An overview of how psychological treatments might best be deployed in early schizophrenia can be framed in the context of what is known about prognostic factors in first-episode schizophrenia. A range of these has been established from follow-up studies, although most factors, such as family history of psychosis, are not changeable. Table 1 lists those replicated predictors of outcome for which an intervention is at least plausible. Discussion of interventions will be considered alongside these known outcome predictors.
Table 1 Early schizophrenia: established predictors of outcome and psychological interventions
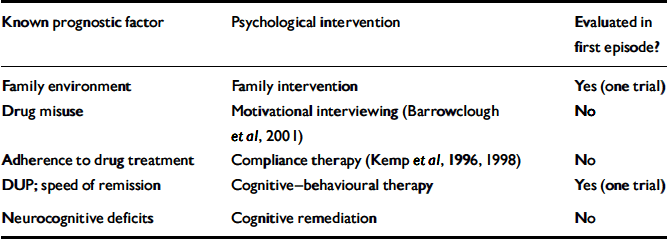
| Known prognostic factor | Psychological intervention | Evaluated in first episode? |
|---|---|---|
| Family environment | Family intervention | Yes (one trial) |
| Drug misuse | Motivational interviewing (Reference Barrowclough, Haddock and TarrierBarrowclough et al, 2001) | No |
| Adherence to drug treatment | Compliance therapy (Kemp et al, Reference Kemp, Hayward and Applethwaite1996, Reference Kemp, Kirov and Everitt1998) | No |
| DUP; speed of remission | Cognitive—behavioural therapy | Yes (one trial) |
| Neurocognitive deficits | Cognitive remediation | No |
In general, individual psychological treatments have been evaluated in established schizophrenia, where they have been delivered alongside routine care, in particular, drug treatment. There are particular issues in early schizophrenia, some of which parallel drug treatment issues. Are potential longer-term gains greater in first-episode schizophrenia possibly preventing the emergence of more chronic functional deficits? Might they be effective in preventing or postponing first relapse? Are they only effective during the treatment period or do they confer lasting benefit: how long should they be continued? How is it best to integrate drug and psychological treatments? Can psychological treatments ever be an alternative to drug treatments, rather than an adjunct?
FAMILY ENVIRONMENT AND FAMILY INTERVENTIONS
One well-replicated predictor of outcome is family environment. Early studies showed that patients persistently exposed to families who speak about them in emotional and critical terms have higher relapse rates (Vaughan & Leff, 1976). A recent meta-analysis of 27 studies supports this (Reference Butzlaff and HooleyButzlaff & Hooley, 1998). This ‘expressed emotion’ (in particular criticism) is a core target for these interventions. The most effective family interventions have a cognitive–behavioural basis and common features. One is education about the illness, symptoms and their likely effects, such as avolition and consequent inactivity. Another is promotion of a problem-solving approach. Problems are identified, their causes and consequences clarified and families are encouraged to develop strategies to combat them. A third feature of effective interventions is that they address the problems families may have in coming to terms with the guilt and anger they may experience at the patient's diagnosis and illness, and the challenge to their expectations for the patient. A fourth feature is that they include the patient and address the family atmosphere itself, by identifying sources of stress within the family and identifying ways of alleviating them. Assessing systematically the unmet needs of family members can be helpful (Reference Barrowclough, Tarrier and LewisBarrowclough et al, 1999; Reference Sellwood, Barrowclough and TarrierSellwood et al, 2001). Finally, it is important to include the patient sufficiently often and have enough sessions. Most interventions of this type have monthly or twice monthly sessions for 6–9 months. Groups of families have been treated successfully and cost-effectively (Reference McFarlane, Lukens and LinkMcFarlane et al, 1995).
There is now considerable evidence of efficacy for family intervention, including a Cochrane meta-analytical review (Reference Pharoah, Mari and StreinerPharoah et al, 2003) and other systematic meta-analyses meta-analyses (e.g. Pitschel-Walz et al, 2001). The main measure of outcome used in family intervention studies has been reduction in relapse rates. The overall effective size in the Cochrane review of randomised, controlled trials (0.2) was smaller than that in interventions of the type described above but it included less effective designs in the analysis. Studies sharing the features described tend to reduce relapse rate by up to 40% compared with controls over follow-up periods of 9–18 months, representing an effect size of about 0.4. Those who benefit show improved adherence to medication and their families have reduction in expressed emotion. The benefits in some studies (Reference Tarrier, Sharpe and BeckettTarrier et al, 1993) have continued up to 8 years, but diminished over time.
Family interventions in the first episode have not been widely evaluated, although there is good reason to expect that they should be effective (Reference GoldsteinGoldstein, 1996). The most informative trial was that of Lenior et al (Reference Lenior, Dingemans and Linszen2001) who randomised 64 patients with early schizophrenia, 55% in their first episode, to receive family intervention or routine care alone. Relapse rates were reduced during the actual 12-month experimental treatment period, but did not differ significantly between the two groups at 5-year follow-up, although the total time spent in in-patient care was reduced in the family intervention group.
SUBSTANCE MISUSE AND MOTIVATIONAL INTERVENTIONS
It is clear that drug misuse increases relapse risk in schizophrenia and may even constitute a risk factor for the disorder itself (Arsenault et al, 2002; Reference Zammit, Allebeck and AndreassonZammit et al, 2002). Cannabis and amphetamine-like drugs appear particularly toxic in this regard. In the first-episode follow-up study of Linszen et al (Reference Linszen, Dingemans and Lenior1994), those people who used significant amounts of cannabis showed a twofold increase in relapse rates over the next year, compared with those people who used small amounts or none. People with schizophrenia who misuse substances (‘dual diagnosis’) represent a special challenge since services are poorly configured to deal with both problems and the evidence base for drug and psychological interventions is sparse. There is anecdotal evidence that clozapine can reduce drug misuse in schizophrenia (Reference Drake, Haley and AkhtarDrake et al, 2000,Reference Drake, Xie and Mc HugoDrake et al, 2000), perhaps by the same mechanism that it appears to reduce other areas of impulsive behaviour in schizophrenia, such as violence and deliberate self-harm (Reference Buckley, Noffsinger and SmithBuckley et al, 2003).
The only therapeutic trial of an effective patient-level intervention is that of Barrowclough et al (Reference Barrowclough, Haddock and Tarrier2001). Here, patients (not first-episode) with dual diagnosis were randomised to routine care or to a psychological treatment package of motivational interviewing directed at reducing the substance misuse, cognitive therapy aimed at psychotic symptom control and family intervention. Motivational interviewing has been used to treat uncomplicated substance dependence. Patients are encouraged to explore the problems their substance misuse causes and the ways in which it prevents them achieving their goals. They are also encouraged to explore how they could address these problems, including reduction in substance misuse, strategies for relapse prevention and possibly engagement with services and use of drugs to reduce craving or block the effects of illicit drugs. The results of the trial showed at 12 months a significant increase in global functioning, and a halving in relapse rates from 56% to 28% in the experimental group (Reference Haddock, Barrowclough and TarrierHaddock et al, 2003).
Drake & Mueser (Reference Drake, Mueser, Lieberman and Murray2001) have argued that the most successful programmes share an integrated approach, so that substance misuse interventions, case management, assessment, family education, medication management and social and rehabilitation aspects are related, and all have features that reflect awareness of the special needs of this group. They also involve active monitoring, outreach and gradual engagement.
DRUG TREATMENT NON-ADHERENCE AND COMPLIANCE THERAPY
Motivational interviewing aimed at reducing misuse is a good example of a psychological treatment whose effect is mediated by explicitly reducing a known risk factor. Similar techniques have been used in attempts to improve outcomes by enhancing a known protective factor, antipsychotic drug treatment. People in the first episode of schizophrenia respond well to low doses of medication, but are sensitive to the adverse effects (Reference Remington, Kapur and ZipurskyRemington et al, 1998), which contribute to high rates of non-adherence to maintenance drug treatment and consequent poor outcome (Reference Verdoux, Lengronne and LiraudVerdoux et al, 2000).
Kemp et al (Reference Kemp, Kirov and Everitt1998) used a form of therapy derived from motivational interviewing to enhance adherence to medication. In a clinical trial, patients admitted for acute relapse (not in the first episode) were randomised to receive routine care or routine care plus a brief package of motivational interviewing, adapted for those with schizophrenia and concentrating on ways to treat symptoms, reduce problems and prevent relapse using antipsychotic medication. The experimental group showed clinically and statistically significant reduction in readmission rates and improvements in compliance over 18 months, and improvements in symptoms at the end of therapy but not after 18 months.
NEUROCOGNITIVE DEFICITS AND COGNITIVE REMEDIATION
There are well-replicated neurocognitive deficits in schizophrenia. Most evidence supports the existence of deficits in working memory and executive function and these deficits strongly predict outcome (Reference GreenGreen, 1996). Still at issue is the natural history of these deficits. Individuals at high genetic risk for schizophrenia show deficits in motor coordination, attention and executive function, in particular (e.g. Reference Hans, Marcus and NuechterleinHans et al, 1999; Reference Byrne, Hodges and GrantByrne et al, 2000; Reference Erlenmeyer-Kimling, Rock and RobertsErlenmeyer-Kimling et al, 2000). There is some evidence that at the onset of positive symptoms such individuals also show decrements in neurocognitive function, in dorsolateral prefrontal cortical tasks, in particular (Reference Bilder, Lipschutz and ReiterBilder et al, 1992; Reference Cosway, Byrne and ClaffertyCosway et al, 2000). There is mixed evidence about the effect of long periods without treatment, some of which shows greater deterioration in frontal tests (Reference Scully, Coakley and KinsellaScully et al, 1997; Reference Amminger, Edwards and BrewerAmminger et al, 2002; Reference Joyce, Hutton and MutsatsaJoyce et al, 2002), although other authors disagree (Reference Ho, Alicata and WardHo et al, 2003). It is clear that during and after initial presentation with schizophrenia there is a generalised neurocognitive deficit with particular impairment in planning, executive functions and memory (Reference Hutton, Puri and DuncanHutton et al, 1998; Reference Mohamed, Paulsen and O'LearyMohamed et al, 1999; Reference Bilder, Goldman and RobinsonBilder et al, 2000; Mojtabi et al, 2000; Reference Riley, McGovern and MocklerRiley et al, 2000). There is evidence that planning deficits subsequently improve (Reference Joyce, Hutton and MutsatsaJoyce et al, 2002), whereas deficits in attentional set-shifting and paired visual associate tasks appear to deteriorate (Pantelis et al, 2001; Reference Joyce, Hutton and MutsatsaJoyce et al, 2002). A 10-year follow-up study of a consecutively ascertained ascertained cohort of 110 patients with first-episode first-episode psychosis has shown that executive function tends to improve but progressive visuospatial deficits emerge; the scale of both these changes predicts long-term clinical outcome (Reference Stirling, White and LewisStirling et al, 2003). Other follow-up studies over the short to medium term in early schizophrenia have shown little or no change in cognitive function (Reference RundRund, 1998).
Cognitive remediation centres on direct remediation of the cognitive deficits that are presumed to lead to symptoms and difficulty in social function (Reference Brenner, Roder and HodelBrenner et al, 1994). For some years this approach has demonstrated limited success, with a failure of any benefits in cognitive function to feed through into improved social function, but recently more refined models of cognitive deficits and improvements in tailoring training to those with schizophrenia have suggested greater benefits may be realised. In a small sample, Wykes et al (Reference Wykes, Reeder and Corner1999, Reference Wykes, Reeder and Williams2003) used techniques, such as errorless learning (in which tasks are taught taking care to avoid the subject being confused by making mistakes), scaffolding (where strategies are demonstrated to subjects initially but gradually support is reduced) and massed practice (repeated exercises at least 3–5 times per week) to produce persistent gains in executive function, memory and self-esteem.
Patterns of cognitive deficit relate to a greater or lesser extent to symptomatology and an extension of cognitive remediation to early schizophrenia may be the targeting of individual deficits that relate to core, emerging symptoms. Poor insight is one important clinical example, where there is mounting evidence of an association with a specific executive neurocognitive task – set-shifting. Drake & Lewis (Reference Drake and Lewis2003) found set-shifting errors involving perseveration to be strongly linked to the core component of poor insight, the inability to relabel symptoms, in a sample with predominantly first-episode psychosis, leading to the proposal that defective self-monitoring contributes both to perseveration and poor insight. Koren and colleagues (Reference Viksman, Poyurovsky and BalushViksman et al, 2002) similarly confirmed that good insight in the first episode was linked to the ability to act appropriately on the basis of self-monitoring. Correction of this deficit might improve insight.
EARLY INTERVENTION AND COGNITIVE – BEHAVIOURAL THERAPY
The relationship between duration of untreated psychosis and clinical outcome has been confirmed by a systematic review (Reference Norman, Lewis and MarshallNorman et al, 2005, this issue). The relationship is probably causal, at least in part (Reference Harrigan, McGorry and KrstevHarrigan et al, 2003), although alternative mechanisms may contribute, such as pre-existing poor premorbid adjustment or families with high-expressed emotion, when detecting problems earlier. The shape of the dose–response curve is nonlinear (Reference Drake, Haley and AkhtarDrake et al, 2000,Reference Drake, Xie and Mc HugoDrake et al, 2000; Reference Harrigan, McGorry and KrstevHarrigan et al, 2003), suggesting that the most gains in outcome will be realised by reducing the duration of untreated psychosis further in cases where it is already fairly brief. Service-level interventions are those that will allow early detection. The individual components of the intervention should probably include cognitive–behavioural therapy (CBT).
Individual CBT has generated a sizeable body of evidence, although the first trials in this area were undertaken only 10 years ago. The accepted findings are that CBT, if delivered over a period of at least 6 months, will reduce positive and to some extent negative symptoms in otherwise treatment-resistant schizophrenia. In these trials, as with all others so far, CBT has been delivered as an adjunct to drug treatment as usual. A convergence between independent randomised controlled trials in this patient group is striking. Four good quality trials (Tarrier et al, Reference Tarrier, Sharpe and Beckett1993, Reference Tarrier, Yusopoff and Kinney1998; Reference Kuipers, Garety and FowlerKuipers et al, 1997; Reference Sensky, Turkington and KingdonSensky et al, 2000) have used similar inclusion criteria with similar experimental treatments in terms of content and duration. The trials have differed in other respects, particularly the selection and rationale of control interventions and the use or otherwise of blinded assessments of outcome. The effect size for improvement of positive symptoms in these trials is about 0.6. The effect of the intervention extends to improvement in negative symptoms and, in some circumstances, social functioning. In addition, the effect appears to be durable at 6–9 months post-treatment and beyond. The patient population identified for these trials is closely similar to that used in the earlier clozapine efficacy studies and the effect size, according to systematic review, is not dissimilar (Reference Wahlbeck, Tuunainen and GilbodyWahlbeck et al, 2000). An important statistical issue here is that the population and the samples for these trials are selected on the basis of having persistent and stable positive symptoms, so maximising the power of a trial to test the efficacy of an add-on treatment. This statistical advantage may not be present when other patient populations are targeted, such as those with first-episode psychosis or acutely ill patients, or those in remission open to relapse.
The effectiveness of CBT in addition to routine care in first-episode schizophrenia has been evaluated in the SoCRATES trial (Reference Lewis, Tarrier and HaddockLewis et al, 2002; Reference Tarrier, Lewis and HaddockTarrier et al, 2004). Taking as its starting point the demonstrated effectiveness of CBT in schizophrenia patients with persistent, treatment-resistant symptoms, the hypotheses of this trial were that CBT, in addition to routine care (drugs), would accelerate resolution of acute symptoms in first-episode schizophrenia, improve 18-month outcomes and delay future relapse. Consecutive first- or second-episode acute inpatient or day patient admissions with DSM–IV (American Psychiatric Assocation, 1994) schizophrenia-spectrum psychoses were randomised into the trial from 11 centres. Consenting subjects were randomised within 14 days to one of three treatment arms. The experimental treatment was a 5-week package of CBT, plus three boosters over 3 months, in addition to routine care. A second psychological treatment arm aimed to control for non-specific therapist effects and involved supportive counselling over a similar period plus routine care. The third arm was routine care alone. Outcome assessments were made blind to treatment group and were performed weekly over the first 6 weeks, then at 9 and 18 months.
Interventions were commenced within 3 days of randomisation and in the case of the CBT and supportive counselling were manual-based and supervised. In addition, psychological treatment sessions were audiotaped and rated masked to evaluate and confirm treatment fidelity. The randomised sample for analysis was 309 patients: 101 patients received CBT, 106 received supportive counselling and 102 received routine care alone. The median age of the sample was 27.4 years, 70% were male and 83% were in their first admission. Mean total score on the Positive and Negative Syndrome Scale (PANSS; Reference Kay, Fiszbein and OplerKay et al, 1987) at baseline was 87, confirming that this was a severely ill sample. Blind assessments over the first 6 weeks showed a trend towards more rapid resolution of acute symptoms in the CBT groups.
Post hoc analyses confirmed that the CBT group was significantly more improved than the routine care group at 4 weeks on PANSS positive symptom and delusion scale scores, but that this effect had disappeared by 6 weeks (Reference Lewis, Tarrier and HaddockLewis et al, 2002). Follow-up at 18 months showed that the group who had received CBT in the first 5 weeks had a significantly lower PANSS total score (P=0.03; effect size 0.44) and lower PANSS positive score (P=0.01; effect size 0.43) than the routine care group, after adjusting for baseline score, time to assessment, clinical centre, sex, in-patient v. day patient status, first-v. second-episode psychosis and duration of untreated psychosis at baseline. On the primary outcome measures at 18 months, the supportive counselling group showed symptom scores intermediate between the CBT and routine care groups (Reference Tarrier, Lewis and HaddockTarrier et al, 2004). Analysis of relapse and readmission rates showed that the experimental treatment had no affect on this measure.
The overall conclusions from this trial were that a brief package of CBT in acute early schizophrenia accelerated improvement in target symptoms but that these gains were lost by 6 weeks, perhaps due to the powerful main effect of routine care, i.e. drug treatment. The intervention also led to improved symptomatic outcomes at 18 months compared with routine care alone. However, these effects were small, although measurable and durable, and there is no effect on time to relapse.
THE INTERFACE BETWEEN DRUG AND PSYCHOLOGICAL TREATMENTS
Methodological issues
The results of these trials have been taken up enthusiastically by a clinical community looking for alternative strategies to drug treatments, on the basis of their intellectual appeal. It is not surprising that people with, or caring for those with, severe psychological symptoms should expect treatments based on primary psychological approaches to be available. However, it is important to recognise methodological limitations in this area, particularly those that might in some circumstances lead to a type 1 statistical error. In broad terms, these limitations can be divided into general design issues for randomised controlled trials and those involving theoretical issues about the content of the treatment itself. These areas overlap.
The generally desirable features of a clinical trial are shown in the Appendix. In general, it can be argued that the ground rules for establishing the effectiveness of psychological treatment should be no different from those used to establish the effectiveness of pharmacological therapy. However, there are a number of challenges in evaluating psychological treatments that are not present in drug trials. Cognitive–behavioural therapy, unlike drug treatment, involves modifying behavioural contingencies and the cognitive architecture.
Looking at specific issues, the use of a double-blind design, as is the benchmark approach in phase III clinical trials of a drug treatment, is not possible with psychological treatments. This makes it more, rather than less, important that when a masked parameter is possible, it is used. There have been debates about whether or not it is possible to maintain masking to treatment allocation when assessing outcome. Because of the known potency of the use of open assessments in introducing bias, it is vital to attempt to use independent, masked assessments of outcome, with assessment of the quality of the masking if possible. In addition, the choice of outcomes should include those which are relatively impervious to the effects of masking, such as relapse, hospitalisation and instrumental outcomes, such as employment status.
The choice of control group in these studies is also important. The choice should be based on the hypothesis of the study and take into account that, in general, psychological treatments are used in addition to treatment as usual, rather than an alternative. The hypothesis in this area is usually that CBT has a specific effect over and above a supportive counselling approach. Ideally, this means the use of two control groups, one controlling for non-specific effects of talking treatments (Reference Lewis, Tarrier and HaddockLewis et al, 2002).
There has been a trend of late to focus on pragmatic trials in healthcare evaluations generally. These are trials which tend to be large with simple outcomes that solely address the question of effectiveness. It can be argued that in an area such as psychological treatments of psychosis it is vital to derive treatments from a sound theoretical base and build into the trial design an explanatory component to test whether this hypothesised mechanism is actually that which mediates any effect. One example of an alternative explanation would be that the clinical effect of a psychological treatment is actually mediated inadvertently through another therapeutic mechanism, such as improved adherence to drug treatments. Another important issue specific to this area is replicability. With CBT, this typically involves a range of psychological techniques focusing on different aspects of psychopathology. The emphasis, particularly in Europe, is for the approach to be individually tailored according to individual clinical priorities and case formulation (Reference Tarrier and CalamTarrier & Calam, 2002). In North America, the approach tends to be more a standardised, less flexible, manualised approach. Issues of replicability are important, given this situation. At the very least, the semi-objective demonstration of treatment fidelity, i.e. that the treatment given adheres to a written procedural protocol, is measured and reported.
The prediction of response to the most suitable interventions is one obvious area of further study. Further development of therapies like individual CBT and family intervention in the light of this, and investigation of the processes of therapy are others. Development of cognitive remediation and integration into other methods of social rehabilitation offers some promise. Investigation of compliance therapy and other interventions delivered by other less highly trained staff is important and potentially problematic because of the complex skills needed. Turkington et al (Reference Turkington, Kingdon and Turner2002) found a CBT programme delivered by trained community nurses to patients and families was effective in a randomised controlled trial. Development of model programmes integrating different approaches is also a current area of research, although the weakness of many of these studies is that it remains unclear which elements of complex programmes, sometimes given to heterogeneous samples, are effective.
Results of trials of psychodynamic therapy have been discouraging (Reference Mueser and BerenbaumMueser & Berenbaum, 1990) but a form of psychotherapy designed to address interpersonal and social difficulties without overstimulating patients had mixed success (Hogarty et al, Reference Hogarty, Greenwald and Ulrich1997a ,Reference Hogarty, Kornblith and Greenwald b ). Relapse rates were reduced for patients living with families, but increased for the remainder. There was some evidence of improved social function over 3 years (although this was not rated with masking) and almost none of symptomatic benefit.
Do drug and psychological treatments enhance each other?
It has been suggested that the effect of family intervention is independent of medication (Reference Kuipers, Bebbington and PillingKuipers et al, 1999), but it may be that optimal antipsychotic treatment can benefit non-drug therapy. Examples exist in the literature of how psychological treatments can enhance the effect of drug treatments, or vice versa. A good example of how a psychological treatment can enhance the effect of a drug treatment in schizophrenia is the compliance therapy trial of Kemp et al (Reference Kemp, Kirov and Everitt1998). The observed reduction in relapse rates in the experimental treatment group was not due to any direct effect of psychological treatment but rather an improved efficiency of the pharmacological treatment. Conversely, a good example of how an antipsychotic drug treatment can enhance the effect of a psychosocial treatment can be found in the double-blind randomised controlled comparison between clozapine and haloperidol in treatment-resistant patients (Reference Rosenheck, Tekell and PetersRosenheck et al, 1998). This trial confirmed the effectiveness of clozapine compared with haloperidol using a design where the identity of the drugs was double-blind and patients allocated to haloperidol treatment received blood tests to mimic the monitoring system for clozapine. The clinical trial was run in the context of a mental health service setting where a range of psychosocial treatments were on offer of varying degrees of complexity. A post hoc analysis (Reference Rosenheck, Tekell and PetersRosenheck et al, 1998) showed that not only were the clozapine-treated patients more likely to show an improvement in symptoms, but over the first 12 months of the trial they were progressively more likely to be able to take up psychosocial treatments of greater complexity. This represents an example of an effective drug treatment allowing individuals to use psychological treatments more efficiently. There may be different expectations after termination of treatment. Relapse after discontinuing drug treatment implies efficacy of the drug and the need for continual treatment, whereas release after stopping a psychological treatment implies a failure to maintain treatment gains.
Can psychological treatments work in the absence of drug treatments?
Psychological interventions have always been investigated as adjuncts to antipsychotic medication. The evidence for the efficacy of drug treatment is so well established that it may be ethically dubious to attempt to test empirically whether psychological treatments on their own are effective. One new area may throw light on this. A small number of studies have examined the possibility of detecting individuals in the prodromal stage, prior to the development of full psychosis. Yung et al (Reference Yung, Phillips and McGorry1998) have developed operational criteria to identify four subgroups at ultra-high risk of incipient psychosis. The improved ability to define high risk accurately has led to the possibility of intervention to prevent psychosis in this group.
Three clinical trials have reported interim or final results. McGorry et al (Reference McGorry, Yung and Phillips2002) in Melbourne found that specific pharmacotherapy (low-dose risperidone) plus CBT, in comparison with supportive therapy and case management, reduced the risk of early transition to psychosis in an open, randomised trial of 59 young people at ultra-high risk. There was reduction in progression to psychosis at end of the 6-month treatment, but not at follow-up after a further 6-month period of no treatment. However, the relative contribution of psychotherapy could not be determined since theirs was a combined treatment. Woods et al (Reference Woods, Breier and Zipursky2003) at Yale compared olanzapine with placebo double-blind in 60 individuals at ultra-high risk. Interim data at 1 year showed that olanzapine was more effective than placebo in reducing the prodromal symptoms themselves, with a trend towards reduction in transition to psychosis. The Morrison et al (Reference Morrison, Bentall and French2002) trial in Manchester, UK is an open randomised trial of CBT over 6 months v. monitoring in 58 individuals from the same group at ultra-high risk. Participants were assessed for suitability and monitored on a monthly basis using the PANSS, which was also used to determine transition. Full details regarding entry criteria, study design and treatment protocol have been reported (Reference Morrison, Bentall and FrenchMorrison et al, 2002). An interim analysis of the rate of transition to psychosis suggested an effect of CBT in reducing transmission and reducing severity of subclinical symptoms (Reference Morrison, Bentall and FrenchMorrison et al, 2002). This will be, to our knowledge, the first study to attempt to evaluate whether CBT alone is effective in any stage of the psychotic disorder (in this case, preventing the progression of subclinical symptoms in the absence of drug treatment). Combining data from these trials will provide information about the relative acceptability, safety and efficacy of drug and non-drug treatments in this emerging area.
CONCLUSIONS
A range of psychological interventions now exists for established psychotic disorders and there is a rationale for their use during, following and even prior to the first episode, although formal evaluations are needed. User and carer preferences for available treatments is an issue that deserves further study, and the mental health needs of carers is a legitimate area of study in its own right. Combining interventions, such as CBT and family interventions, is an approach that needs to be evaluated. At least 80% of people in their first episode will achieve good remission relatively quickly, but at least 80% will relapse by 5 years. Preventing or ameliorating first relapse will be of crucial importance. Rates of non-adherence to drug treatment are high in this group and the possible role of intermittent, targeted drug treatment (Reference Gaebel, Janner and FrommannGaebel et al, 2002), in tandem with psychological strategies aimed at relapse prevention (Reference Gumley, O'Grady and McNayGumley et al, 2003), needs to be assessed. Where early intervention services are to be set up, it is important to have clear evidence about the effectiveness of treatment for individuals in contact with the service.
APPENDIX
Design characteristics of a high-quality psychological treatment trial
-
(a) Large, representative sample from a clinically relevant, specified population
-
(b) Sample size justified by power calculation
-
(c) Independent, concealed randomisation
-
(d) Well-specified intervention with fidelity independently assessed
-
(e) Outcome assessed blind to treatment allocation
-
(f) Reliable and valid primary outcome measure
-
(g) Intent-to-treat analysis with characterisation of those lost to follow-up
Clinical Implications and Limitations
CLINICAL IMPLICATIONS
-
▪ Psychological treatments in the prodromal phase of schizophrenia are probably important and often modify risk factors for poor outcome.
-
▪ Preventing or ameliorating first-episode psychosis will be a key area for future evaluation.
-
▪ Integrating research and interventions across the interface between drug and psychological treatments is potentially fruitful.
LIMITATIONS
-
▪ Many potentially useful psychological treatments have only been evaluated in established, rather than early, schizophrenia.
-
▪ This article focuses on patient-level, rather than service-level, interventions.
-
▪ First-episode service users’ and caregivers’ preferences for type of care have not been well assessed.






eLetters
No eLetters have been published for this article.