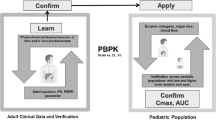
Abstract
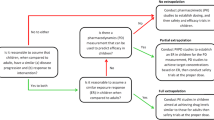
The use of physiologically based pharmacokinetic (PBPK) models in the field of pediatric drug development has garnered much interest of late due to a recent Food and Drug Administration recommendation. The purpose of this study is to illustrate the developmental processes involved in creation of a pediatric PBPK model incorporating existing adult drug data. Lorazepam, a benzodiazepine utilized in both adults and children, was used as an example. A population-PBPK model was developed in PK-Sim v4.2® and scaled to account for age-related changes in size and composition of tissue compartments, protein binding, and growth/maturation of elimination processes. Dose (milligrams per kilogram) requirements for children aged 0–18 years were calculated based on simulations that achieved targeted exposures based on adult references. Predictive accuracy of the PBPK model for producing comparable plasma concentrations among 63 pediatric subjects was assessed using average-fold error (AFE). Estimates of clearance (CL) and volume of distribution (V ss) were compared with observed values for a subset of 15 children using fold error (FE). Pediatric dose requirements in young children (1–3 years) exceeded adult levels on a linear weight-adjusted (milligrams per kilogram) basis. AFE values for model-derived concentration estimates were within 1.5- and 2-fold deviation from observed values for 73% and 92% of patients, respectively. For CL, 60% and 80% of predictions were within 1.5 and 2 FE, respectively. Comparatively, predictions of V ss were more accurate with 80% and 100% of estimates within 1.5 and 2 FE, respectively. Using the presented workflow, the developed pediatric model estimated lorazepam pharmacokinetics in children as a function of age.





Similar content being viewed by others
References
Leong R, Vieira ML, Zhao P, Mulugeta Y, Lee CS, Huang SM, et al. Regulatory experience with physiologically based pharmacokinetic modeling for pediatric drug trials. Clin Pharmacol Ther. 2012;91(5):926–31.
Bjorkman S. Prediction of drug disposition in infants and children by means of physiologically based pharmacokinetic (PBPK) modelling: theophylline and midazolam as model drugs. Br J Clin Pharmacol. 2005;59(6):691–704.
Edginton AN, Schmitt W, Willmann S. Development and evaluation of a generic physiologically based pharmacokinetic model for children. Clin Pharmacokinet. 2006;45(10):1013–34.
Johnson TN, Rostami-Hodjegan A. Resurgence in the use of physiologically based pharmacokinetic models in pediatric clinical pharmacology: parallel shift in incorporating the knowledge of biological elements and increased applicability to drug development and clinical practice. Paediatr Anaesth. 2011;21(3):291–301.
Edginton AN, Ritter L. Predicting plasma concentrations of bisphenol A in children younger than 2 years of age after typical feeding schedules, using a physiologically based toxicokinetic model. Environ Health Perspect. 2009;117(4):645–52.
Barrett JS, Della Casa AO, Laer S, Meibohm B. Physiologically based pharmacokinetic (PBPK) modeling in children. Clin Pharmacol Ther. 2012;92(1):40–9.
Lexi-Comp Online (2012) Pediatric & neonatal Lexi-drugs online. Hudson, Ohio, Lexi-Comp, Inc. 18-6-2012. Ref Type: Online Source.
Kumar P, Walker JK, Hurt KM, Bennett KM, Grosshans N, Fotis MA. Medication use in the neonatal intensive care unit: current patterns and off-label use of parenteral medications. J Pediatr. 2008;152(3):412–5.
Twite MD, Rashid A, Zuk J, Friesen RH. Sedation, analgesia, and neuromuscular blockade in the pediatric intensive care unit: survey of fellowship training programs. Pediatr Crit Care Med. 2004;5(6):521–32.
Federal Register (2003) List of drugs for which pediatric studies are needed. Federal Register 2003 Jan 9;68(13):2789–90.
Chin PK, Jensen BP, Larsen HS, Begg EJ. Adult age and ex vivo protein binding of lorazepam, oxazepam and temazepam in healthy subjects. Br J Clin Pharmacol. 2011;72(6):985–9.
Divoll M, Greenblatt DJ. Effect of age and sex on lorazepam protein binding. J Pharm Pharmacol. 1982;34(2):122–3.
Crom WR, Relling MV, Christensen ML, Rivera GK, Evans WE. Age-related differences in hepatic drug clearance in children: studies with lorazepam and antipyrine. Clin Pharmacol Ther. 1991;50(2):132–40.
Greenblatt DJ, Schillings RT, Kyriakopoulos AA, Shader RI, Sisenwine SF, Knowles JA, et al. Clinical pharmacokinetics of lorazepam. I. Absorption and disposition of oral 14C-lorazepam. Clin Pharmacol Ther. 1976;20(3):329–41.
Greenblatt DJ, Shader RI, Franke K, MacLaughlin DS, Harmatz JS, Allen MD, et al. Pharmacokinetics and bioavailability of intravenous, intramuscular, and oral lorazepam in humans. J Pharm Sci. 1979;68(1):57–63.
Verbeeck R, Tjandramaga TB, Verberckmoes R, De Schepper PJ. Biotransformation and excretion of lorazepam in patients with chronic renal failure. Br J Clin Pharmacol. 1976;3(6):1033–9.
Rodgers T, Rowland M. Physiologically based pharmacokinetic modelling 2: predicting the tissue distribution of acids, very weak bases, neutrals and zwitterions. J Pharm Sci. 2006;95(6):1238–57.
Rodgers T, Leahy D, Rowland M. Physiologically based pharmacokinetic modeling 1: predicting the tissue distribution of moderate-to-strong bases. J Pharm Sci. 2005;94(6):1259–76.
Rodgers T, Leahy D, Rowland M. Tissue distribution of basic drugs: accounting for enantiomeric, compound and regional differences amongst beta-blocking drugs in rat. J Pharm Sci. 2005;94(6):1237–48.
Greenblatt DJ, Comer WH, Elliott HW, Shader RI, Knowles JA, Ruelius HW. Clinical pharmacokinetics of lorazepam. III. Intravenous injection. Preliminary results. J Clin Pharmacol. 1977;17(8–9):490–4.
Greenblatt DJ, Divoll M, Harmatz JS, Shader RI. Pharmacokinetic comparison of sublingual lorazepam with intravenous, intramuscular, and oral lorazepam. J Pharm Sci. 1982;71(2):248–52.
Wermeling DP, Miller JL, Archer SM, Manaligod JM, Rudy AC. Bioavailability and pharmacokinetics of lorazepam after intranasal, intravenous, and intramuscular administration. J Clin Pharmacol. 2001;41(11):1225–31.
Willmann S, Hohn K, Edginton A, Sevestre M, Solodenko J, Weiss W, et al. Development of a physiology-based whole-body population model for assessing the influence of individual variability on the pharmacokinetics of drugs. J Pharmacokinet Pharmacodyn. 2007;34(3):401–31.
Edginton AN, Schmitt W, Voith B, Willmann S. A mechanistic approach for the scaling of clearance in children. Clin Pharmacokinet. 2006;45(7):683–704.
Sim SM, Back DJ, Breckenridge AM. The effect of various drugs on the glucuronidation of zidovudine (azidothymidine; AZT) by human liver microsomes. Br J Clin Pharmacol. 1991;32(1):17–21.
McNamara PJ, Alcorn J. Protein binding predictions in infants. AAPS PharmSci. 2002;4(1):E4.
Alcorn J, McNamara PJ. Ontogeny of hepatic and renal systemic clearance pathways in infants: part II. Clin Pharmacokinet. 2002;41(13):1077–94.
Irwin JJ, Kirchner JT. Anemia in children. Am Fam Physician. 2001;64(8):1379–87.
Hayton WL. Maturation and growth of renal function: dosing renally cleared drugs in children. AAPS PharmSci. 2000;2(1):E3.
Chamberlain JM, Capparelli EV, Brown KM, Vance CW, Lillis K, Mahajan P, et al. Pharmacokinetics of intravenous lorazepam in pediatric patients with and without status epilepticus. J Pediatr 2011;160:667-672; Nov 1.
Zhao P. Regulatory experience in physiologically-based pharmacokinetic (PBPK) models in pediatric submissions. FDA—Advisory Committee for Pharmaceutical Science and Clinical Pharmacology. 14-3-2012. Conference Presentation.
Edginton AN. Knowledge-driven approaches for the guidance of first-in-children dosing. Paediatr Anaesth. 2011;21(3):206–13.
Chiou WL. Potential pitfalls in the conventional pharmacokinetic studies: effects of the initial mixing of drug in blood and the pulmonary first-pass elimination. J Pharmacokinet Biopharm. 1979;7(5):527–36.
Pyka A, Babuska M, Zachariasz M. A comparison of theoretical methods of calculation of partition coefficients for selected drugs. Acta Pol Pharm. 2006;63(3):159–67.
Popovic GV, Sladic DM, Stefanovic VM, Pfendt LB. Study on protolytic equilibria of lorazepam and oxazepam by UV and NMR spectroscopy. J Pharm Biomed Anal. 2003;31(4):693–9.
Boxenbaum H. Comparative pharmacokinetics of benzodiazepines in dog and man. J Pharmacokinet Biopharm. 1982;10(4):411–26.
Acknowledgments
This work was funded by the Natural Sciences and Engineering Research Council (NSERC) of Canada.
Author information
Authors and Affiliations
Corresponding author
Additional information
Guest Editors: Bernd Meibohm, Jeffrey S. Barrett, and Gregory Knipp
Rights and permissions
About this article
Cite this article
Maharaj, A.R., Barrett, J.S. & Edginton, A.N. A Workflow Example of PBPK Modeling to Support Pediatric Research and Development: Case Study with Lorazepam. AAPS J 15, 455–464 (2013). https://doi.org/10.1208/s12248-013-9451-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1208/s12248-013-9451-0