Abstract
Sirolimus (rapamycin, RAPAMUNE, RAPA) is an immunosuppressive agent used for the prophylaxis of renal allograft rejection and exhibits an immunosuppressive mechanism that is distinct from that for cyclosporine and tacrolimus. The purpose of this manuscript is to discuss the exposure-response relationships and drug interactiosn of sirolimus. The various factors affecting sirolimus whole blood exposure included first-pass extraction, formulation, food, demographics, liver disease, assay method, and interacting drugs. Clinically significant effects caused by food, pediatric age, hepatic impairment, and interacting drugs require recommendations for the safe and efficacious use of sirolimus in renal allograft patients. An exposure-response model based on multivariate logistic regression was developed using the interstudy data from 1832 renal allograft patients. The analysis revealed an increased probability of acute rejection for sirolimus troughs <5 ng/mL, cyclosporine troughs <150 ng/mL, human leukocyte antigen (HLA) mismatches ≥4, and females. The outcomes suggested that individualization of sirolimus doses immediately after transplantation, based on HLA mismatch and sex, would likely decrease the probability of acute rejections in renal allograft recipients who receive concomitant sirolimus, cyclosporine (full-dose), and corticosteroid therapy. Sirolimus is a substrate for both Cytochrome P450 3A (CYP3A) and P-glycoprotein (P-gp) and undergoes extensive first-pass extraction. Drugs that are known to inhibit or induce these proteins may potentially affect sirolimus whole blood exposure. In healthy volunteers, cyclosporine, diltiazem, erythromycin, ketoconazole, and verapamil significantly increased sirolimus whole blood exposure, and rifampin significantly decreased sirolimus exposure. However, sirolimus whole blood exposure was not affected by acyclovir, atorvastatin, digoxin, ethinyl estradiol/norgestrel, glyburide, nifedipine, or tacrolimus. Among the 15 drugs studied, sirolimus significantly increased the exposures of only erythromycin and S-(−)verapamil.
Similar content being viewed by others
References
Physicians' Desk Reference, 57th ed. Montvale, NJ: Medical Economics Company; 2004.
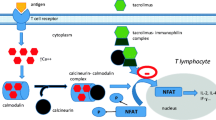
Sehgal SN. Rapamune (sirolimus, rapamycin): an overview and mechanism of action.Ther Drug Monit. 1995;17:660–665.
United States Pharmacopeial Convention.USP Dictionary of USAN and International Drug Names. Rockville, MD:United States Pharmacopeial Convention.
Liu J, Farmer JD Jr, Lane WS, Friedman J, Weissman I, Schreiber SL. Calcineurin is a common target of cyclophilin-cyclosporin A and FKBP-FK506 complexes.Cell. 1991;66:807–815.
Diasio RB, LoBuglio AF. Immunomodulators: immunosuppressive agents and immunostimulants. In: Hardman JG, Limbird LE, eds.Goodman and Gilman's The Pharmacological Basis of Therapeutics. 9th ed, New York, NY: McGraw-Hill, 1995:1291–1308.
Sabers CJ, Martin MM, Brunn GJ, et al. Isolation of a protein target of the FKBP12-rapamycin complex in mammalian cells.J Biol Chem. 1995;270:815–822.
Choi J, Chen J, Schreiber SL, Clardy J. Structure of the FKBP12-rapamycin complex interacting with the binding domain of human FRAP.Science. 1996;273:239–242.
Terada N, Patel HR, Takase K, Kohno K, Nairn AC, Gelfand EW. Rapamycin selectively inhibits translation of mRNAs encoding elongation factors and ribosomal proteins.Proc Natl Acad Sci USA. 1994;91:11477–11481.
Feuerstein N, Huang D, Prystowsky MB. Rapamycin selectively blocks interleukin-2 induced proliferating cell nuclear antigen gene expression in T lymphocyte.J Biol Chem. 1995;270:9454–9458.
Nourse J, Firpo E, Flanagan WM, et al. Interleukin-2-mediated elimination of the p27Kipl cyclin-dependent kinase inhibitor prevented by rapamycin.Nature. 1994;372:570–573.
Jefferies HBJ, Reinhard C, Kozma SC, Thomas G. Rapamycin selectively represses translation of the “polypyrimidine tract” mRNA family.Proc Natl Acad Sci USA. 1994;91:4441–4445.
Zheng J, Sambol NC, Zimmerman J, Zaidi A. Population pharmacokinetics (PK) of sirolimus [abstract].Clin Pharmacol Ther. 1996;59:150.
Van Buren CT. Sirolimus oral solution and tablets demonstrate equivalent safety and efficacy in renal allograft patients.Transplantation. 2000;69(suppl):S153-S154.
Zimmerman JJ, Ferron GM, Lim HK, Parker V. The effect of a highfat meal on the oral bioavailability of the immunosuppressant sirolimus (rapamycin).J Clin Pharmacol. 1999;39:1155–1161.
Pentikis H, Knebel W, Reis P, Zimmerman JJ. The effect of ethnicity on the pharmacokinetics of sirolimus: a population pharmacokinetic analysis of phase 1 data [abstract].AAPS Pharm Sci. 2003;5(S4):Abstract 2353.
Tejani A, Alexander S, Ettenger R, et al. Safety and pharmacokinetics of ascending single doses of sirolimus (Rapamune£, rapamycin) in pediatric patients with stable chronic renal failure undergoing dialysis.Pediatr Transplant. In Press.
Shaw LM, Kaplan B, Brayman KL. Introduction and overview (Advances in therapeutic drug monitoring for immunosuppressants: a review of sirolimus).Clin Ther. 2000;22(suppl B):B1-B13.
Jones K, Saadat-Lajevardi S, Lee T, et al. An immunoassay for the measurement of sirolimus.Clin Ther. 2000;22(suppl B):B49-B61.
Zimmerman JJ, Harper D, Getsy J, Jusko WJ. Pharmacokinetic interactions between sirolimus and microemulsion cyclosporine when orally administered jointly and 4 hours apart.J Clin Pharmacol. 2003;43:1168–1176.
SAS Institute Inc.SAS/STAT, User's Guide, Version 8. Cary, NC: SAS Institute Inc, 2000.
Box GEP, Tidwell PW. Transformation of the independent variables.Technometrics. 1962;4:531–550.
Sattler M, Guengerich FP, Yun CH, et al. Cytochrome P-450 3A enzymes are responsible for biotransformation of FK506 and rapamycin in man and rat.Drug Metab Disp. 1992;20:753–761.
Lampen A, Zhang Y, Hackbarth I, et al. Metabolism and transport of the macrolide immunosuppressant sirolimus in the small intestine.J Pharmacol Exp Ther. 1998;285:1104–1112.
Leung LY, Zimmerman J, Lim HK, et al. Metabolic disposition of14C-Rapamucin (sirolimus) in healthy male subjects after a single oral dose [abstract].ISSX Proc. 1997;12:26.
Crowe A, Lemaire M. In vitro and in situ absorption of SDZ-RAD using a human intestinal cell line (Caco-2) and a single pass perfusion model in rats: comparison with rapamycin.Pharm Res. 1998;15:1666–1672.
Thiebaut F, Tsuruo T, Hamada H, Gottesman MM, Pastan I, Willingham MC. Cellular localization of the multidrug-resistance gene product P-glycoprotein in normal human tissues.Proc Natl Acad Sci USA. 1987;84:7735–7738.
Wacher VJ, Salphati L, Benet LZ. Active secretion and enterocytic drug metabolism barriers to drug absorption.Adv Drug Deliv Rev. 1996;20:99–112.
Zhang Y, Benet LZ. The gut as a barrier to drug absorption: combined role of cytochrome P450 3A and P-glycoprotein.Clin Pharmacokinet. 2001;40:159–168.
Bottiger Y, Sawe J, Brattstrom C, et al. Pharmacokinetic interaction between single oral doses of diltiazem and sirolimus in healthy volunteers.Clin Pharmacol Ther. 2001;69:32–40.
Floren LC, Christians U, Zimmerman JJ, et al. Sirolimus oral bioavailability increases eleven-fold with concomitant ketoconazole [abstract].Clin Pharmacol Ther. 1999;65:159.
Patat A, Zimmerman JJ, Parks V, Richards J. Pharmacokinetic (PK) interaction study of sirolimus (SRL) and cyclosporine (CsA) [abstract].Clin Pharmacol Ther. 2004;75:33.
Zimmerman JJ, Harper D, Speth JT, Fruncillo RJ, Getsy J, eds. Potential pharmacokinetic interactions between sirolimus and atorvastatin [abstract].ESOT. 2001;(Oct 6–11):145.
Zimmerman JJ, Patat A, Souan J-L, Paty I, Cadieu G. Potential pharmacokinetic interactions between sirolimus and tacrolimus [abstract].Am J Transplant. 2001;1(suppl 1):384.
Pichard L, Gillet G, Fabre I, et al. Identification of the rabbit and human cytochromes P-450IIIA as the major enzymes involved in the N-demethylation of diltiazem.Drug Metab Dispos. 1990;18:711–719.
Pichard L, Fabre I, Fabre G, et al. Screening for inducers and inhibitors of cytochrome P-450 (cyclosporin A Oxidase) in primary cultures of human hepatocytes and liver microsomes.Drug Metab Dispos. 1990;18:595–606.
Saeki T, Ueda K, Tanigawara Y, Hori R, Komano T. P-glycoprotein-mediated transcellular transport of MDR-reversing agents.Fed Europ Biochem Soc. 1993;324:99–102.
Emi Y, Tsunashima D, Ogawara K-I, Higaki K, Kimura T. Role of P-glycoprotein as a secretory mechanism in quinidine absorption from rat small intestine.J Pharm Sci. 1998;87:295–299.
Kroemer HK, Gautier J-C, Beaune P, Henderson C, Wolf CR, Eichelbaum M. Identification of P450 enzymes involved in metabolism of verapamil in humans.Arch Pharmacol. 1993;348:332–337.
Renton KW. Inhibition of hepatic microsomal drug metabolism by the calcium channel blockers diltiazem and verapamil.Biochem Pharmacol. 1985;34:2549–2533.
Yusa K, Tsurua T. Reversal mechanism of multidrug resistance by verapamil: direct binding of verapamil to P-glycoprotein on specific sites and transport of verapamil outward across the plasma membrane of K562/ADM cells.Cancer Res. 1989;49:5002–5006.
Hollt V, Kouba M, Dietel M, Vogt G. Stereoisomers of calcium antagonists which differ markedly in their potencies as calcium blockers are equally effective in modulating drug transport by P-glycoprotein.Biochem Pharmacol. 1992;43:2601–2608.
Kolars JC, Awni W, Merion RM, Watkins PB. First-pass metabolism of cyclosporin by the gut.Lancet. 1991;338:1488–1490.
Wandel C, Kim RB, Kajiji S, Guengerich FP, Wilkinson GR, Wood AJJ. P-glycoprotein and cytochrome P-450 3A inhibition: dissociation of inhibitory potencies.Cancer Res. 1999:3944–3948.
Saeki T, Ueda K, Tanigawara Y, Hori R, Komano T. Human P-glycoprotein transports cyclosporin A and FK506.J Biol Chem. 1993;268:6077–6080.
Hunt CM, Watkins PB, Saenger P, et al. Heterogeneity of CYP3A isoforms metabolizing erythromycin and cortisol.Clin Pharmacol Ther. 1992;51:18–23.
Larrey D, Funck-Brentano C, Breil P, et al. Effects of erythromycin on hepatic drug-metabolizing enzymes in humans.Biochem Pharmacol. 1983;32:1063–1068.
Kim RB, Wandel C, Leake B, et al. Interrelationship between substrates and inhibitors of human CYP3A and P-glycoprotein.Pharm Res. 1999;16:408–413.
Hofsli E, Nissen-Meyer J. Reversal of drug resistance by erythromycin: erythromycin increases the accumulation of actinomycin D and doxorubicin in multidrug-resistant cells.Int J Cancer. 1989;44:149–154.
Baldwin SJ, Bloomer JC, Smith GJ, Ayrton AD, Clarke SE, Chenery RJ. Ketoconazole and sulphaphenazole as the respective selective inhibitors of P4503A and 2C9.Xenobiotica. 1995;25:261–270.
Siegsmund MJ, Cardarelli C, Aksentiwevich I, Sugimoto Y, Pastan I, Gottesman MM. Ketoconazole effectively reverses multidrug resistance in highly resistant KB cells.J Urol. 1994;151:485–491.
Kolars JC, Schmiedlin-Ren P, Schuetz JD, Fang C, Watkins PB. Identification of rifampin-inducible P450IIIA4 (CYP3A4) in human small bowel enterocytes.J Clin Invest. 1992;90:1871–1878.
Westphal K, Weinbrenner A, Zschiesche M, et al. Induction of P-glycoprotein by rifampin increases intestinal secretion of talinolol in human beings: a new type of drug/drug interaction.Clin Pharmacol Ther. 2000;68:345–355.
Jacobsen W, Kuhn B, Soldner A, et al. Lactonization is the critical first step in the disposition of the 3-hydroxy-3-methylglutaryl-CoA reductase inhibitor atorvastatin.Drug Metab Dispos. 2000;28:1369–1378.
Boyd RA, Stern RH, Stewart BH, et al. Atorvastatin coadministration may increase digoxin concentrations by inhibition of intestinal P-glycoprotein-mediated secretion.J Clin Pharmacol. 2000;40:91–98.
De Lannoy IA, Silverman M. The MDR1 gene product, p-glycoprotein, mediates the transport of the cardiac glycoside, digoxin.Biochem Biophys Res Commun. 1992;189:51–557.
Brian WR, Sari M-A, Iwasaki M, Shimada T, Kaminsky LS, Guengerich FP. Catalytic activities of human liver cytochrome P-450 IIIA4 expressed in saccharomyces cerevisiae.Biochemistry. 1990;29:11280–11292.
Guengerich FP, Brian WR, Iwasaki M, Sari M-A, Baarnhielm C, Berntsson P. Oxidation of dihydropyridine calcium channel blockers and analogues by human liver cytochrome P-450 IIIA4.J Med Chem. 1991;34:1838–1844.
Arceci RJ, Stieglitz K, Bierer BE. Immunosuppressants FK506 and rapamycin function as reversal agents of the multidrug resistance phenotype.Blood. 1992;80:1528–1536.
Author information
Authors and Affiliations
Corresponding author
Additional information
Published: October 15, 2004.
Rights and permissions
About this article
Cite this article
Zimmerman, J.J. Exposure-response relationships and drug interactions of sirolimus. AAPS J 6, 28 (2004). https://doi.org/10.1208/aapsj060428
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/aapsj060428