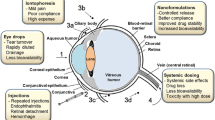
Abstract
Despite its apparent easy accessibility, the eye is, in fact, well protected against the absorption of foreign materials, including therapeutic agents, by the eyelids, by the tearflow, and by the permeability barriers imposed by the cornea on one side and the blood-retinal barrier on the other. Most existing ophthalmic drugs were adapted from other therapeutic applications and were not specifically developed for the treatment of eye diseases; hence, they are not well suited to provide eye-specific effects without causing systemic side effects. A real breakthrough in the area of ophthalmic therapeutics can be achieved only by specifically designing new drugs for ophthalmic applications to incorporate the possibility of eye targeting into their chemical structure. Possibilities provided along these lines by designing chemical delivery systems (CDSs) and soft drugs within the framework of retrometabolic drug design are reviewed here. Both are general concept applicable in almost any therapeutic area. This review will concentrate on \-adrenergic agonists and anti-inflammatory corticosteroids, where clinical results obtained with new chemical entities, such as betaxoxime, adaprolol, loteprednol etabonate, and etiprednol dicloacetate, exist to support the advantages of such metabolism-focused, ophthalmic-specific drug design approaches.
Similar content being viewed by others
References
Stewart PA, Tuor UI. Blood-eye barriers in the rat: correlation of ultrastructure with function.J Comp Neurol. 1994;340:566–576.
Schoenwald RD. Ocular drug delivery: pharmacokinetic considerations.Clin Pharmacokinet. 1990;18:255–269.
Mitra AK, Mikkelson TJ. Mechanism of transcorneal permeation of pilocarpine.J Pharm Sci. 1988;77:771–775.
Davies NM. Biopharmaceutical considerations in topical ocular drug delivery.Clin Exp Pharmacol Physiol. 2000;27:558–562.
Grass GM, Robinson JR. Mechanism of corneal penetration. I. In vivo and in vitro kinetics.J Pharm Sci. 1988;77:3–14.
Prausnitz MR, Noonan JS. Permeability of cornea, sclera, and conjuctiva: a literature analysis for drug delivery to the eye.J Pharm Sci. 1998;87:1479–1488.
Edwards A, Prausnitz MR. Predicted permeability of the cornea to topical drugs.Pharm Res. 2001;18:1497–1508.
Lee VH, Robinson JR. Topical ocular drug delivery: recent developments and future challenges.J Ocul Pharmacol. 1986;2:67–108.
Sasaki H, Yamamura K, Mukai T, et al. Enhancement of ocular drug penetration.Crit Rev Ther Drug Carrier Syst. 1999;16:85–146.
Bundgaard H, ed.Design of Prodrugs. Amsterdam, The Netherlands: Elsevier Science; 1985.
Bodor N, Kaminski JJ. Prodrugs and site-specific chemical delivery systems.Annu Rep Med Chem. 1987;22:303–313.
Wermuth CG, Gaignault J-C, Marchandeau C. Designing prodrugs and bioprecursors I: Carrier prodrugs. In: Wermuth CG, ed.The Practice of Medicinal Chemistry. London, UK: Academic Press; 1996:671–696.
Ettmayer P, Amidon GL, Clement B, Testa B. Lessons learned from marketed and investigational prodrugs.J Med Chem. 2004;47:2393–2404.
Bodor N. Drug targeting and retrom etabolic drug design approaches.Adv Drug Deliv Rev. 1994;14:157–166.
Bodor N. Buchwald P. Drug targeting via retrometabolic approaches.Pharmacol Ther. 1997;76:1–27.
Bodor N, Buchwald P. Retrometabolism-based drug design and targeting. In: Abraham DJ, ed.Drug Discovery and Drug Development. Burger’s Medicinal Chemistry and Drug Discovery. Vol 2. 6th ed. New York, NY: John Wiley and Sons; 2003:533–608.
Bodor N, El-Koussi A, Kano M, Nakamuro T. Improved delivery through biological membranes. 26. Design, synthesis, and pharmacological activity of a novel chemical delivery system for β-adrenergic blocking agents.J Med Chem. 1988;31:100–106.
El-Koussi A, Bodor N. Formation of propanolol in the iris-ciliary body from its propranolol ketoxime precursor—a potential antiglaucoma drug.Int J Pharm. 1989;53:189–194.
Bodor N, Prokai L. Site- and stereospecific ocular drug delivery by sequential enzymatic bioactivation.Pharm Res. 1990;7:723–725.
Bodor N, El-Koussi A. Improved delivery through biological membranes. LVI. Pharmacological evaluation of alprenoxime—a new potential antiglaucoma agent.Pharm Res. 1991;8:1389–1395.
Simay A, Prokai L, Bodor N. Oxidation of aryloxy-β-amino alcohols with activated dimethylsulfoxide: a novel C-N oxidation facilitated by neighboring group effect.Tetrahedron. 1989;45:4091–4102.
Simay A, Bodor N. Site- and stereospecific drug delivery to the eye. In: Sarel S, Mechoulam R, Agranat I, eds.Trends in Medicinal Chemistry ’90. Oxford, UK: Blackwell Scientific Publications; 1992:361–368.
Bodor N. Retrometabolic drug design concepts in ophthalmic targetspecific drug delivery.Adv Drug Deliv Rev. 1995;16:21–38.
Polgar P, Bodor N. Minimal cardiac electrophysiological activity of alprenoxime, a site-activated ocular β-blocker, in dogs.Life Sci. 1995;56:1207–1213.
Prokai L, Wu W-M, Somogyi G, Bodor N. Ocular delivery of the β-adrenergic antagonist alprenolol by sequential bioactivation of its methoxime analog.J Med Chem. 1995;38:2018–2020.
Bodor N, Farag HH, Somogyi G, Wu W-M, Barros MDC, Prokai L. Ocular-specific delivery of timolol by sequential bioactivation of its oxime and methoxime analogs.J Ocul Pharmacol. 1997;13:389–403.
Farag HH, Wu W-M, Barros MDC, Somogyi G, Prokai L, Bodor N. Ocular-specific chemical delivery system of betaxolol for safe local treatment of glaucoma.Drug Des Discov. 1997;15:117–130.
Nathanson JA. Stereospecificity of beta adrenergic antagonists: R-enantiomers show increased selectivity for beta-2 receptors in ciliary process.J Pharmacol Exp Ther. 1988;245:94–101.
Mehvar R, Brocks DR. Stereospecific pharmacokinetics and pharmacodynamics of beta-adrenergic blockers in humans.J Pharm Pharm Sci. 2001;4:185–200.
Sharif NA, Xu SX, Crider JY, McLaughlin M, Davis TL. Levobetaxolol (Betaxon) and other beta-adrenergic antagonists: preclinical pharmacology, IOP-lowering activity and sites of action in human eyes.J Ocul Pharmacol Ther. 2001;17:305–317.
Nandel FS, Dhaliwal RK, Singh B. Modeling, design, chiral aspects and role of para-substituents in aryloxypropranolamine based beta-blockers.Indian J Biochem Biophys. 1999;36:29–35.
Quigley H. How common is glaucoma worldwide?Int Glaucoma Rev [serial online]. 2002; Available at: http://www.glaucom.com/Mettings/3-3/worldwide.php. Accessed August 16, 2005.
Moroi SE, Lichter PR. Ocular pharmacology. In: Hardman JG, Limbird LE, eds.Goodman & Gilman’s The Pharmacological Basis of Therapeutics. New York, NY: McGraw-Hill; 1996:1619–1645.
Alward WL. Biomedicine: a new angle on ocular development.Science. 2003;299:1527–1528.
Radius RL, Diamond GR, Pollack IP, Langham ME. Timolol: a new drug for management of chronic simple glaucoma.Arch Ophthalmol. 1978;96:1003–1008.
Sugrue MF. New approaches to antiglaucoma therapy.J Med Chem. 1997;40:2793–2809.
Stamper RL, Wigginton SA, Higginbotham EJ. Primary drug treatment for glaucoma: beta-blockers versus other medications.Surv Ophthalmol. 2002;47:63–73.
Taniguchi T, Kitazawa Y. The potential systemic effect of topically applied beta-blockers in glaucoma therapy.Curr Opin Ophthalmol. 1997;8:55–58.
Hayreh SS, Podhajsky P, Zimmerman MB. Beta-blocker eyedrops and nocturnal arterial hypotension.Am J Ophthalmol. 1999;128:301–309.
Nelson WL, Fraunfelder FT, Sills JM, Arrowsmith JB, Kuritsky JN. Adverse respiratory and cardiovascular events attributed to timolol ophthalmic solution, 1978–1985.Am J Ophthalmol. 1986;102:606–611.
Lynch MG, Whitson JT, Brown RH, Nguyen H, Drake MM. Topical β-blocker therapy and central nervous system side effects: a preliminary study comparing betaxolol and timolol.Arch Ophthalmol. 1988;106:908–911.
Fraunfelder FT, Meyer SM. Sexual dysfunction secondary to topical ophthalmic timolol.JAMA. 1985;253:3092–3093.
Sorensen SJ, Abel SR. Comparison of the ocular beta-blockers.Ann Pharmacother. 1996;30:43–54.
Schoene RB, Martin TR, Charan NB, French CL. Timolol-induced bronchospasm in asthmatic bronchitis.JAMA. 1981;245:1460–1461.
Van Buskirk EM, Weinreb RN, Berry DP, Lustgarten JS, Podos SM, Drake MM. Betaxolol in patients with glaucoma and asthma.Am J Ophthalmol. 1986;101:531–534.
Harris LS, Greenstein SH, Bloom AF. Respiratory difficulties with betaxolol.Am J Ophthalmol. 1986;102:274–275.
Pfitzner KE, Moffat JG, Sulfoxide-carbodiimide reactions. I. A facile oxidation of alcohols.J Am Chem Soc. 1965;87:5661–5670.
Bodor N. The soft drug approach.Chemtech. 1984;14:28–38.
Bodor N, Buchwald P. Soft drug design: general principles and recent applications.Med Res Rev. 2000;20:58–101.
Bodor N. Designing safer ophthalmic drugs. In:Trends in Medicinal Chemistry ’88: Proceedings of the Xth International Symposium on Medicinal Chemistry. Amsterdam, The Netherlands: Elsevier; 1989:145–164.
Bodor N. The use of retrometabolic drug design concepts in ophthalmic drug discovery. In: Reddy IK, ed.Ocular Therapeutics and Drug Delivery: A Multidisciplinary Approach. Lancaster, PA: Technomic; 1996:335–361.
Albert A.Selective Toxicity: The Physico-Chemical Basis of Therapy. London, UK: Chapman and Hall; 1985.
Gillette JR. Effects of induction of cytochrome P-450 enzymes on the concentration of foreign compounds and their metabolites and on the toxicological effects of these compounds.Drug Metab Rev. 1979;10:59–87.
Mannering GJ. Hepatic cytochrome P-450-linked drug-metabolizing systems. In: Testa B, Jenner P, eds.Concepts in Drug Metabolism. Part B. New York, NY: Marcel Dekker Inc; 1981:53–166.
Borg KO, Carlsson E, Hoffmann K-J, Jönsson K-J, Thorin H, Wallin B. Metabolism of metoprolol-(3H) in man, the dog and the rat.Acta Pharmacol Toxicol (Copenh). 1975;36:125–135.
Regardh CG, Johnsson G. Clinical pharmacokinetics of metoprolol.Clin Pharmacokinet. 1980;5:557–569.
Benfield P, Clissold SP, Brogden RN. Metoprolol: an updated review of its pharmacodynamic and pharmacokinetic properties, and therapeutic efficacy, in hypertension, ischaemic heart disease and related cardiovascular disorders.Durgs. 1986;31:376–429.
Bodor N, Oshiro Y, Loftsson T, Katovich M, Caldwell W. Soft drugs. 6. The application of the inactive metabolite approach for design of soft β-blockers.Pharm Res. 1984;1:120–125.
Bodor N, El-Koussi A, Kano M, Khalifa MM. Soft drugs. 7. β-Blockers for systemic and ophthalmic use.J Med Chem. 1988;31:1651–1656.
Bodor N, El-Koussi A. Novel ‘soft’ β-blockers as potential safe antiglaucoma agents.Curr Eye Res. 1988;7:369–374.
Polgar P, Bodor N. Cardiac electrophysiologic effects of adaprolol maleate, a new β-blocker, in closed chest dogs.Life Sci. 1991;48:1519–1528.
Bodor N, El-Koussi A, Zuobi K, Kovacs P. Synthesis and pharmacological activity of adaprolol enantiomers: a new soft drug for treating glaucoma.J Ocul Pharmacol Ther. 1996;12:115–122.
McGhee CN, Dean S, Danesh-Meyer H. Locally administered ocular corticosteroids: benefits and risks.Drug Saf. 2002;25:33–55.
Buchman AL. Side effects of corticosteroid therapy.J Clin Gastroenterol. 2001;33:289–294.
Raizman M. Corticosteroid therapy of eye disease: fifty years later.Arch Ophthalmol. 1996;114:1000–1001.
Dickerson JE Jr, Dotzel E, Clark AF. Steroid-induced cataract: new perspectives from in vitro and lens culture studies.Exp Eye Res. 1997;65:507–516.
Heyns K, Koch W. Über die bildung eines aminozuckers ausd-fruktose und ammoniak.Z Naturforsch [B]. 1952;7B:486–488.
Bucala R, Fishman J, Cerami A. Formation of covalent adducts between cortisol and 16α-hydroxyestrone and protein: possible role in the pathogenesis of cortisol toxicity and systemic lupus erythematosus.Proc Natl Acad Sci USA. 1982;79:3320–3324.
Manabe S, Bucala R, Cerami A. Nonenzymatic addition of glucocorticoids to lens proteins in steroid-induced cataracts.J Clin Invest. 1984;74:1803–1810.
Bucala R, Gallati M, Manabe S, Cotlier E, Cerami A. Glucocorticoid-lens protein adducts in experimentally induced steroid cataracts.Exp Eye Res. 1985;40:853–863.
Urban RC, Jr, Cotlier E. Corticosteroid-induced cataracts.Surv Ophthalmol. 1986;31:102–110.
Noble S, Goa KL. Loteprednol etabonate: clinical potential in the management of ocular inflammation.Bio Drugs. 1998;10:329–339.
Howes JF. Loteprednol etabonate: a review of ophthalmic clinical studies.Pharmazie. 2000;55:178–183.
Bodor N, Buchwald P. Design and development of a soft corticosteroid, loteprednol etabonate. In: Schleimer RP, O’Byrne PM, Szefler SJ, Brattsand R, eds.Inhaled Steroids in Asthma. Optimizing Effects in the Airways. New York, NY: Marcel Dekker, 2002:541–564.
Bodor N, inventor.Stéroïds doux exerçant une activité antiinflammatoire (Steroids having antiinflammatory activity). Belgian patent BE889,563 (Internat Classif C07J/A61K). November 3, 1981.
Bodor N, Varga M. Effect of a novel soft steroid on the wound healing of rabbit cornea.Exp Eye Res. 1990;50:183–187.
Druzgala P, Hochhaus G, Bodor N. Soft drugs. 10. Blanching activity and receptor binding affinity of a new type of glucocorticoid: loteprednol etabonate.J Steroid Biochem. 1991;38:149–154.
Bodor N, Loftsson T, Wu W-M. Metabolism, distribution, and transdermal permeability of a soft corticosteroid, loteprednol etabonate.Pharm Res. 1992;9:1275–1278.
Hochhaus G, Chen L-S, Ratka A, et al. Pharmacokinetic characterization and tissue distribution of the new glucocorticoid soft drug loteprednol etabonate in rats and dogs.J Pharm Sci. 1992;81:1210–1215.
Bodor N, Murakami T, Wu W-M. Soft drugs. 18. Oral and rectal delivery of loteprednol etabonate, a novel soft corticosteroid, in rats—for safer treatment of gastrointestinal inflammation.Pharm Res. 1995;12:869–874.
Bodor N, Wu W-M, Murakami T, Engel S. Soft drugs. 19. Pharmacokinetics, metabolism and excretion of a novel soft corticosteroid, loteprednol etabonate, in rats.Pharm Res. 1995;12:875–879.
Monder C, Bradlow HL. Cortoic acids: explorations at the frontier of corticosteroid metabolism.Recent Prog Horm Res. 1980;36:345–400.
Bodor N. Novel approaches for the design of membrane transport properties of drugs. In: Roche EB, ed.Design of Biopharmaceutical Properties Through Prodrugs and Analogs. Washington, DC: Academy of Pharmaceutical Sciences; 1977:98–135.
Bodor N. Designing safer drugs based on the soft drug approach.Trends Pharmacol Sci. 1982;3:53–56.
Bodor N. Soft drugs: strategies for design of safer drugs.Metabolisme et Conception Medicaments: Quo Vadis? Proceedings of Symposium at Montpellier, France; November 26–27, 1981; Montpellier, France, CLIN MIDY; 1983. 217–251.
Druzgala P, Bodor N. Regioselective O-alkylation of cortienic acid and synthesis of a new class of glucocorticoids containing a 17α-alkoxy, a 17α-(1’-alkoxyethyloxy), a 17α-alkoxymethyloxy, or a 17α-methylthiomethyloxy function.Steroids. 1991;56:490–494.
Bodor N. The application of soft drug approaches to the design of safer corticosteroids. In: Christophers E, Kligman AM, Schöpf E, Stoughton RB, eds.Topical Corticosteroid Therapy: A Novel Approach to Safer Drugs. New York, NY: Raven Press Ltd; 1988:13–25.
Buchwald P, Bodor N. Soft glucocorticoid design: structural elements and physicochemical parameters determining receptor-binding affinity.Pharmazie. 2004;59:396–404.
Buchwald P. General linearized biexponential model for QSAR data showing bilinear-type distribution.J Pharm Sci. 2005;94:2355–2379.
Druzgala P, Wu W-M, Bodor N. Ocular absorption and distribution of loteprednol etabonate, a soft steroid, in rabbit eyes.Curr Eye Res. 1991;10:933–937.
Bodor N, Bodor N, Wu W-M. A comparison of intraocular pressure elevating activity of loteprednol etabonate and dexamethasone in rabbits.Curr Eye Res. 1992;11:525–530.
Novack GD, Howes J, Crockett RS, Sherwood MB. Change in intraocular pressure during long-term use of loteprednol etabonate.J Glaucoma. 1998;7:266–269.
Howes J, Novack GD. Failure to detect systemic levels and effects of loteprednol etabonate and its metabolite, PJ-91, following chronic ocular administration.J Ocul Pharmacol Ther. 1998;14:153–158.
Lotemax (Loteprednol Etabonate Ophthalmic Suspension 0.5%) [product monograph]. Rochester, NY: Bausch & Lomb Pharmaceuticals. 1998.
Ilyas H, Slonim CB, Braswell GR, Favetta JR, Schulman M. Longterm safety of loteprednol etabonate 0.2% in the treatment of seasonal and perennial allergic conjunctivitis.Eye Contact Lens. 2004;30:10–13.
Szelenyi I, Hermann R, Petzold U, Pahl A, Hochhaus G. Possibilities in improvement of glucocorticoid treatments in asthma with special reference to loteprednol etabonate.Pharmazie. 2004;59:409–411.
Szelenyi I, Hochhaus G, Heer S, et al. Loteprednol etabonate: a soft steroid for the treatment of allergic diseases of the airways.Drugs Today (Barc). 2000;36:313–320.
Bodor N, inventor.Androstene derivatives. US patent 5 981 517. November 9, 1999.
Barton P, Laws AP, Page MI. Structure-activity relationships in the esterase-catalysed hydrolysis and transesterification of esters and lactones.J Chem Soc, Perkin Trans 2. 1994; 2021–2029.
Miklós A, Magyar Z, Kiss É, et al. 28-Day oral toxicity study with soft corticosteroid BNP-166 in rats and dogs, followed by a 14-day recovery period.Pharmazie. 2002;57:142–146.
Kurucz I, Tóth S, Németh K, et al. Potency and specificity of the pharmacological action of a new, antiasthmatic, topically administered soft steroid, etiprednol dicloacetate (BNP-166).J Pharmacol Exp Ther. 2003;307:83–92.
Kurucz I, Németh K, Mészáros S, et al. Anti-inflammatory effect and soft properties of etiprednol dicloacetate (BNP-166), a new, antiasthmatic steroid.Pharmazie. 2004;59:412–416.
Jaffuel D, Demoly P, Gougat C, et al. Transcriptional potencies of inhaled glucocorticoids.Am J Respir Crit Care Med. 2000;162:57–63.
Bhalay G, Sandham DA. Recent advances in corticosteroids for the treatment of asthma.Curr Opin Investig Drugs. 2002;3:1149–1156.
Author information
Authors and Affiliations
Corresponding author
Additional information
Published: December 7, 2005.
Rights and permissions
About this article
Cite this article
Bodor, N., Buchwald, P. Ophthalmic drug design based on the metabolic activity of the eye: Soft drugs and chemical delivery systems. AAPS J 7, 79 (2005). https://doi.org/10.1208/aapsj070479
Received:
Accepted:
DOI: https://doi.org/10.1208/aapsj070479