-
PDF
- Split View
-
Views
-
Cite
Cite
Jacques Balthazart, Michelle Baillien, Gregory F. Ball, Rapid Control of Brain Aromatase Activity by Glutamatergic Inputs, Endocrinology, Volume 147, Issue 1, 1 January 2006, Pages 359–366, https://doi.org/10.1210/en.2005-0845
Close - Share Icon Share
Estrogens derived from the neural aromatization of testosterone play a key role in the activation of male sexual behavior in many vertebrates and have now been recognized to have rapid membrane effects on brain function. Such changes in aromatase activity and hence in local estrogen concentrations could rapidly modulate behavioral responses. We show here that there is a very rapid (within minutes) decrease in aromatase activity in quail hypothalamic explants exposed to treatments affecting intracellular Ca2+ concentrations, such as the addition of glutamate agonists (kainate, α-amino-3-hydroxymethyl-4-isoxazole propionic acid, and, to a much lesser extent, N-methyl-d-aspartate), but not of γ-aminobutyric acid. The kainate effects, which reduce aromatase activity by 25–50%, are observed within 5 min, are completely blocked in explants exposed to specific kainate antagonists (6-cyano-7-nitroquinoxaline-2,3-dione disodium or 1,2,3,4-tetrahydro-6-nitro-2,3-dioxo-benzo[f]quinoxaline-7-sulfonamide disodium), and are also rapidly reversible when effectors are washed out. Together, these data support the idea that the synthesis of estrogen can be rapidly regulated in the brain, thus producing rapid changes in local estrogen bioavailability that could rapidly modify brain function with a time course similar to what has previously been described for neurotransmitters and neuromodulators.
TESTOSTERONE AND OTHER androgenic steroids exert a profound effect on brain function in many vertebrate species (1, 2), but it is often underappreciated that cellular actions of testosterone often require that it first be metabolized to another steroid. For example, in several species the role played by testosterone in the regulation of male-typical sexual behaviors, aggression, and negative feedback effects on gonadotropin secretion all require that it be converted to 17β-estradiol via the P450 enzyme aromatase (estrogen synthase, EC 1.14.14.1) (3–5).
The Japanese quail provides an excellent model species to analyze the mechanisms that control brain aromatase activity (AA), because aromatase expression is high in the brain of this species, which facilitates the measurement of enzyme activity and immunocytochemical detection of the protein (6, 7). The function of aromatase, especially in relation to the control of male sexual behavior, has been well characterized (8, 9). Also, the mechanisms that control AA in quail seem generally applicable to other species, including mammals (10).
Many fundamental questions remain concerning the dynamics of estrogen synthesis and action in the brain, such as the timing and degree of its local availability and the time course and mechanism of its cellular action (11–13). Brain AA is regulated in a relatively slow fashion (hours to days) via changes in transcription of the corresponding Cyp 19 gene (14–16). Accordingly, many effects of estrogens are mediated through binding to nuclear receptors, which then act as transcription factors that will ultimately influence neurotransmission (17). However, estrogens also have rapid central effects (seconds to minutes) on physiology and behavior (12, 18–21). Understanding how and why estrogen acts rapidly in the brain is one important question facing contemporary neuroendocrinology (12, 17). One aspect of the problem that has been largely ignored concerns the possibility that brain AA and therefore estrogen synthesis are rapidly regulated. We recently reported that AA in brain homogenates is regulated within minutes by Ca2+-dependent phosphorylations of the aromatase protein (22). For example, we demonstrated that AA in brain homogenates is rapidly inhibited under conditions that favor protein phosphorylation (presence of ATP, Ca2+, and Mg2+), that this process is inhibited by kinase inhibitors (specifically inhibitors of protein kinases C and A), and that phosphorylating conditions enhance the density of phospho residues (serine, threonine, and tyrosine) on Western blots of aromatase partially purified by immunoprecipitation (22, 23). The implication of phosphorylation processes in the control of AA is also consistent with the identification of several consensus sites of phosphorylation on the deduced aromatase sequence, and comparison of the specificity of these phosphorylation sites with the effects of various specific kinase inhibitors suggests that AA is actually regulated by the phosphorylation of threonine 455 or 486 (22).
However, the physiological relevance of these findings for intact neurons is unknown. If such rapid regulation of aromatase occurs in defined neural systems in response to well-characterized neurotransmitters, this could fundamentally change our views about the dynamics of estrogen availability in the brain.
To elucidate the mechanisms rapidly regulating estrogens in intact neural systems, we investigated the effects of endogenous neurotransmitters on AA in hypothalamic explants. These studies indicate that excitatory amino acid transmitters can rapidly and reversibly down-regulate AA. A form of regulation of brain estrogen thus exhibits a time course reminiscent of what has been described for neurotransmitters or neuromodulators.
Materials and Methods
Experiments were carried out on young adult (between 8 and 16 wk old) sexually mature male Japanese quail (Coturnix japonica) that were obtained from a local breeder in Belgium and housed as previously described (24). Birds were housed, manipulated, and killed in agreement with the Belgian laws on protection and welfare of animals and the protection of experimental animals, and protocols were approved by the ethics committee for the use of animals at University of Liege.
Birds were killed by rapid decapitation, because AA is likely to be affected in a profound manner by anesthesia. The hypothalamus-preoptic area (HPOA) block was dissected by two coronal cuts at the level of the tractus septopallio-mesencephalicus (rostral edge of the POA) and the oculomotor nerves (caudal edge of hypothalamus), two parasagittal cuts placed approximately 1–1.5 mm lateral to the brain midline, and one horizontal cut about 2 mm above the floor of the brain. The width of the HPOA block was minimized to optimize the diffusion of oxygen and experimental compounds into the explant. This block, however, still contains the majority of the aromatase-expressing cells (6, 7).
This isolated block of tissue (40–60 mg) is naturally separated into two halves by the third ventricle. Each half block was immediately transferred in a test tube containing 300 μl physiological saline [25 mm glucose, 150 mm NaCl, 4.4 mm KCl, 3.1 mm CaCl2, 1.3 mm MgSO4, and 10 mm Tris-HEPES (pH 7.2)] containing 25 nm [1β-3H]androstenedione (NET-926, NEN Life Science Products, Boston MA) and oxygenated with pure O2. One hemiexplant (the left or the right, selected randomly) always served as the control, while the matched other half was submitted for a duration of 10 min to experimental treatments with neurotransmitters designed to modulate the intracellular Ca2+ concentration. The explant was then returned to control conditions to test the reversibility of effects (see Ref.23 for detail of methods).
Every 5 min, the incubation medium was aspirated with a syringe and replaced by 300 μl fresh saline containing 25 nm [1β-3H]androstenedione. When appropriate, the new medium also contained the different receptor agonists and antagonists that were tested. Because the effects of these compounds were tested for a duration of 10 min, they were thus added twice to the explants (at 20 and 25 min in the experiments; see below).
Withdrawn samples were immediately cooled in an ice bath, and 400 μl ice-cold 10% trichloroacetic acid containing 2% activated charcoal were added. They were further processed to isolate the tritiated water produced by aromatization from the remaining radioactive steroids as described previously (25).
Briefly, tubes were centrifuged at 1200 × g for 15 min. Supernatants were applied to small columns made of Pasteur pipettes plugged with glass beads and filled (3 cm high) with a Dowex cation exchange resin (AG 50W-X4, 100–200 mesh; Bio-Rad Laboratories, Richmond, CA). The columns were eluted with 3 × 0.6 ml distilled water, and effluents were collected in scintillation vials. Ten milliliters of Ecoscint A (National Diagnostics, Atlanta, GA) was added, and vials were counted for 4 min on a Packard Tri-Carb 1600 TR liquid scintillation analyzer (Downers Grove, IL).
Enzyme activity was expressed in femtomoles of tritiated water produced per 5 min in the half HPOA explant after correction of the counts for quenching, recovery, blank values (obtained by running four aliquots of radioactive incubation medium that had not been exposed to the explant through the entire purification procedure), and percentage of tritium in the β position in the substrate (see Refs.23 and 25 for technical details). In the case of the commercially available [1β-3H]androstenedione, only 76.8% of the tritium is in the 1β position, the rest being in 1α according to the manufacturer’s specification sheet. The amount of tritiated water produced during aromatization is thus underestimated and must be corrected. This is, however, a constant correction for all results that does not affect the pattern of results, only the absolute magnitude of the measured enzymatic activities. We also showed previously that the addition of a specific aromatase inhibitor (R76713 or vorozole) completely blocks tritiated water formation in brain homogenates (22, 26) and in explants incubated under the conditions used in the present experiments (27). This inhibition of tritiated water release is observed within minutes of the addition of vorozole (28). The tritiated water assay therefore provides a specific and accurate measure of aromatase activity in quail brain explants.
To allow direct comparisons between different explants with slightly different baseline levels of activity, all measures were expressed as a ratio of each specific data point to the value obtained under control conditions for the same explant when the steady-state level of AA had been reached (15- to 20-min period; see below). The average enzymatic activity observed in control explants in these steady-state conditions was equal to 108.8 ± 4.26 fmol/5 min/hemiexplant (n = 56).
Data were analyzed by one- or two-way ANOVA including repeated designs as appropriate. These ANOVA were then followed, when appropriate, by post hoc tests (Tukey highest significant tests) adapted for repeated measures using the relevant mean square of the ANOVA as a basis for comparisons. Data are expressed in the text and figures as the mean ± sem, and differences are considered significant at P ≤ 0.05.
Results
Effects of glutamate agonists on aromatase activity in HPOA explants
It was previously shown that AA in HPOA explants can be measured repeatedly over periods of 1 to several hours by quantifying the amount of tritiated water released from [1β-3H]androstenedione (23). As was the case in previous studies, when AA was measured every 5 min, it took about 20 min after the addition of the radioactive substrate for explants to reach an equilibrium between the entering tritiated androstenedione and the output of tritiated water (equilibration period; see Figs. 1 and 2). This inertia, related to the thickness of the implants, limits the temporal resolution of the measures that can be made. Diffusion also presumably limits the concentration of effectors that reach the target aromatase-containing cells located at the center of the implants (mostly the medial preoptic area) (6). High concentrations of effectors (usually above the physiological range) were therefore used in the experiments.
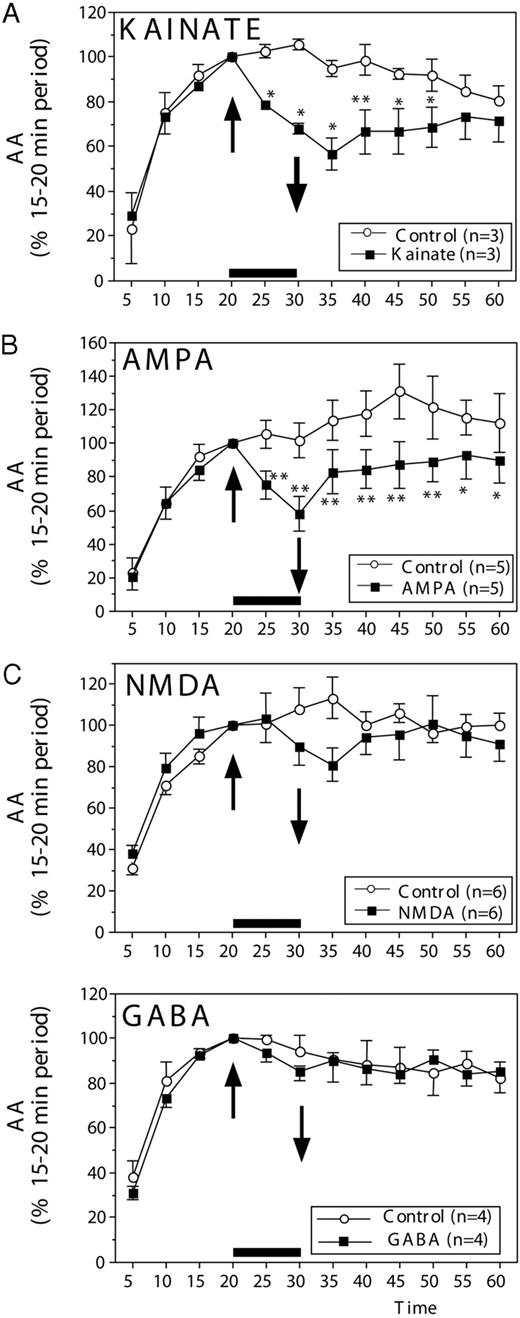
AA (mean ± sem) in paired HPOA explants incubated in vitro and exposed for 10 min (up arrow) to 100 μm kainate (A), 100 μm AMPA (B), 100 μm NMDA (C), or 100 μm GABA. In all experiments, normal saline was restored at 30 min (down arrow). All data are expressed as a percentage of the basal release, defined as the activity during the period preceding the experimental manipulation (time, 15–20 min). The number of data points available for each compound and the corresponding controls is indicated in each panel in the inset explaining the symbols used for the different lines. Data were analyzed by two-way ANOVA for repeated measures, followed, when appropriate, by Tukey highest significant difference tests, comparing control and experimental conditions at each time point. Statistically significant results are indicated by asterisks next to the corresponding data points: *, P < 0.05; **, P < 0.01 (vs. corresponding control).
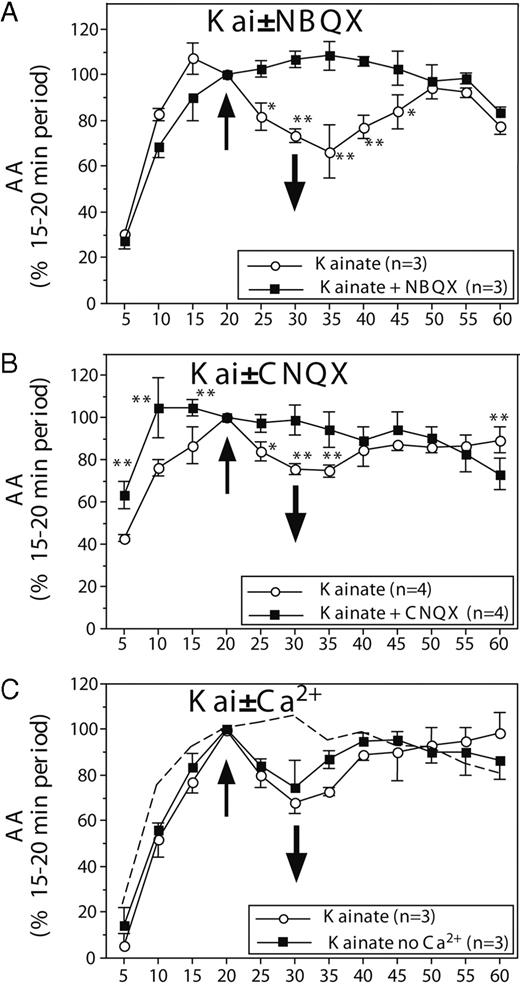
AA (mean ± sem) in paired HPOA explants incubated in vitro and exposed for 10 min (time, 20–30 min) to 100 μm kainate (A and B) with or without preincubation with the non-NMDA glutamate antagonist, NBQX (A) or CNQX (B). C, Results of experiments in which explants were exposed to 100 μm kainate from 20–30 min in the presence or absence of Ca2+ in the extracellular medium. The dotted line replicates the control condition observed in the absence of kainate in Fig. 1A (see text). The number of data points available for each condition and the corresponding controls is indicated in each panel in the inset explaining the symbols used for the different lines.
When AA measures had reached equilibrium, the addition of kainate (100 μm; Sigma-Aldrich Corp., Bornem, Belgium) or α-amino-3-hydroxymethyl-4-isoxazole propionic acid (AMPA; 100 μm; Sigma-Aldrich Corp.) rapidly (within 5 min) decreased the amount of tritiated water released from the explants. Enzymatic activity progressively recovered after washout of the agonist. Forty minutes later (time, 60 min), it had returned to the level seen in control explants (Fig. 1, A and B).
Analysis of these data (from 5–60 min) revealed significant differences between groups (control vs. experimental explants: kainate: F1,2 = 108.96; P = 0.0091; AMPA: F1,4 = 20.878; P = 0.0103), significant changes with time (kainate: F11,22 = 16.041; P < 0.0001; AMPA: F11,44 = 8.075; P < 0.0001), and, most importantly, significant interactions between these two factors (kainate: F11,22 = 3.291; P = 0.0084; AMPA: F11,44 = 3.071; P = 0.00739). To identify the origins of these interactions, separate ANOVA with two repeated factors as described above were subsequently performed on data collected before (5–15 min) and after (25–60 min) exposure of the explants to the glutamate agonists. Interactions largely resulted from differences between controls and experimental explants after, but not before, the treatment. Before treatment (5–15 min), time had a very significant effect, reflecting the establishment of the equilibrium between the explants and their medium (kainate: F2,4 = 67.362; P < 0.008; AMPA: F2,8 = 129.12; P < 0.0001), but there was no difference between groups (kainate: F2,1 = 0.001; P = 0.9739; AMPA: F2,1 = 0.894; P = 0.3979) and no interaction of time with groups (kainate: F2,4 = 0.577; P = 0.6020; AMPA: F2,8 = 1.112; P = 0.3476). In contrast, after treatment (25–60 min), overall changes in time were still observed even if they did not reach statistical significance in each case (kainate: F7,14 = 2.349; P = 0.0823; AMPA: F7,28 = 2.686; P = 0.0292), but they were associated with significant differences between groups (kainate: F1,2 = 28.121; P = 0.0338; AMPA: F1,4 = 19.572; P < 0.0115) and with a significant interaction of groups with time for kainate (F7,14 = 3.069; P = 0.0352), but not for AMPA (F7,28 = 0.654; P = 0.7080), reflecting the fact that after the initial inhibition of AA, a marked recovery was observed in explants that had been exposed to kainate, but not to the same degree as in those exposed to AMPA.
Post hoc analyses comparing control and experimental values at each time point confirmed that exposure to kainate significantly inhibited AA within 5 min (time, 25 min). The effect reached its maximum after 15 min (time, 35 min), and a progressive recovery was then observed so that no significant difference between control explants and explants exposed to kainate was present at the end of the experiment (time, 55 or 60 min). A similar rapid effect was observed in explants exposed to AMPA, and some recovery was observed, but a significant effect was still present at the end of the experiment even if its magnitude had decreased (see asterisks in Fig. 1, A and B).
Superficially similar effects were observed after the addition of N-methyl-d-aspartate (NMDA; 100 μm, Sigma-Aldrich Corp.), but the magnitude of the decrease in AA was much lower (∼20% vs. 30–45%), and the effect took longer to become apparent (Fig. 1C). Although a significant decrease in AA was detected by 5 min after the addition of kainate or AMPA, it took 10 min before any change in average enzymatic activity could be detected after NMDA application, and this effect never reached statistical significance (see statistics below). Indeed, analysis of the effects of NMDA by two-way ANOVA (data points from 5–60 min) revealed significant overall variations with time (F11,55 = 18.639; P < 0.0001), but no overall group difference (F1,5 = 0.335; P = 0.5876) and no significant interaction (F11,55 = 1.708; P = 0.0958). The effect of time in the general analysis obviously reflected the progressive establishment of the steady-state condition, as confirmed by the presence of a significant effect of time in the analysis of data collected before application of the experimental treatment (time, 5–15 min; F2,10 = 86.577; P < 0.0001). Both groups were similar during this period, however (group effect: F1,5 = 1.361; P = 0.296; interaction: F2,10 = 0.157; P = 0.857). After treatment (time, 25–60 min), no significant change in time, no group difference, and no interaction could be detected (time: F7,35 = 0.224; P = 0.977; group: F1,5 = 0.981; P = 0.367; interaction: F7,35 = 1.530; P = 0.189).
Effects of γ-aminobutyric acid (GABA) on aromatase activity in HPOA explants
Additional experiments investigated the potential effect on AA of GABA (100 μm, Sigma-Aldrich). After 20 min of exposure to tritiated androstenedione, application for 10 min of 100 μm GABA had no apparent effect on the enzyme activity (Fig. 1D). Accordingly, the ANOVA of these data only showed the presence of a significant effect of time (F11.33 = 31.719; P < 0.0001), reflecting, as usual, changes observed during the equilibration period. There was no difference between conditions and no interaction between conditions and time (F1,3 = 0.152; P = 0.7220 and F11,33 = 0.590; P = 0.8225, respectively).
Effect of kainate antagonists
To obtain additional evidence that the kainate/AMPA-dependent inhibitions of AA are mediated by an interaction with glutamate receptors, the effects of kainate were tested in the presence of the specific non-NMDA glutamate receptor antagonists 1,2,3,4-tetrahydro-6-nitro-2,3-dioxo-benzo[f]quinoxaline-7-sulfonamide disodium (NBQX; 1 mm, Sigma-Aldrich) and 6-cyano-7-nitroquinoxaline-2,3-dione disodium (CNQX; 1 mm; Sigma-Aldrich Corp.). In these experiments, one of the hemiexplants was exposed throughout the incubation period (0–60 min) to the receptor antagonist, and kainate was added to both hemiexplants for 10 min from 20–30 min. These experiments fully replicated the rapid inhibitory effect of kainate on AA; although minor differences were present between experiments, addition of kainate induced in each case a significant decrease in AA that lasted 15–30 min (compare the kainate curves in Figs. 1A and 2, A and B). This inhibitory effect of kainate was completely blocked in the presence of both antagonists.
Analysis of the data summarized in Fig. 2, A and B, by two-way ANOVA identified in both cases a significant effect of time (NBQX: F11,22 = 35.257; P < 0.0001; CNQX: F11,33 = 11.934; P < 0.0001) and, importantly, a significant interaction between time and treatments (NBQX: F11,22 = 4.604; P = 0.0011; CNQX: F11,33 = 6.028; P < 0.0001). There was an overall effect of treatment for NBQX (F1,2 = 32.093; P = 0.0298), but not for CNQX (F1,3 = 2.102; P = 0.242). Post hoc tests clearly identified the origin of the significant interaction: after the application of kainate (time, 20 min), AA was significantly different in the kainate alone and in the kainate plus antagonist for a period that lasted 15 (CNQX) to 25 min (NBQX; see asterisks on Fig. 2, A and B). This apparently resulted from a pronounced drop in AA in explants that had not been exposed to the antagonist, but this drop was completely prevented by incubation with NBQX or CNQX. It should also be noted that in the presence of CNQX, AA tended to increase more rapidly to reach its equilibrium value (maximal level reached at 10 instead of 20 min normally observed), and the post hoc tests therefore indicated a difference between CNQX and control conditions before the addition of kainate at 20 min. This increased AA in the presence of a kainate antagonist could logically be expected if there is an endogenous glutamatergic activity in the explants. This effect should, however, be considered very cautiously because 1) it was observed during a period when aromatase activity is not stabilized, which makes it susceptible to experimental artifacts; and 2) the faster rate of increase in AA in the presence of CNQX was associated, for unknown reasons, with a difference in the maximal level that was reached. Although in almost every experiment, the levels of AA at 20 min were very similar in the two hemiexplants that were compared, in this specific experiment, a higher level of activity was reached by the control explants than by explants exposed to CNQX. We therefore believe that the reliability of this result is questionable. It is possible that addition of a glutamatergic antagonist effectively blocks the inhibitory effects on AA of an endogenous glutamatergic tone in the explants but this conclusion should not be accepted in the absence of additional experimental data.
Effects of kainate in the absence of calcium
The observed difference between effects of kainate/AMPA, on the one hand, and NMDA, on the other, may relate to the well-established different behaviors of the corresponding receptors toward Ca2+ ions. The NMDA receptor is a relatively effective Ca2+ channel that allows substantial amount of Ca2+ ions to enter neurons, whereas kainate and AMPA receptors would affect the intracellular Ca2+ mostly by inducing a release from intracellular stores (29) (see Discussion). The fact that kainate and AMPA had a more pronounced effect on AA than NMDA would then suggest that the Ca2+ that affects enzymatic activity essentially has its origin in intracellular stores. To obtain an additional test of this idea, the effects of kainate were assessed under conditions where a decrease in the extracellular Ca2+ concentration was present in the medium.
After an equilibration period of 20 min (see above), 100 μm kainate was applied to both hemiexplants, but a medium without Ca2+ was used for bathing one explant in each pair. This resulted in a marginal decrease in the osmolarity of the solution, but this is not a concern in the interpretation of the results, because, as is apparent in Fig. 2C, removal of Ca2+ in the medium did not affect the magnitude of the kainate-induced aromatase inhibition.
Accordingly, the two-way ANOVA of these data only identified an effect of time (F11,22 = 20.110; P < 0.0001), but no group difference and no interaction (F1,2 = 6.062; P = 0.1329 and F11,22 = 1.0776; P = 0.4217, respectively). The subanalysis focusing on the experimental period (time, 25–60 min) also revealed no group difference and no time by group interaction (P > 0.30 in both cases). There was, nevertheless, a clear decrease in AA in during the 10 min when kainate had been added in comparison with the control condition illustrated in Fig. 1A (and reproduced as a dotted line in Fig. 2C). Because the control data in the absence of kainate were from a different experiment and were thus not matched with those of explants exposed to kainate, these two sets of data were not compared by ANOVA, but it appears obvious that the kainate-induced decline in AA was observed again independently of whether Ca2+ was present in the medium.
Discussion
Our goal was to elucidate how brain estrogen production might be rapidly regulated by endogenous neurotransmitters in intact neural systems. We therefore administered, in our in vitro quail brain explant preparation (23), well-characterized ligands that stimulate glutamatergic activity in a manner consistent with in vivo actions of this common excitatory amino acid transmitter. These explants are useful in that they maintain many intrinsic aspects of the hypothalamic neural system and thereby provide us with physiologically relevant data. An analysis of such regulatory systems of AA by afferent inputs would indeed be impossible if we employed dissociated cell cultures or investigated the regulation of aromatase as revealed based on studies in expression vectors. These two potential alternative experimental systems do provide a better window on protein regulation from an individual cell perspective, but do not preserve the anatomical connectivity of the POA. Specific agonists of the three glutamate receptor subtypes (kainate, AMPA, and NMDA) were administered. As would be expected based on studies in other vertebrates, all three of these receptors are present in the quail preoptic-hypothalamic area, with kainate being the most common, and they exhibit pharmacological properties generally similar to those described in mammals (30, 31). Kainate and AMPA inhibited AA within minutes in quail HPOA explants. These inhibitions are specific to the stimulation of non-NMDA glutamate receptors as indicated by the facts that 1) no statistically significant effect was observed after addition of NMDA; 2) all effects were blocked by preincubation with the specific antagonists NBQX and CNQX; and 3) no effect was observed after treatment with GABA. The physiological nature of these effects is also supported by the observation that AA was restored to the control value of the paired explant after a washout period, indicating that the effects do not result from excitotoxic injury to neurons.
These inhibitions of enzyme activity were already statistically significant after 5 min of exposure to the effector, presumably mimicking in vivo physiological activations. The amount of tritiated water released into the medium was decreased by about 25% only 5 min after the addition of kainate or AMPA. The rate of decrease in tritiated water in the explant (reflecting aromatization; see Materials and Methods) is obviously underestimated by these measures due to the time required for diffusion of the effector in, and the water out, of the explant. This idea is clearly illustrated by the fact that 20 min were needed to reach an equilibrium in the amount of tritiated water present in the medium after the first addition of radioactive substrate. One can therefore reasonably assume that the response of AA to glutamate agonists is actually much faster, presumably in the minute or even second range. Technological limitations currently limit the true assessment of this aspect of the response.
It might seem surprising that stimulation of excitatory amino acid receptors resulted in an inhibition of AA. Based on previous work, we assume that the addition of kainate and AMPA increased the intracellular concentration of free Ca2+ that stimulated Ca2+-dependent kinases and, thus, aromatase phosphorylation (22, 23). Phosphorylations regulate the activity of a variety of enzymes and receptors, and very often the phosphorylated form of an enzyme is more active than its nonphosphorylated version. For example, phosphorylation of serine 40 increases the activity of tyrosine hydroxylase, the limiting step in catecholamine synthesis (32, 33). However, other enzymes are inhibited by phosphorylations (33, 34), and it is clearly the case for aromatase (22, 23). Many questions remain, however, regarding the molecular mechanisms mediating these effects of glutamate agonists.
Because kainate and AMPA were more active than NMDA, but the NMDA receptor is usually assumed to be a more effective Ca2+ channel than kainate and AMPA receptors (29), we speculated that kainate and AMPA acted mainly by releasing intracellular Ca2+ stores rather than allowing influx of extracellular Ca2+. This interpretation is consistent with the observation that removal of Ca2+ from the incubation medium at the time of kainate application had no detectable effect on AA inhibition. It remains possible nevertheless that sufficient amounts of Ca2+ were trapped in the extracellular space of the explant that could enter neurons after kainate application to activate the intracellular kinases. A role for internal stores, however, is consistent with the fact that similar inhibitions of AA are observed after application on HPOA explants of thapsigargin, a lactone that releases intracellular Ca2+ stores (23). It is also supported by our previous experiments showing that the addition of Ca2+ channel blockers, such as nifedipine, verapamil, ω-conotoxin, and Ni2+, does not affect the AA inhibition resulting from a K+-induced massive depolarization, another manipulation supposed to increase intracellular Ca2+ (23). At this time, all these facts converge to indicate that the rapid changes in brain AA result mostly from mobilizations of intracellular Ca2+ stores, rather than from an influx of extracellular Ca2+. The present experiment, indicating that a decrease in the extracellular Ca2+ concentration does not modify the effects of kainate on AA, is consistent with this interpretation, but because it was impossible to completely remove Ca2+ from the extracellular compartment of the explants (even if no Ca2+ was present in the incubation medium), the respective roles of intra- and extracellular Ca2+ should nonetheless be investigated further.
Our previous work indicated that AA can be down-regulated via Ca2+-dependent phosphorylations in a more rapid manner than previously suspected (22, 23) (see introduction for a summary of these findings). However, our manipulations were pharmacological and/or we did not have good control over the time course of the effects. Based on the current data, we can relate the rapid regulation of aromatase to well-characterized amino acid transmitters in a physiologically relevant neural system, and we have a good grasp of the time course of effects. It is likely that the changes in AA observed here after stimulation of glutamate receptor subtypes are mediated by the calcium-dependent phosphorylations that have been identified in brain homogenates (see introduction). Additional studies assessing the effects of kainate in the presence of kinase inhibitors would be helpful in this regard. Calcium-dependent phosphorylations, however, represent the only known mechanism that could affect aromatase activity with short latencies on the order of a few minutes. Other currently unknown mechanisms, however, could also play a role and should also be investigated.
Independently of the cellular/biochemical mechanisms that mediate the fast changes in AA identified in the present study, these data also support the idea that the synthesis of estrogen, and thus estrogen action, can be rapidly regulated in the brain with a time course similar to what has previously been described for neurotransmitters and neuromodulators. Several criteria should be met for a neurochemical substance to be considered a neurotransmitter or neuromodulator (35, 36), and estrogens now fulfill most of these criteria. 1) Aromatase immunoreactivity is observed to be present in presynaptic boutons (37, 38), and AA is also present (39) and sometimes enriched (40, 41) in synaptosomes, indicating that estrogen is produced and released from presynaptic boutons. 2) Rapid neuronal responses to estrogen have been described in many biological systems (12, 18–20, 42, 43) even if multiple questions regarding the transduction mechanisms mediating these responses at the level of the neuronal membrane remain open (13, 21). 3) We describe a mechanism by which estrogen production could be switched on and off rapidly based on local changes in excitatory neurotransmission. It is probable that in local populations of aromatase-expressing cells, AA is tonically inhibited by the constant spontaneous glutamatergic electrical activity recently described by electrophysiological techniques (44). Inhibition of this spontaneous electrical activity in relation to various sensory inputs would then rapidly up-regulate estrogen production. Conversely, estrogen synthesis in specified neuronal populations could be rapidly down-regulated by local increases in glutamatergic activity. This spontaneous electrical activity is presumably decreased or no longer present in the large explants maintained in vitro. Its effects, however, are clearly mimicked by the application of glutamate agonists to the incubation medium. 4) Finally, even if estrogen synthesis varies rapidly, rapid changes in estrogen signaling also imply that locally produced estrogens should rapidly be cleared from the tissue when AA is interrupted. Indirect evidence that this is actually the case comes from recent behavioral experiments showing that a single systemic injection of an aromatase inhibitor decreases within 15–30 min the expression of various aspects of male sexual behavior in quail (28). Estrogens are catabolized to inactive (or less active) water-soluble metabolites by a variety of enzymes (2- and 4-hydroxylases, glucuronidase, sulfonotransferase, and O-methylase) that are located mainly in the liver, but are also expressed in the brain (45–52). High levels of 2- and 4-hydroxylases, for example, have been identified in the brain, notably the HPOA, in various species, including quail (45, 53, 54). The blockade of estrogen production could thus result in a rapid drop in the local estrogen concentration, even if the actual rate of decrease remains to be established. Furthermore, convincing evidence has been presented demonstrating that the enzyme that catalyzes the 2-hydroxylation of estrogens into catecholestrogens is actually the same enzyme that catalyzes the production of estrogen, that is, aromatase (55). The same protein thus functions as estrogen synthase or as catecholestrogen-forming enzyme as a function of the available substrate and local pH, which should provide a very effective way for rapidly controlling local concentrations of bioactive estrogens.
The findings reported here advance our knowledge about the regulation of brain estrogen by demonstrating that the activity of the estrogen-synthesizing enzyme aromatase is rapidly regulated by glutamatergic neurotransmission in intact neural systems. Thus, estrogen synthesis can be rapidly down-regulated under physiological conditions. These observations combined with recent studies of fast membrane actions of estrogen are challenging the idea that the cellular actions of a hormone such as estrogen are distinct from those of neurotransmitters and neuromodulators.
This work was supported by grants from the National Institute of Mental Health (MH-50388; to G.F.B. and J.B.) and a grant from the Belgian Fonds de la Recherche Fondamentale Collective (2.4562.05; to J.B.).
Abbreviations
- AA,
Aromatase activity;
- AMPA,
α-amino-3-hydroxymethyl-4-isoxazole propionic acid;
- CNQX,
6-cyano-7-nitroquinoxaline-2,3-dione disodium;
- GABA,
γ-aminobutyric acid;
- HPOA,
hypothalamus-preoptic area;
- NBQX,
1,2,3,4-tetrahydro-6-nitro-2,3-dioxo-benzo[f]quinoxaline-7-sulfonamide disodium;
- NMDA,
N-methyl-d-aspartate;
- POA,
preoptic area.