ECT), hepatic or renal diseases, genetic defects in xenobioticFootnote c metabolising enzymes, and combinations of psychotropic medication with inhibitors of their enzymes, for example an SSRI with an MAOI.
TABLE 1 Comparison of clinical features of serotonin syndrome with those of neuroleptic malignant syndrome
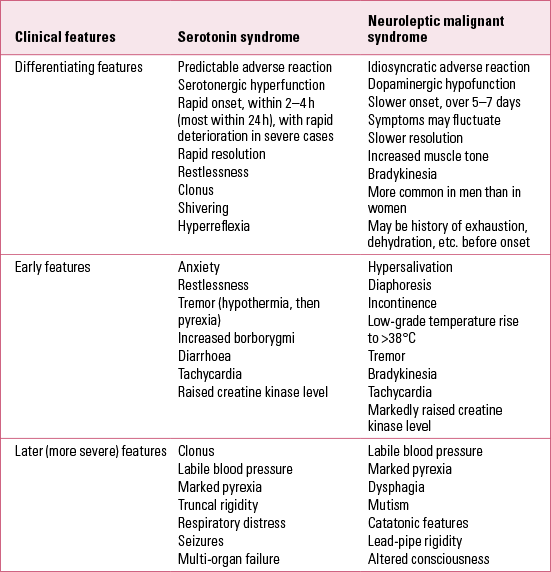
What to look for
Higher risk of serotonin syndrome is associated with increasing age, increasing dose of serotonergic agents and serotonergic agents in combination with medications that have high CYP2D6 inhibitory function. It is important to exercise greater vigilance where patients present with comorbid depressive symptoms and chronic pain. Commonly prescribed medications, such as antidepressants with tramadol, may interact, and at higher doses, tramadol is reported as being able to both block reuptake and induce release of serotonin (Reference Park, Wackermah and StimmelPark 2014). A higher incidence of serotonin syndrome has been reported in patients who are undergoing haemodialysis for end-stage renal disease and who are taking SSRIS; this may be due to decreased renal function (Reference Chander, Singh and MukhiyaChander 2011). Although rare, transient serotonin syndrome has been reported following a single dose of ECT in at least one patient receiving paroxetine. One of the mechanisms proposed was that ECT had increased the permeability of the blood–brain barrier, thus facilitating accumulation of more toxic levels of the SSRI in the brain (Reference Okamoto, Sakomoto and YamadaOkamoto 2012). Reference MorrishMorrish (2014) describes presentations with fever, rigidity and confusion in patients with Parkinson’s disease treated with MAO-B inhibitors who were also being treated for depression.
Serotonin syndrome and non-prescribed drugs
The first-generation antihistamine clorphenamine is thought to display serotonin reuptake inhibiting activity when ingested with dextromethorphan, such as in overdose of over-the-counter cough medicines (Reference Monte, Chuang and BodmerMonte 2010). Recreational drugs, prominently MDMA/ecstasy, in creative combinations with amphetamines, cocaine and variously obtained other drugs, such as attention-deficit hyperactivity disorder medication, sildenafil and novel psychoactive substances (also known as ‘legal highs’), tend to be used by younger, relatively well educated and employed males, who thus may be at increased risk of developing serotonin syndrome. Those who combine ecstasy with antidepressants more often report potentially serious symptoms such as muscle rigidity and nystagmus compared with those who use ecstasy alone (Reference Copeland, Dillon and GascoigneCopeland 2006). Several cases of serotonin syndrome following combined overdose with ecstasy and unprescribed moclobemide have been reported; in the more severe of these cases the person developed sudden onset of hypoxia associated with bronchospasm secondary to the rigidity of respiratory muscles (Reference Silins, Copeland and DillonSilins 2007).
Serotonin syndrome and headache
Serotonin is thought to modulate headache via specific receptor subtypes; many serotonergic medications have also been associated with headache as a side-effect (Reference FerrariFerrari 2006). A recent case series of four patients thought to have serotonin syndrome associated with fluoxetine, paroxetine and tramadol use reported headache as a prominent presenting feature (Reference Prakash, Belani and TrivediPrakash 2014).
Since 2006, the US Food and Drug Administration (FDA) has alerted healthcare professionals of the ‘potential for life-threatening serotonin syndrome’ in patients taking serotonergic medications (specifically SSRIs or serotonin–noradrenaline reuptake inhibitors – SNRIs – who are co-prescribed triptans for migraine headaches (FDA 2006). However, the clinical evidence underpinning the FDA’s warning has been questioned (Reference Evans, Tepper and ShapiroEvans 2010). Further, triptans are selective agonists at 5-HT1B/1D/1F receptor subtypes, with a much weaker affinity at 1A receptors and not thought to possess agonist activity at 2A receptors; there is thus also a view that triptans lack a plausible pharmacological role in precipitating or contributing to the severe toxicity observed in serotonin syndrome (Reference GillmanGillman 2010a). Nevertheless, the FDA has maintained its stance; there is general consensus that patient advice, caution and close monitoring are warranted (Reference Evans, Tepper and ShapiroEvans 2010).
Serotonin syndrome and interface with other specialties
Serotonin syndrome has been reported perioperatively as a result of serotonergic potentiation secondary to the coadministration of fentanyl during anaesthesia (Reference GillmanGillman 2005), to the use of methylene blue dye during parathyroid surgery (Reference GillmanGillman 2010b) and to the use of linezolid in the treatment of severe infection (Reference Shaikh, Krueper and MalinsShaikh 2011). Linezolid is a synthetic oxazolidinone antibiotic with a half-life of 5 h; it was originally developed for use as an antidepressant owing to its reversible non-selective MAO inhibitory properties. Its coincidental antimicrobial properties led to its further development and subsequent use in treating specific Gram-positive infections such as methicillin-resistant Staphylococcus aureus (MRSA) (Reference Jones, Athan and O'BrienJones 2004). Serotonin syndrome has recently been described following rewarming in patients who had been treated with therapeutic hypothermia for cardiac arrest (Reference Fugate, White and RabinsteinFugate 2014) and in a breastfed neonate whose mother had been taking 60 mg fluoxetine daily (Reference Morris and MatthesMorris 2015).
Management of serotonin syndrome
Treatment in mild to moderate cases consists of identifying and discontinuing the serotonergic medication(s), whereupon resolution usually follows within 1–3 days. There is currently no specific test to confirm the diagnosis of serotonin syndrome. Non-specific abnormalities, including raised white cell count, raised creatine kinase, and reduced magnesium, calcium and sodium levels, have been noted. However, monitoring of haematological and biochemical parameters, including a drug screen, may be useful where the diagnosis is unclear and in the treatment of patients who exhibit severe toxicity (Reference Iqbal, Miles and KaplanIqbal 2012). Maintain fluid balance, paying careful attention to urinary output and undertake regular observations of pulse, blood pressure and temperature (Reference Buckley, Dawson and IsbisterBuckley 2014).
An electrocardiogram (ECG) should be undertaken, for example where tricyclic antidepressants have been ingested; arrhythmias and postural hypo tension may not be self-evident. Liver toxicity may ensue either directly from large doses of lofepramine or as a secondary effect of the serotonin syndrome. An electroencephalogram may help in excluding non-convulsive epileptic states. Care should be taken where slow-release preparations or benzodiazepines have been ingested: observation times should be increased to 12 h or more in order to monitor for a delay in emergent side-effects. This should not detract from managing the patient’s immediate presenting symptoms in the meantime. Measurement of urinary dopamine and serotonin metabolites has been proposed as a possible adjunct to clinical assessment.
In moderate to severe serotonin syndrome individuals are likely to exhibit a degree of agitation and this can be treated with oral diazepam. However, it is important not to underestimate the possibility of rapid deterioration, especially in situations where even low doses of both an MAOI and an SSRI have been taken together, and input from the medical team should be sought. This should be done immediately if the patient has consumed large quantities of drugs, if there is doubt about the quantity or type of medication ingested, if novel psychoactive substances have been used, if combinations of medications have been taken or if there is suicidal intent with an unknown quantity of medication. There may also be cardiotoxic effects, such as prolonged QT interval, as in the case of overdose with citalopram. Norfluoxetine has a half-life of about 17 days, while its parent compound fluoxetine has a half-life of about 7 days. Together, the two compounds can precipitate serotonin syndrome several weeks after the last dose, especially if an irreversible MAOI is subsequently prescribed.
Drug screening should be undertaken and early contact with toxicology services considered. Benzodiazepines may help with muscle rigidity/hyperactivity, myoclonus, seizures and agitation. Lorazepam or oxazepam have been suggested as the most appropriate benzodiazepine, the rationale being their shorter duration of action and lack of active metabolites (Reference Ahuja and ColeAhuja 2009). Diazepam use has also been described (Reference Buckley, Dawson and IsbisterBuckley 2014). Prudence is required in the use of benzodiazepines in elderly patients, who may develop delirium or hypotension, especially where there is impaired liver or kidney function.
Common antipyretic drugs are not indicated in serotonin syndrome. Excess muscle activity contributes to the hyperthermia, so muscle paralysis may be required to curb this in extreme cases (Reference Boyer and ShannonBoyer 2005; Reference Volpie-Abadie, Kaye and KayeVolpie-Abadie 2013). Avoid the use of restraint, as active resistance can contribute to metabolic acidosis and lead to further rise in body temperature. Pyrexia of 38.5°C or higher, and/or markedly increased truncal rigidity may herald imminent respiratory distress: in such cases senior medical advice should be sought immediately, as rapid deterioration is likely to ensue (Reference Dunkley, Isbister and SibbrittDunkley 2003).
Further interventions include the antihistamine cyproheptadine (an antagonist at both 5-HT1A and 5-HT2A receptors) and chlorpromazine, a 5-HT2A antagonist which may require prior fluid loading to prevent hypotension (Reference Buckley, Dawson and IsbisterBuckley 2014). Although both these drugs have recognised serotonin receptor antagonist properties, their use has been described on a theoretical basis only and they remain unlicensed for this purpose. Owing to the common symptom profile shared by serotonin syndrome and neuroleptic malignant syndrome, it may be prudent to confine the use of chlorpromazine to severe cases treated by experienced prescribers of the drug. There is a lack of supporting evidence for the use of propranolol or haloperidol in serotonin syndrome.
Highly toxic states, where the body temperature rises to over 40°C, are associated with a risk of multi -organ failure and death; active cooling is imperative (Reference Buckley, Dawson and IsbisterBuckley 2014). It has been suggested that lowering of body temperature reduces functionality of the 5-HT2A receptors (Reference Krishnamoorthy, Ma and ZhangKrishnamoorthy 2010), which may minimise triggering of neurotoxic cascades (Reference Fugate, White and RabinsteinFugate 2014). Rapid cooling may be achieved by covering the body in ice packs in conjunction with the use of cooled fluids for bladder and/or peritoneal lavage and for intravenous administration. Disseminated intravascular coagulation, kidney failure, acidosis and acute respiratory distress syndrome are also possible secondary complications of severe serotonin syndrome and intensive supportive care is likely to be necessary.
Further management
A detailed review of the patient’s prescribed drugs should be undertaken 1–2 weeks after the serotonin syndrome has resolved. Alternatives should be considered where possible. Some of the causative medications may be individually retitrated, beginning at lower doses. Most SSRIs have a half-life of 12–36 h and require a washout period of 7–14 days before restarting. Where an MAOI was involved in the cause of serotonin syndrome, serotonergic agents should not be restarted for at least 14 days. Fluoxetine has active metabolites with a 5–7 day half-life, thereby requiring a 5-week washout period; serotonergic interactions may thus occur several weeks after its discontinuation. Patients should be monitored for increased anxiety, agitation, tremor, pulse, blood pressure, temperature, headache and reflexes. Care should be exercised; recurrence of serotonin syndrome following exposure to a different serotonergic drug has been described in the presence of pre-existing liver disease (Reference Tomaselli and ModestinTomaselli 2004).
Prognosis
The prognosis is good, especially where there has been early recognition, immediate discontinuation of the causal medication and rapid establishment of appropriate supportive measures (Reference Sun-Edelstein, Tepper and ShapiroSun-Edelstein 2008). A degree of confusion may persist for a few days, and sometimes muscle pain for longer. Reference ChechaniChechani (2002) reported four severe episodes of serotonin syndrome in the same woman; with two different SSRIS, she continued to experience muscle aches and tremors 2 months after resolution of the syndrome. This may be partly due to the necessity of abrupt cessation of potent CYP2D6 inhibitors such as paroxetine, or occasionally to SSRI-induced extrapyramidal side-effects of medications with long half-lives, such as fluoxetine, along with their active metabolites (Reference LaneLane 1998).
Prevention
We have an aging population often treated with complex regimens of multiple drugs. This poses particular challenges if several medical conditions co-occur. Reference Lingam and ScottLingam & Scott (2002) state that 10– 60% of patients do not take their antidepressant medication as prescribed; introduction of the SSRIs appears to have had little impact on this. Patterns of adherence to prescribed antidepressants vary from taking excessive amounts sporadically, to periodic use, to frequent stop–starts, to abrupt cessation; around a third of patients who discontinue their medication may not reveal this to their doctor. There is thus not only the risk of toxicity but of experiencing discontinuation symptoms, which include sleep disturbance, agitation, anxiety and depression (Reference Demyttenaere and HaddadDemyttenaere 2000). Patient- and/or illness-related factors may contribute to this; sensitively exploring patients’ attitudes, associated health beliefs and treatment expectations may be helpful in assessing the degree of medication adherence. Routine enquiry about various foods, including caffeine consumption, and the use of recreational drugs may assist in the face of apparent treatment non-response before prescribing second- or even third-line medication. The importance of advising patients against mixing other substances with prescribed drugs should not be underestimated. Medication reconciliation and communication with other people who may be prescribing for patients are key.
MCQs
Select the single best option for each question stem
-
1 How many families of serotonin receptor subtype have been identified?
-
a 3
-
b 9
-
c 2
-
d 7
-
e 5.
-
-
2 Which body organ contains the highest density of the cytochrome P450 enzymes?
-
a lungs
-
b spleen
-
c liver
-
d kidneys
-
e brain.
-
-
3 Caffeine is both metabolised by and an inhibitor of which CYP enzyme?
-
a CYP2D6
-
b CYP1A2
-
c CYP2C19
-
d CYP2C9
-
e CYP3A4.
-
-
4 Which of the following was noted in severely toxic presentations of serotonin syndrome and added to the Hunter criteria?
-
a tremor
-
b diaphoresis
-
c hyperreflexia
-
d rigidity
-
e agitation.
-
-
5 Which treatment option has not been found to be useful in the clinical management of serotonin syndrome?
-
a antipyretic medication
-
b discontinuation of serotonergic medication
-
c chlorpromazine
-
d benzodiazepines
-
e cyproheptadine.
-
MCQ answers
| 1 | d | 2 | c | 3 | b | 4 | d | 5 | a |
Acknowledgement
I wish to thank Dr Mark Hugson, consultant psychiatrist (retired), and Mr Paul Davies, prescribing management pharmacist with Mental Health Services – NHS Greater Glasgow and Clyde, for their encouraging scrutiny of the draft manuscript.






eLetters
No eLetters have been published for this article.