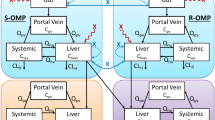
Abstract
Prasugrel HCl may convert to prasugrel base during manufacturing or storage. It was reported that formulations with different ratios of salt to base were bioequivalent in healthy subjects, but formulations with a higher extent of conversion were not bioequivalent in subjects taking proton pump inhibitor (PPI) whose stomach pH is elevated. The objective of this study was to assess the magnitude of impact of salt-to-base conversion on prasugrel HCl products BE evaluation in healthy subjects on PPI. A physiologically based pharmacokinetic (PBPK) absorption model was constructed to predict pharmacokinetic (PK) profiles of active metabolite after oral administration of prasugrel HCl products containing various fractions of base based on the prasugrel salt and base intrinsic solubility. The intrinsic solubility was obtained by deconvoluting the model against the observed active metabolite PK profiles with various fractions of base in healthy subjects with or without PPI. The developed PBPK absorption model accurately predicted the average active metabolite PK profiles in healthy subjects without PPI for the product containing 100% salt. A model based on assumptions of the fraction of a dose absorbed remaining unchanged for formulations containing different fractions of base over predicted the reduction of bioavailability upon conversion to the base. Therefore, this represented the conservative estimate with respect to the impact of free base in a product on BE evaluation. Virtual BE trial simulation predicted that less than 20% free base in prasugrel HCl product ensures in vivo BE of the generic product including in subjects that may be taking PPI.






Similar content being viewed by others
References
Wijeyeratne YD, Heptinstall S. Anti-platelet therapy: ADP receptor antagonists. Br J Clin Pharmacol. 2011;72(4):647–57.
Thomas, M.R. and R.F. Storey, Clinical significance of residual platelet reactivity in patients treated with platelet P2Y12 inhibitors. Vasc Pharmacol, 2016.
EMA Assessment Report http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Public_assessment_report/human/000984/WC500021975.pdf.
Bhatt DL, et al. ACCF/ACG/AHA 2008 expert consensus document on reducing the gastrointestinal risks of antiplatelet therapy and NSAID use: a report of the American College of Cardiology Foundation Task Force on Clinical Expert Consensus Documents. Circulation. 2008;118(18):1894–909.
Mitra A, et al. Using absorption simulation and gastric pH modulated dog model for formulation development to overcome achlorhydria effect. Mol Pharm. 2011;8(6):2216–23.
Badawy SI, et al. Formulation of solid dosage forms to overcome gastric pH interaction of the factor Xa inhibitor, BMS-561389. Pharm Res. 2006;23(5):989–96.
Farid NA, Kurihara A, Wrighton SA. Metabolism and disposition of the thienopyridine antiplatelet drugs ticlopidine, clopidogrel, and prasugrel in humans. J Clin Pharmacol. 2010;50(2):126–42.
Clinical Pharmacology Review NDA 22307, Part 32 http://www.accessdata.fda.gov/drugsatfda_docs/nda/2009/022307s000_ClinPharmR_p32.pdf.
Drugs@FDA http://www.accessdata.fda.gov/drugsatfda_docs/nda/2009/022307s000_CrossR_P12.pdf.
Patent EP2275087A1 http://www.google.com/patents/EP2275087A1?cl=en.
FDA Clinical Pharmacology Review NDA 22307, Part 12 http://www.accessdata.fda.gov/drugsatfda_docs/nda/2009/022307s000_CrossR_P12.pdf.
John CT, et al. Formulating weakly basic HCl salts: relative ability of common excipients to induce disproportionation and the unique deleterious effects of magnesium stearate. Pharm Res. 2013;30(6):1628–41.
Guerrieri P, Taylor LS. Role of salt and excipient properties on disproportionation in the solid-state. Pharm Res. 2009;26(8):2015–26.
Rehmel JL, et al. Interactions of two major metabolites of prasugrel, a thienopyridine antiplatelet agent, with the cytochromes P450. Drug Metab Dispos. 2006;34(4):600–7.
Wang J, Flanagan DR. General solution for diffusion-controlled dissolution of spherical particles. 2. Evaluation of experimental data. J Pharm Sci. 2002;91(2):534–42.
Wang J, Flanagan DR. General solution for diffusion-controlled dissolution of spherical particles. 1. Theory. J Pharm Sci. 1999;88(7):731–8.
Sugano K. Biopharmaceutics modeling and simulations, theory, practice, methods, and applications. Hoboken: John Wiley & SOns, Inc.; 2012.
Seiler D, Doser K, Salem I. Relative bioavailability of prasugrel free base in comparison to prasugrel hydrochloride in the presence and in the absence of a proton pump inhibitor. Arzneimittelforschung. 2011;61(4):247–51.
Cristofoletti R, Patel N, Dressman JB. Assessment of bioequivalence of weak base formulations under various dosing conditions using physiologically based pharmacokinetic simulations in virtual populations. Case examples: ketoconazole and posaconazole. J Pharm Sci. 2017;106(2):560–9.
FDA Clinical Pharmacology Review NDA 22307, Part 5. http://www.accessdata.fda.gov/drugsatfda_docs/nda/2009/022307s000_ClinPharmR_p5.pdf.
Mathew Leigh BK, Schaich M. Comparison of the solubility and dissolution of drugs in fasted-state biorelevant media (FaSSIF and FaSSIF-V2). Dissolution Technologies. 2013;20:7.
Gao P, Shi Y. Characterization of supersaturatable formulations for improved absorption of poorly soluble drugs. AAPS J. 2012;14(4):703–13.
FDA Clinical Pharmacology Review NDA 22307, http://www.accessdata.fda.gov/drugsatfda_docs/nda/2009/022307s000_SumR.pdf.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article reflects the views of the authors and should not be construed to represent FDA’s views or policies.
Rights and permissions
About this article
Cite this article
Fan, J., Zhang, X. & Zhao, L. Utility of Physiologically Based Pharmacokinetic Absorption Modeling to Predict the Impact of Salt-to-Base Conversion on Prasugrel HCl Product Bioequivalence in the Presence of Proton Pump Inhibitors. AAPS J 19, 1479–1486 (2017). https://doi.org/10.1208/s12248-017-0116-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1208/s12248-017-0116-2