-
PDF
- Split View
-
Views
-
Cite
Cite
Wolfgang Wick, Michael Platten, Michael Weller, New (alternative) temozolomide regimens for the treatment of glioma, Neuro-Oncology, Volume 11, Issue 1, February 2009, Pages 69–79, https://doi.org/10.1215/15228517-2008-078
Close - Share Icon Share
Abstract
One barrier to successful treatment of malignant glioma is resistance to alkylating agents such as temozolomide. The cytotoxic activity of temozolomide and other alkylating agents is believed to manifest largely by the formation of O6-methylguanine DNA adducts. Consequently, the primary mechanism of resistance to temozolomide is a function of the activity of the DNA repair enzyme O6-methylguanine DNA methyltransferase (MGMT). Fortuitously, MGMT is inactivated after each reaction (i.e., suicide enzyme). Therefore, if the rate of DNA alkylation were to outpace the rate of MGMT protein synthesis, the enzyme could, in theory, be depleted. Several studies have shown that prolonged exposure to temozolomide can deplete MGMT activity in blood cells, a process that could potentially increase the antitumor activity of the drug. To date, however, there are limited data demonstrating the depletion of MGMT activity in tumor tissue exposed to temozolomide. A variety of dosing schedules that increase the duration of exposure and the cumulative dose of temozolomide are currently being investigated for the treatment of glioma, with the goal of improving antitumor activity and overcoming resistance. These alternative dosing regimens have been shown to deplete MGMT activity in peripheral blood mononuclear cells, but the regimen that provides the best balance between enhanced antitumor activity and acceptable hematologic toxicity has yet to be determined.
For decades, the mainstay of treatment for malignant glioma has been radiotherapy (RT) followed by alkylating agents such as the nitrosoureas, procarbazine, and, more recently, temozolomide. Initially temozolomide was approved for the treatment of recurrent high-grade glioma at a dose of 150–200 mg/m2/day for 5 days every 28-day cycle (i.e., standard 5-day regimen).1–3 This clinical dosing regimen was determined based on preclinical studies conducted by Stevens and colleagues in the late 1980s showing schedule-dependent activity4 and phase I clinical studies conducted by Newlands and colleagues in the early 1990s.5,6 Subsequently, Brock et al.7 demonstrated that a continuous daily regimen at a dose of 75 mg/m2/day was active and well tolerated for periods up to 6 or 7 weeks. This study began a very fruitful period of investigation into the clinical activity of a variety of alternative temozolomide dosing regimens.
The cytotoxicity of temozolomide, like many other alkylating agents, manifests primarily by methylation of the O6 position of guanine. Although these DNA adducts represent <9% of the total DNA methylation events caused by temozolomide,8–10O6-methylguanine induces DNA mismatch repair, which is unable to successfully repair the lesion, and the resulting double-strand breaks ultimately drive the cell to undergo apoptosis.11,12 In contrast, N7-methylguanine and N3-methyladenine, which constitute approximately 80% of the total methylation events, are readily repaired by the base excision repair pathway and are generally not cytotoxic.9,10 Therefore, generation of O6-methylguanine and a functional DNA mismatch repair pathway are both critical to the cytotoxic potential of temozolomide. The only cellular mechanism capable of repairing these adducts is the enzyme O6-methylguanine DNA methyltransferase (MGMT). Consequently, the cellular levels of MGMT can affect the cytotoxicity of temozolomide and other alkylating agents, and the amount of MGMT in tumor cells is often regulated by epigenetic silencing of the gene via hypermethylation of CpG islands within the MGMT gene promoter. Tumor cells with a methylated MGMT promoter produce less MGMT protein. This is particularly relevant in light of recent studies showing that MGMT promoter methylation correlates with the clinical benefit of alkylating agents, particularly temozolomide.
Recently, temozolomide was shown to significantly improve survival in patients with newly diagnosed glioblastoma (GBM) when administered concomitantly with RT and as maintenance therapy thereafter.3,13 A randomized, phase III trial jointly conducted by the European Organisation for Research and Treatment of Cancer (EORTC) and the National Cancer Institute of Canada (NCIC) Clinical Trials Group analyzed standard RT (60 Gy) alone compared with RT plus concomitant temozolomide (75 mg/m2/day × 6 weeks) followed by up to six cycles of maintenance temozolomide using the standard 5-day schedule.3 Median survival was 12.1 months with RT alone versus 14.6 months for patients treated with RT plus temozolomide (p < 0.001), and 2-year survival was 27% in the temozolomide group compared with 10% in the control group.3 This trial also showed that methylation of the MGMT promoter correlated with improved survival in patients treated with RT plus temozolomide,14 suggesting that tumors presumably with low levels of MGMT protein caused by epigenetic gene silencing may respond better to temozolomide than do tumors with high MGMT levels. These data also indicated that GBM with an unmethylated MGMT promoter may show only a marginal response to temozolomide. The retrospective MGMT promoter methylation analysis performed in the context of this large, randomized trial confirmed previous reports from several smaller studies and provided further evidence to support the hypothesis that MGMT promoter methylation status can profoundly affect sensitivity to alkylating agents.15,16 The growing body of evidence demonstrating the clinical importance of MGMT has generated considerable interest in the exploration of strategies to overcome MGMT-mediated resistance to alkylating agents. Research efforts have focused on either disabling the enzyme using inhibitors such as O6-benzylguanine (O6-BG) or depleting MGMT enzyme activity in tumor cells.
When MGMT repairs O6-methylguanine lesions, it transfers the alkyl group from guanine to a cysteine moiety in its active site, thereby repairing the DNA. In the process, however, MGMT is irreversibly inactivated such that new MGMT protein synthesis is required for recovery of MGMT activity.17–19 In theory, it may be possible to exploit this feature to overcome chemoresistance. If the number of O6-methylguanine DNA adducts formed were to exceed the synthesis of cellular MGMT, then MGMT activity could be depleted, thus increasing the cytotoxic potential of the alkylating agent. However, MGMT is also depleted in normal cells, particularly hematopoietic stem cells, resulting in hematologic toxicity. The therapeutic window is largest when tumor cells have lower levels of MGMT activity relative to normal tissue. This is often the case in tumor cells with a hypermethylated MGMT promoter, which effectively silences the gene.20,21 Epigenetic gene silencing of the MGMT promoter is found in 45%–70% of high-grade gliomas.22–25 This reflects a general mechanism whereby tumor cells turn off many genes that might otherwise act to reduce mutations, to slow cell division, or to induce apoptosis in response to DNA damage or mutations.
Unfortunately, the kinetics of MGMT depletion and recovery in tumor tissue is not well understood. Most studies have measured MGMT activity only in peripheral blood mononuclear cells (PBMCs) taken from cancer patients receiving treatment with alkylating agents. However, it is not known whether MGMT activity in PBMCs is a good surrogate for MGMT activity in tumor tissue. In fact, there is some evidence to suggest that depletion or inactivation of MGMT occurs more readily in PBMCs and that MGMT activity in PBMCs is not a reliable surrogate for MGMT activity in tumor tissue.26,27 This may reflect the fact that tumor cells are more metabolically active than are PBMCs and may synthesize new MGMT more rapidly. Depletion of MGMT activity was first described in patients with metastatic melanoma who were treated with temozolomide on the standard 5-day dosing schedule.28 This study revealed that MGMT levels in PBMCs dropped within 4 h of the initial dose to a median nadir of 53% below pretreatment levels, and continued dosing over subsequent days resulted in cumulative and progressive MGMT depletion. In some patients, recovery of MGMT protein levels to approximately 45% of pretreatment level occurred in only 48 h after the last dose, suggesting that prolonged temozolomide exposure may effectively deplete MGMT in PBMCs. To date, however, few studies have directly measured MGMT enzyme activity in tumor tissue.29,30 In one of these studies,29 patients with primary brain tumors were treated with O6-BG at various time points before surgery, and MGMT activity was measured in the resected tumor. The results showed that MGMT activity in primary brain tumors was nearly completely inhibited 6 h after exposure to 120 mg/m2O6-BG but was partially recovered 18 h after exposure to O6-BG.29 This continues to be an area of controversy and active research. The key unanswered question is whether temozolomide dosing regimens that deliver a higher cumulative dose over a prolonged period can effectively deplete MGMT activity in the tumor and overcome chemoresistance.
Alternative Temozolomide Dosing Schedules
Early clinical studies investigating compressed and extended dosing schedules suggested that continuous daily administration of temozolomide was more effective than a single dose.5,6 More frequent administration (e.g., twice daily)6 yielded higher levels of O6-methylguanine DNA adducts, suggesting that the capacity of tumor cells to repair these adducts can be saturated. Unfortunately, hematologic toxicity was dose limiting with some schedules. For example, in a phase II trial in which 30 patients with metastatic melanoma received five doses of temozolomide (200 mg/m2) within 16 h (total dose = 1,000 mg/m2), the majority (68%) of patients developed grade 3/4 thrombocytopenia, and 54% of patients developed grade 3/4 leukopenia,31 which is rarely seen with the standard dosing regimen. Interestingly, pretreatment MGMT levels in PBMCs correlated with the temozolomide dose intensity that patients were able to tolerate. Profound lymphopenia has also been reported in a retrospective analysis of melanoma patients who received temozolomide at a dose of 75 mg/m2/day for extended periods,32 and this may leave patients susceptible to Pneumocystis jiroveci pneumonia and other opportunistic infections, particularly when lymphocyte counts drop below 200 cells/μl. Patients in this study had received temozolomide either alone or in combination with thalidomide or interferon-alpha, and 60% of patients developed lymphopenia. There was a trend toward more frequent lymphopenia among patients who failed to take a 2-week drug holiday every 8-week cycle. These clinical experiences highlight the challenge, which is to find a dosing regimen that allows normal cells ample time to recover, thus avoiding dose-limiting hematologic toxicity, while maximizing depletion of MGMT and cytotoxic activity in the tumor.
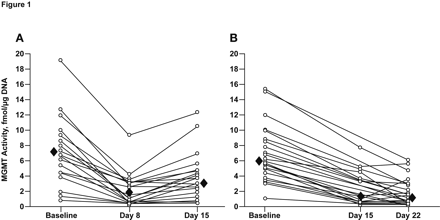
Since these early studies, a variety of alternative dosing schedules have been tested in clinical trials for the treatment of glioma. In general, these alternative regimens increase exposure to temozolomide over a 28-day cycle by approximately 2-fold compared with the standard 5-day regimen (Table 1).19 The extent to which two of these alternative regimens were able to deplete MGMT was rigorously tested in a study reported by Tolcher et al.33 In this study, patients were treated with either an alternating weekly schedule (7 days on/7 days off) or for 21 consecutive days every 28-day cycle (21/28-day schedule), and MGMT enzyme activity was assayed in PBMCs collected at various time points during treatment. The results showed a time- and dose-dependent decrease in MGMT activity with both regimens. Continuous daily dosing for 7 days at 75–175 mg/m2/day reduced mean baseline MGMT activity by 72% on day 8 in a dose-dependent manner, but during the 7-day rest period, MGMT activity recovered to 55% of baseline on day 15 (Fig. 1).33 The 21-day continuous schedule at a lower daily dose (85–125 mg/m2) produced a similar reduction in mean MGMT activity by day 14 that was sustained through day 21. MGMT activity was not measured on day 28, so it is not known to what extent MGMT activity recovered after 7 days of rest following 21 days of continuous dosing.
Alternative temozolomide dosing schedules
| Regimen . | Daily Dose, mg/m2 . | Schedule . | Dose Intensity, mg/m2 per 28-Day Cycle . | Relative Dose Intensity . |
|---|---|---|---|---|
| 5/28 days | 200 | 5 days every 28 days | 1,000 | 1 |
| Daily | 75 | Daily × 6–7 weeks | 1,575 | 1.6 |
| 10/28 days | 150 | Days 1–5 and 15–19 every 28 days | 1,500 | 1.5 |
| 14/28 days | 150 | 7 days on/7 days off | 2,100 | 2.1 |
| 21/28 days | 100 | 21 days on/7 days off | 2,100 | 2.1 |
| Regimen . | Daily Dose, mg/m2 . | Schedule . | Dose Intensity, mg/m2 per 28-Day Cycle . | Relative Dose Intensity . |
|---|---|---|---|---|
| 5/28 days | 200 | 5 days every 28 days | 1,000 | 1 |
| Daily | 75 | Daily × 6–7 weeks | 1,575 | 1.6 |
| 10/28 days | 150 | Days 1–5 and 15–19 every 28 days | 1,500 | 1.5 |
| 14/28 days | 150 | 7 days on/7 days off | 2,100 | 2.1 |
| 21/28 days | 100 | 21 days on/7 days off | 2,100 | 2.1 |
Reprinted with permission from Hegi et al.19
Alternative temozolomide dosing schedules
| Regimen . | Daily Dose, mg/m2 . | Schedule . | Dose Intensity, mg/m2 per 28-Day Cycle . | Relative Dose Intensity . |
|---|---|---|---|---|
| 5/28 days | 200 | 5 days every 28 days | 1,000 | 1 |
| Daily | 75 | Daily × 6–7 weeks | 1,575 | 1.6 |
| 10/28 days | 150 | Days 1–5 and 15–19 every 28 days | 1,500 | 1.5 |
| 14/28 days | 150 | 7 days on/7 days off | 2,100 | 2.1 |
| 21/28 days | 100 | 21 days on/7 days off | 2,100 | 2.1 |
| Regimen . | Daily Dose, mg/m2 . | Schedule . | Dose Intensity, mg/m2 per 28-Day Cycle . | Relative Dose Intensity . |
|---|---|---|---|---|
| 5/28 days | 200 | 5 days every 28 days | 1,000 | 1 |
| Daily | 75 | Daily × 6–7 weeks | 1,575 | 1.6 |
| 10/28 days | 150 | Days 1–5 and 15–19 every 28 days | 1,500 | 1.5 |
| 14/28 days | 150 | 7 days on/7 days off | 2,100 | 2.1 |
| 21/28 days | 100 | 21 days on/7 days off | 2,100 | 2.1 |
Reprinted with permission from Hegi et al.19
This seminal study by Tolcher et al.33 highlighted several aspects of MGMT depletion with temozolomide. Most important, depletion of MGMT activity was a nonlinear function of both the total cumulative dose and the area under the concentration time curve (best described by a maximum effect response model). At high daily doses, temozolomide appears to deplete MGMT levels more rapidly than at lower daily doses, but because of dose-limiting hematologic toxicity, high daily doses can be tolerated only for short periods of time. Temozolomide administration for 21 consecutive days at a low daily dose achieved a comparable cumulative dose and resulted in a comparable depletion of MGMT activity compared with the 7-days-on/7-days-off regimen at a higher daily dose. These data suggest that the 21-day schedule may provide more sustained depletion of MGMT in PBMCs, whereas the 7-days-on/7-days-off regimen may allow for recovery of MGMT and potentially less hematologic toxicity.
Although this study established an important benchmark for evaluating alternative regimens, it has some limitations.33 First and foremost, it is important to recognize that Tolcher et al. measured MGMT activity only in PBMCs, not directly in tumor tissue. In addition, no comparisons were made with the standard 5-day regimen. Therefore, it remains unknown whether these alternative regimens are more effective than the standard regimen in terms of MGMT depletion. Clearly, the schedule that most effectively depletes MGMT in tumor cells and strikes the best balance between antitumor activity and hematologic toxicity has yet to be defined.
Brain Tumor Trials Investigating Alternative Temozolomide Schedules
Newly Diagnosed High-Grade Glioma
All available published reports of alternative temozolomide dosing schedules that have been investigated in patients with newly diagnosed high-grade glioma are summarized in Table 2.3,14,36–42,44,50 These studies have investigated alternative regimens in a variety of different settings within the context of initial therapy: as maintenance therapy after concomitant RT plus temozolomide, as an alternative to the standard 75 mg/m2/day concomitant with RT, or in the neoadjuvant setting. Clearly, a number of key questions remain unanswered regarding the optimal dosing schedule for temozolomide in the first-line setting. First, what is the optimal dose and schedule to combine with RT? Second, what benefit is derived from maintenance therapy? Third, what is the optimal dose and schedule to use as maintenance therapy?
Clinical studies of alternative temozolomide dosing regimens in patients with newly diagnosed high-grade glioma
| Study . | n . | Patient Population . | Schedule . | Median PFS, Months . | Median OS, Months . |
|---|---|---|---|---|---|
| Stupp et al. (2005)3 | 573 | Newly Dx GBM | RT + TMZ (75 mg/m2) followed by 50–200 mg/m2 days 1–5 q28d | 6.9 | 14.6 |
| vs. RT alone (60 Gy) | 5.0 | 12.1 | |||
| Athanassiou et al. (2005)34 | 110 | Newly Dx GBM | RT + TMZ (75 mg/m2) followed by 150 mg/m2 days 1–5 and 15–19 q28d | 10.8 | 13.4 |
| vs. RT alone (60 Gy) | 5.2 | 7.7 | |||
| Caroli et al. (2007)36 | 38 | Newly Dx GBM | After surgery and RT, 150 mg/m2 days 1–5, then 75 mg/m2 days 6–10 q28d | 10 | 16 |
| Chinot et al. (2007)37 | 29 | Inoperable, newly Dx GBM | 150 mg/m2 days 1–7 and 15–21 q28d as neoadjuvant tx before RT and maintenance after RT | 3.8 | 6 |
| Combs et al. (2005, 2007)38,39 | 53 | Newly Dx GBM | RT + TMZ (50 mg/m2) concomitant; no maintenance | 8 | 19 |
| D'Agostino et al. (2007)40,a | 41 | Newly Dx GBM (n = 29) or AA (n = 12) | RT + TMZ (75 mg/m2 7 d/w from 1st to last day of RT)a | NA | 19 |
| vs. RT 1 TMZ (75 mg/m2 5 d/w for 1st and last week of RT) | 21 | ||||
| Montemaggi et al. (2005)41 | 40 | Newly Dx high-grade glioma | RT + TMZ (75 mg/m2) followed by 250 mg/m2 days 1–5 q28d | NA | 20 |
| NOA-0842 (open) | 340 planned | Newly Dx GBM or AA, patients >65 years old | 100 mg/m2 days 1–7 and 15–21 q28d or RT alone | NA | NA |
| Clarke et al. (2007)50 | 67 | Newly Dx GBM | RT + TMZ (75 mg/m2) followed by 150 mg/m2 days 1–7 and 15–21 q28d plus cis-retinoic acid | 6.6 | NR |
| vs. 50 mg/m2/day for six 28-day cycles plus cis-retinoic acid | 4.4 | ||||
| Zorlu et al. (2005)44 | 32 | Newly Dx GBM (n = 17) or AA (n = 15) | RT + TMZ (100 mg/m2/day) during weeks 1 and 3 of fractionated RT | NA | 15 (all patients combined) |
| vs. RT + TMZ (150 mg/m2/day) during weeks 1 and 3 of fractionated RT |
| Study . | n . | Patient Population . | Schedule . | Median PFS, Months . | Median OS, Months . |
|---|---|---|---|---|---|
| Stupp et al. (2005)3 | 573 | Newly Dx GBM | RT + TMZ (75 mg/m2) followed by 50–200 mg/m2 days 1–5 q28d | 6.9 | 14.6 |
| vs. RT alone (60 Gy) | 5.0 | 12.1 | |||
| Athanassiou et al. (2005)34 | 110 | Newly Dx GBM | RT + TMZ (75 mg/m2) followed by 150 mg/m2 days 1–5 and 15–19 q28d | 10.8 | 13.4 |
| vs. RT alone (60 Gy) | 5.2 | 7.7 | |||
| Caroli et al. (2007)36 | 38 | Newly Dx GBM | After surgery and RT, 150 mg/m2 days 1–5, then 75 mg/m2 days 6–10 q28d | 10 | 16 |
| Chinot et al. (2007)37 | 29 | Inoperable, newly Dx GBM | 150 mg/m2 days 1–7 and 15–21 q28d as neoadjuvant tx before RT and maintenance after RT | 3.8 | 6 |
| Combs et al. (2005, 2007)38,39 | 53 | Newly Dx GBM | RT + TMZ (50 mg/m2) concomitant; no maintenance | 8 | 19 |
| D'Agostino et al. (2007)40,a | 41 | Newly Dx GBM (n = 29) or AA (n = 12) | RT + TMZ (75 mg/m2 7 d/w from 1st to last day of RT)a | NA | 19 |
| vs. RT 1 TMZ (75 mg/m2 5 d/w for 1st and last week of RT) | 21 | ||||
| Montemaggi et al. (2005)41 | 40 | Newly Dx high-grade glioma | RT + TMZ (75 mg/m2) followed by 250 mg/m2 days 1–5 q28d | NA | 20 |
| NOA-0842 (open) | 340 planned | Newly Dx GBM or AA, patients >65 years old | 100 mg/m2 days 1–7 and 15–21 q28d or RT alone | NA | NA |
| Clarke et al. (2007)50 | 67 | Newly Dx GBM | RT + TMZ (75 mg/m2) followed by 150 mg/m2 days 1–7 and 15–21 q28d plus cis-retinoic acid | 6.6 | NR |
| vs. 50 mg/m2/day for six 28-day cycles plus cis-retinoic acid | 4.4 | ||||
| Zorlu et al. (2005)44 | 32 | Newly Dx GBM (n = 17) or AA (n = 15) | RT + TMZ (100 mg/m2/day) during weeks 1 and 3 of fractionated RT | NA | 15 (all patients combined) |
| vs. RT + TMZ (150 mg/m2/day) during weeks 1 and 3 of fractionated RT |
Abbreviations: PFS, progression-free survival; OS, overall survival; Dx, diagnosed; GBM, glioblastoma; RT, radiotherapy; TMZ, temozolomide; q28d, every 28 days; tx, treatment; AA, anaplastic astrocytoma; d/w, days per week; NA, not available; NR, not reached
This trial compared these results with same-institution, historical results found with a less frequent administration schedule
Clinical studies of alternative temozolomide dosing regimens in patients with newly diagnosed high-grade glioma
| Study . | n . | Patient Population . | Schedule . | Median PFS, Months . | Median OS, Months . |
|---|---|---|---|---|---|
| Stupp et al. (2005)3 | 573 | Newly Dx GBM | RT + TMZ (75 mg/m2) followed by 50–200 mg/m2 days 1–5 q28d | 6.9 | 14.6 |
| vs. RT alone (60 Gy) | 5.0 | 12.1 | |||
| Athanassiou et al. (2005)34 | 110 | Newly Dx GBM | RT + TMZ (75 mg/m2) followed by 150 mg/m2 days 1–5 and 15–19 q28d | 10.8 | 13.4 |
| vs. RT alone (60 Gy) | 5.2 | 7.7 | |||
| Caroli et al. (2007)36 | 38 | Newly Dx GBM | After surgery and RT, 150 mg/m2 days 1–5, then 75 mg/m2 days 6–10 q28d | 10 | 16 |
| Chinot et al. (2007)37 | 29 | Inoperable, newly Dx GBM | 150 mg/m2 days 1–7 and 15–21 q28d as neoadjuvant tx before RT and maintenance after RT | 3.8 | 6 |
| Combs et al. (2005, 2007)38,39 | 53 | Newly Dx GBM | RT + TMZ (50 mg/m2) concomitant; no maintenance | 8 | 19 |
| D'Agostino et al. (2007)40,a | 41 | Newly Dx GBM (n = 29) or AA (n = 12) | RT + TMZ (75 mg/m2 7 d/w from 1st to last day of RT)a | NA | 19 |
| vs. RT 1 TMZ (75 mg/m2 5 d/w for 1st and last week of RT) | 21 | ||||
| Montemaggi et al. (2005)41 | 40 | Newly Dx high-grade glioma | RT + TMZ (75 mg/m2) followed by 250 mg/m2 days 1–5 q28d | NA | 20 |
| NOA-0842 (open) | 340 planned | Newly Dx GBM or AA, patients >65 years old | 100 mg/m2 days 1–7 and 15–21 q28d or RT alone | NA | NA |
| Clarke et al. (2007)50 | 67 | Newly Dx GBM | RT + TMZ (75 mg/m2) followed by 150 mg/m2 days 1–7 and 15–21 q28d plus cis-retinoic acid | 6.6 | NR |
| vs. 50 mg/m2/day for six 28-day cycles plus cis-retinoic acid | 4.4 | ||||
| Zorlu et al. (2005)44 | 32 | Newly Dx GBM (n = 17) or AA (n = 15) | RT + TMZ (100 mg/m2/day) during weeks 1 and 3 of fractionated RT | NA | 15 (all patients combined) |
| vs. RT + TMZ (150 mg/m2/day) during weeks 1 and 3 of fractionated RT |
| Study . | n . | Patient Population . | Schedule . | Median PFS, Months . | Median OS, Months . |
|---|---|---|---|---|---|
| Stupp et al. (2005)3 | 573 | Newly Dx GBM | RT + TMZ (75 mg/m2) followed by 50–200 mg/m2 days 1–5 q28d | 6.9 | 14.6 |
| vs. RT alone (60 Gy) | 5.0 | 12.1 | |||
| Athanassiou et al. (2005)34 | 110 | Newly Dx GBM | RT + TMZ (75 mg/m2) followed by 150 mg/m2 days 1–5 and 15–19 q28d | 10.8 | 13.4 |
| vs. RT alone (60 Gy) | 5.2 | 7.7 | |||
| Caroli et al. (2007)36 | 38 | Newly Dx GBM | After surgery and RT, 150 mg/m2 days 1–5, then 75 mg/m2 days 6–10 q28d | 10 | 16 |
| Chinot et al. (2007)37 | 29 | Inoperable, newly Dx GBM | 150 mg/m2 days 1–7 and 15–21 q28d as neoadjuvant tx before RT and maintenance after RT | 3.8 | 6 |
| Combs et al. (2005, 2007)38,39 | 53 | Newly Dx GBM | RT + TMZ (50 mg/m2) concomitant; no maintenance | 8 | 19 |
| D'Agostino et al. (2007)40,a | 41 | Newly Dx GBM (n = 29) or AA (n = 12) | RT + TMZ (75 mg/m2 7 d/w from 1st to last day of RT)a | NA | 19 |
| vs. RT 1 TMZ (75 mg/m2 5 d/w for 1st and last week of RT) | 21 | ||||
| Montemaggi et al. (2005)41 | 40 | Newly Dx high-grade glioma | RT + TMZ (75 mg/m2) followed by 250 mg/m2 days 1–5 q28d | NA | 20 |
| NOA-0842 (open) | 340 planned | Newly Dx GBM or AA, patients >65 years old | 100 mg/m2 days 1–7 and 15–21 q28d or RT alone | NA | NA |
| Clarke et al. (2007)50 | 67 | Newly Dx GBM | RT + TMZ (75 mg/m2) followed by 150 mg/m2 days 1–7 and 15–21 q28d plus cis-retinoic acid | 6.6 | NR |
| vs. 50 mg/m2/day for six 28-day cycles plus cis-retinoic acid | 4.4 | ||||
| Zorlu et al. (2005)44 | 32 | Newly Dx GBM (n = 17) or AA (n = 15) | RT + TMZ (100 mg/m2/day) during weeks 1 and 3 of fractionated RT | NA | 15 (all patients combined) |
| vs. RT + TMZ (150 mg/m2/day) during weeks 1 and 3 of fractionated RT |
Abbreviations: PFS, progression-free survival; OS, overall survival; Dx, diagnosed; GBM, glioblastoma; RT, radiotherapy; TMZ, temozolomide; q28d, every 28 days; tx, treatment; AA, anaplastic astrocytoma; d/w, days per week; NA, not available; NR, not reached
This trial compared these results with same-institution, historical results found with a less frequent administration schedule
Levels of O6-methylguanine DNA methyltransferase (MGMT) enzyme activity in peripheral blood mononuclear cells at baseline and after treatment with temozolomide using the 7-days-on/7-days-off schedule at doses ranging from 75 to 175 mg/m2/day (A) or the 21/28-day schedule at doses ranging from 85 to 125 mg/m2/day (B). Solid diamonds indicate mean values. Reprinted with permission from Tolcher et al.33
Optimal Dosing Schedule in Combination With RT. Several studies have looked at different dosing schedules concomitant with RT.43 In the pivotal phase III trial in newly diagnosed GBM conducted by the EORTC/NCIC, 75 mg/m2/day was shown to be effective and well tolerated.3 Whether intermittent dosing or low metronomic doses (e.g., 50 mg/m2/day) can improve efficacy remains unclear. Combs et al.38,39 reported a series of 53 patients with GBM who were treated with RT plus temozolomide (50 mg/m2/day), and no maintenance therapy was administered after RT. Surprisingly, median progression-free survival (PFS) was 8 months, and median overall survival was 19 months. This compares favorably to the results reported by Stupp et al.3,13 in both the phase II and phase III trials, which included maintenance therapy after concomitant RT plus temozolomide. Although this was a single-institution study, and the results may have been influenced by patient selection, it nevertheless raises important questions as to whether a low metronomic dose may be effective when given concomitantly with RT and whether maintenance therapy provides added benefit. D'Agostino et al.40 have also investigated whether continuous daily administration of temozolomide during RT is necessary. They compared their results achieved with the standard dosing regimen (75 mg/m2 temozolomide 7 days per week × 6 weeks) to a historical control group treated at the same institution with 75 mg/m2/day × 5 days during only the first and last week of RT (total of 10 days). Median survival was 19 months and 21 months, respectively. However, continuous daily administration of temozolomide resulted in more severe toxicity (27% of patients had grade 3/4 adverse events, compared with 3% in the historical control group). The authors concluded, based on the comparable survival outcomes and favorable safety profile, that intermittent administration of temozolomide during the first and last week of RT is effective.40 However, this would require prospective validation. Similarly, Zorlu et al.44 reported using the 7-days-on/7-days-off regimen at a dose of either 100 or 150 mg/m2/day concomitant with conventional or hyperfractionated RT in 32 patients with GBM or anaplastic astrocytoma (AA), and patients received concomitant temozolomide during only the first and third week of RT. This regimen reduces the dose intensity of temozolomide delivered during RT compared with the 75 mg/m2 daily schedule, which delivers 3,150 mg/m2 over 42 consecutive days. Nevertheless, the median overall survival was 15 months, which compares favorably with the results achieved using the conventional regimen. These studies would have benefited from MGMT analysis, which not only would have provided important data for future patient selection but also would have provided an internal control to determine whether patient selection is an issue.
Seven-Days-On/Seven-Days-Off Regimen. The most widely investigated alternative dosing schedule in the first-line setting is the 7-days-on/7-days-off regimen. In most studies the dose was 150 mg/m2/day administered on days 1–7 and 15–21 of each 28-day cycle. Chinot et al.37 examined the activity of this regimen in the neoadjuvant setting (up to four cycles before conventional RT) in 29 patients with inoperable, newly diagnosed GBM.37,45 Patients also received up to four cycles of temozolomide until disease progression as maintenance therapy after RT on the same schedule. In this setting, it is possible to directly assess the antitumor activity of the regimen, and MGMT expression was prospectively assessed by immunohistochemistry in 25 available tumor biopsy specimens. Patients in this study received a median of three cycles before RT, and best tumor response 1 month after completion of RT was partial response in 24% of patients and stable disease in 31% of patients. The median PFS was 3.8 months, and median survival was 6 months. This schedule appeared to have good antitumor activity in this poor-prognosis population,46 and the response rate was comparable with that achieved with neoadjuvant therapy in similar patient populations using the standard 5-day schedule.47–49 Hematologic toxicity was manageable, with dose reductions required in six (21%) patients because of grade 3 or 4 thrombocytopenia and in five (17%) patients because of grade 3 or 4 neutropenia.37 Five patients also developed febrile interstitial pneumonitis associated with either profound lymphopenia or neutropenia. Analysis of MGMT revealed low expression (<35% of tumor cells) in 11 patients and high expression (>35% of tumor cells) in 14 patients. Notably, the response rate was 55% in patients with low MGMT expression and only 7% in patients with high MGMT expression (p = 0.004). Patients with low MGMT expression also had significantly longer PFS (5.5 vs. 1.9 months, respectively; p = 0.009). Unfortunately, the results of this study did not support the conclusion that this regimen is more active than the standard 5-day regimen. As with most other studies reported to date, this study lacked a control arm for comparison. It is also important to note that the correlation between MGMT levels determined by immunohistochemistry and MGMT promoter methylation status has yet to be established.
Clarke et al.50 reported a randomized study investigating the 7-days-on/7-days-off dose-dense regimen (150 mg/m2/day) as maintenance therapy for up to six cycles following standard RT plus concomitant temozolomide (75 mg/m2/day) in patients (n = 67) with newly diagnosed GBM, and they compared this maintenance regimen with a metronomic daily dose of 50 mg/m2/day. Patients in both arms also received oral cis-retinoic acid following completion of maintenance temozolomide. In the most recent report on this study, median overall survival had not been reached, but median PFS was longer for patients treated with the 7-days-on/7-days-off regimen than for those treated with 50 mg/m2/day (6.6 vs. 4.4 months).50 The most common grade 3/4 hematologic toxicity was leukopenia (13%) and thrombocytopenia (6%). This study suggests that the higher daily dose and dose per cycle achieved with the 7-days-on/7-days-off regimen may be more effective than a low metronomic daily dose as maintenance therapy. Analysis of MGMT methylation status is planned.
The largest clinical experience with the 7-days-on/7-days-off regimen in the first-line setting is NOA-08, a combined German-Swiss, randomized, phase III trial of the Neurooncology Working Group within the German Cancer Society.42 This trial is currently investigating the benefit of temozolomide (100 mg/m2/day, days 1–7 and 15–22) until treatment failure versus RT (60 Gy) alone as primary therapy for elderly patients (>65 years old) with GBM or AA and will ultimately enroll 340 patients. Enrollment is ongoing (220 patients had been enrolled as of October 2007). The intriguing aspect of this study is that it compares dose-dense temozolomide alone versus RT alone, but unfortunately, there is no comparison with the standard dosing regimen, and the benefit of this approach in younger patients will not be addressed.
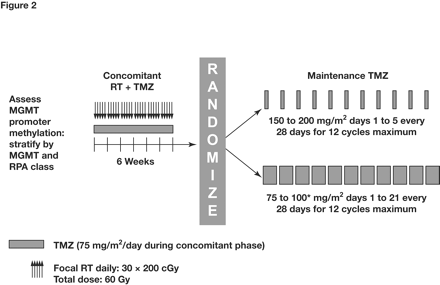
Optimal Maintenance Regimen. With regard to the optimal maintenance regimen, there are as yet no data to suggest that alternative regimens are more effective at delaying disease progression or improving survival than the standard 5-day regimen.35 This question is currently being addressed in the ongoing Radiation Therapy Oncology Group/EORTC trial in newly diagnosed GBM.51,52 This study is testing whether increasing the dose intensity of maintenance temozolomide improves survival compared with the standard regimen (Fig. 2).51,52 Following concomitant RT plus temozolomide, patients will be randomized to standard maintenance temozolomide therapy with 150–200 mg/m2 × 5 days of every 28-day cycle, or to 75–100 mg/m2 × 21 days of every 28-day cycle (21/28-day regimen). Importantly, MGMT promoter methylation status will be available for all randomized patients. It is hoped that this study will provide definitive evidence to either support or refute the hypothesis that dose-dense temozolomide regimens can overcome resistance to alkylating agents.
Study schema for the ongoing Radiation Therapy Oncology Group/European Organisation for Research and Treatment of Cancer trial. MGMT, O6-methylguanine DNA methyltransferase; RPA, recursive partitioning analysis; RT, radiotherapy; TMZ, temozolomide. *Dosing will be initiated at 75 mg/m2 in cycle, with dose escalation to 100 mg/m2 in subsequent cycles if no dose-limiting hematologic toxicity occurs. Data from the National Cancer Institute52 and the European Organisation for Research and Treatment of Cancer.51 Reprinted with permission from Hegi et al.19
Recurrent Glioma
All available published reports of alternative temozolomide dosing schedules that have been investigated in patients with recurrent glioma are summarized in Table 3.30,36,38,39,53–65 In this setting, the 21/28-day and the 7-days-on/7-days-off regimens have been most widely studied, although recent attempts have also been made to improve efficacy by using a compressed twice-daily dosing regimen on the 5/28-day schedule.53 In most cases, the patients enrolled in these studies had not been previously treated with temozolomide, and in other studies, patients treated with standard RT plus temozolomide at initial diagnosis were rechallenged with temozolomide at recurrence. This is important given that patients with high-grade glioma are now commonly exposed to temozolomide at initial diagnosis and may develop resistance at the time of recurrence. Therefore, the question of whether dose-dense temozolomide regimens can overcome resistance is even more relevant today in the recurrent disease setting.
Clinical studies of alternative temozolomide dosing regimens in patients with recurrent glioma
| Study . | n . | Patient Population . | Schedule . | Median PFS, Months . | 6-Month PFS, Rate . | Median OS, Months . |
|---|---|---|---|---|---|---|
| Berrocal et al. (2006)54 | 29 | Glioma | 85 mg/m2 days 1–21 q28d | 2 | NA | 6 |
| Balmaceda et al. (2008)53 | 120 | GBM (n = 68) | 200 mg/m2 loading dose on day 1 followed by nine doses at 90–100 mg/m2 q12h | 4 (GBM) | 35% (GBM) | 9 (GBM) |
| AA (n = 28) | 6 (AA) | 50% (AA) | 15 (AA) | |||
| AO (n = 24) | 8 (AO) | 58% (AO) | 18 (AO) | |||
| Brandes et al. (2005, 2006)55,56 | 33 | GBM | 75 mg/m2 days 1–21 q28d | NA | 30% | NA |
| Caroli et al. (2007)36 | 30 | GBM | 150 mg/m2 days 1–5, then 75 mg/m2 days 6–10 q28d | 6 | NA | 14 |
| Combs et al. (2005, 2007)38,39 | 17 | GBM, AA, astrocytoma, and gliosarcoma | Reirradiation RT concomitant with TMZ (50 mg/m2/d) | NA | 56% | 39 |
| 14 patients also received adjuvant TMZ | ||||||
| D'Amico et al. (2006)57 | 10 | GBM | 50 mg/m2 days 1–7 and 15–21 q28d cycle 1, then escalation in 25 mg/m2 increments to 150 mg/m2 vs. 150 mg/m2 days 1–5 q28d (cycle 1), then 200 mg/m2 days 1–5 q28d | NA | NA | 21 |
| 10 | 14 | |||||
| Donfrancesco et al. (2005)58 | 17 (pediatric) | Neuroblastoma | 180 or 215 mg/m2 days 1–5 q28d | 3.4 | NA | 8 |
| Jauch et al. (2007)59 | 45 | GBM (n = 23) | Rechallenged with one of following: 150–200 mg/m2 days 1–5 q28d (n = 35) 150 mg/m2 days 1–7 q28d (n = 7) 75 mg/m2 days 1–21 q28d (n = 4) 50 mg/m2/day (n = 7) | NA | 41% (GBM) | NA |
| AG (n = 22) | 43% (AG) | |||||
| Khan et al. (2002)60 | 35 | 28 GBM, 3 AA, 2 AO, 2 oligodendroglioma | 75 mg/m2 days 1–42 q70d | 2.5 | NA | 8.7 |
| Neyns et al. (2008)61 | 19 | AA and AO | 100 mg/m2 days 1–21 q28d | NA | 56% | 12.9 |
| Strik et al. (2007)62 | 13 | GBM (n = 11) | First-line: RT + TMZ 200 mg/m2 days 1–5 q28d | NA | 55% (GBM) | |
| AA, AO | Second-line: 8 of 13 patients switched to dose-dense regimen (75–130 mg/m2 days 1–21 q28d) individually tailored to stay below target hematologic toxicity | 44% (AA, AO) | 18 | |||
| Wen et al. (2005)63 | 29 | Glioma | 75 mg/m2 days 1–49 q77d | > 16 | NA | NA |
| Wick et al. (2007)30 | 90 | GBM (n = 64) | 150 mg/m2/day days 1–7 and 15–21 q28d | 6 | 44% (GBM) | 9.5 from progression |
| AG (n = 11) | ||||||
| Low-grade (n = 9) | ||||||
| Other (n = 6) | ||||||
| Wick et al. (2004)65 | 21 | Recurrent GBM | 150 mg/m2 days 1–7 and 15–21 q28d | 5 | 48% | NA |
| Study . | n . | Patient Population . | Schedule . | Median PFS, Months . | 6-Month PFS, Rate . | Median OS, Months . |
|---|---|---|---|---|---|---|
| Berrocal et al. (2006)54 | 29 | Glioma | 85 mg/m2 days 1–21 q28d | 2 | NA | 6 |
| Balmaceda et al. (2008)53 | 120 | GBM (n = 68) | 200 mg/m2 loading dose on day 1 followed by nine doses at 90–100 mg/m2 q12h | 4 (GBM) | 35% (GBM) | 9 (GBM) |
| AA (n = 28) | 6 (AA) | 50% (AA) | 15 (AA) | |||
| AO (n = 24) | 8 (AO) | 58% (AO) | 18 (AO) | |||
| Brandes et al. (2005, 2006)55,56 | 33 | GBM | 75 mg/m2 days 1–21 q28d | NA | 30% | NA |
| Caroli et al. (2007)36 | 30 | GBM | 150 mg/m2 days 1–5, then 75 mg/m2 days 6–10 q28d | 6 | NA | 14 |
| Combs et al. (2005, 2007)38,39 | 17 | GBM, AA, astrocytoma, and gliosarcoma | Reirradiation RT concomitant with TMZ (50 mg/m2/d) | NA | 56% | 39 |
| 14 patients also received adjuvant TMZ | ||||||
| D'Amico et al. (2006)57 | 10 | GBM | 50 mg/m2 days 1–7 and 15–21 q28d cycle 1, then escalation in 25 mg/m2 increments to 150 mg/m2 vs. 150 mg/m2 days 1–5 q28d (cycle 1), then 200 mg/m2 days 1–5 q28d | NA | NA | 21 |
| 10 | 14 | |||||
| Donfrancesco et al. (2005)58 | 17 (pediatric) | Neuroblastoma | 180 or 215 mg/m2 days 1–5 q28d | 3.4 | NA | 8 |
| Jauch et al. (2007)59 | 45 | GBM (n = 23) | Rechallenged with one of following: 150–200 mg/m2 days 1–5 q28d (n = 35) 150 mg/m2 days 1–7 q28d (n = 7) 75 mg/m2 days 1–21 q28d (n = 4) 50 mg/m2/day (n = 7) | NA | 41% (GBM) | NA |
| AG (n = 22) | 43% (AG) | |||||
| Khan et al. (2002)60 | 35 | 28 GBM, 3 AA, 2 AO, 2 oligodendroglioma | 75 mg/m2 days 1–42 q70d | 2.5 | NA | 8.7 |
| Neyns et al. (2008)61 | 19 | AA and AO | 100 mg/m2 days 1–21 q28d | NA | 56% | 12.9 |
| Strik et al. (2007)62 | 13 | GBM (n = 11) | First-line: RT + TMZ 200 mg/m2 days 1–5 q28d | NA | 55% (GBM) | |
| AA, AO | Second-line: 8 of 13 patients switched to dose-dense regimen (75–130 mg/m2 days 1–21 q28d) individually tailored to stay below target hematologic toxicity | 44% (AA, AO) | 18 | |||
| Wen et al. (2005)63 | 29 | Glioma | 75 mg/m2 days 1–49 q77d | > 16 | NA | NA |
| Wick et al. (2007)30 | 90 | GBM (n = 64) | 150 mg/m2/day days 1–7 and 15–21 q28d | 6 | 44% (GBM) | 9.5 from progression |
| AG (n = 11) | ||||||
| Low-grade (n = 9) | ||||||
| Other (n = 6) | ||||||
| Wick et al. (2004)65 | 21 | Recurrent GBM | 150 mg/m2 days 1–7 and 15–21 q28d | 5 | 48% | NA |
Abbreviations: PFS, progression-free survival; OS, overall survival; qXd, every X days; NA, not available; Dx, diagnosed; GBM, glioblastoma; AA, anaplastic astrocytoma; AO, anaplastic oligoastrocytoma; q12h, every 12 h; RT, radiotherapy; TMZ, temozolomide; AG, anaplastic glioma
Clinical studies of alternative temozolomide dosing regimens in patients with recurrent glioma
| Study . | n . | Patient Population . | Schedule . | Median PFS, Months . | 6-Month PFS, Rate . | Median OS, Months . |
|---|---|---|---|---|---|---|
| Berrocal et al. (2006)54 | 29 | Glioma | 85 mg/m2 days 1–21 q28d | 2 | NA | 6 |
| Balmaceda et al. (2008)53 | 120 | GBM (n = 68) | 200 mg/m2 loading dose on day 1 followed by nine doses at 90–100 mg/m2 q12h | 4 (GBM) | 35% (GBM) | 9 (GBM) |
| AA (n = 28) | 6 (AA) | 50% (AA) | 15 (AA) | |||
| AO (n = 24) | 8 (AO) | 58% (AO) | 18 (AO) | |||
| Brandes et al. (2005, 2006)55,56 | 33 | GBM | 75 mg/m2 days 1–21 q28d | NA | 30% | NA |
| Caroli et al. (2007)36 | 30 | GBM | 150 mg/m2 days 1–5, then 75 mg/m2 days 6–10 q28d | 6 | NA | 14 |
| Combs et al. (2005, 2007)38,39 | 17 | GBM, AA, astrocytoma, and gliosarcoma | Reirradiation RT concomitant with TMZ (50 mg/m2/d) | NA | 56% | 39 |
| 14 patients also received adjuvant TMZ | ||||||
| D'Amico et al. (2006)57 | 10 | GBM | 50 mg/m2 days 1–7 and 15–21 q28d cycle 1, then escalation in 25 mg/m2 increments to 150 mg/m2 vs. 150 mg/m2 days 1–5 q28d (cycle 1), then 200 mg/m2 days 1–5 q28d | NA | NA | 21 |
| 10 | 14 | |||||
| Donfrancesco et al. (2005)58 | 17 (pediatric) | Neuroblastoma | 180 or 215 mg/m2 days 1–5 q28d | 3.4 | NA | 8 |
| Jauch et al. (2007)59 | 45 | GBM (n = 23) | Rechallenged with one of following: 150–200 mg/m2 days 1–5 q28d (n = 35) 150 mg/m2 days 1–7 q28d (n = 7) 75 mg/m2 days 1–21 q28d (n = 4) 50 mg/m2/day (n = 7) | NA | 41% (GBM) | NA |
| AG (n = 22) | 43% (AG) | |||||
| Khan et al. (2002)60 | 35 | 28 GBM, 3 AA, 2 AO, 2 oligodendroglioma | 75 mg/m2 days 1–42 q70d | 2.5 | NA | 8.7 |
| Neyns et al. (2008)61 | 19 | AA and AO | 100 mg/m2 days 1–21 q28d | NA | 56% | 12.9 |
| Strik et al. (2007)62 | 13 | GBM (n = 11) | First-line: RT + TMZ 200 mg/m2 days 1–5 q28d | NA | 55% (GBM) | |
| AA, AO | Second-line: 8 of 13 patients switched to dose-dense regimen (75–130 mg/m2 days 1–21 q28d) individually tailored to stay below target hematologic toxicity | 44% (AA, AO) | 18 | |||
| Wen et al. (2005)63 | 29 | Glioma | 75 mg/m2 days 1–49 q77d | > 16 | NA | NA |
| Wick et al. (2007)30 | 90 | GBM (n = 64) | 150 mg/m2/day days 1–7 and 15–21 q28d | 6 | 44% (GBM) | 9.5 from progression |
| AG (n = 11) | ||||||
| Low-grade (n = 9) | ||||||
| Other (n = 6) | ||||||
| Wick et al. (2004)65 | 21 | Recurrent GBM | 150 mg/m2 days 1–7 and 15–21 q28d | 5 | 48% | NA |
| Study . | n . | Patient Population . | Schedule . | Median PFS, Months . | 6-Month PFS, Rate . | Median OS, Months . |
|---|---|---|---|---|---|---|
| Berrocal et al. (2006)54 | 29 | Glioma | 85 mg/m2 days 1–21 q28d | 2 | NA | 6 |
| Balmaceda et al. (2008)53 | 120 | GBM (n = 68) | 200 mg/m2 loading dose on day 1 followed by nine doses at 90–100 mg/m2 q12h | 4 (GBM) | 35% (GBM) | 9 (GBM) |
| AA (n = 28) | 6 (AA) | 50% (AA) | 15 (AA) | |||
| AO (n = 24) | 8 (AO) | 58% (AO) | 18 (AO) | |||
| Brandes et al. (2005, 2006)55,56 | 33 | GBM | 75 mg/m2 days 1–21 q28d | NA | 30% | NA |
| Caroli et al. (2007)36 | 30 | GBM | 150 mg/m2 days 1–5, then 75 mg/m2 days 6–10 q28d | 6 | NA | 14 |
| Combs et al. (2005, 2007)38,39 | 17 | GBM, AA, astrocytoma, and gliosarcoma | Reirradiation RT concomitant with TMZ (50 mg/m2/d) | NA | 56% | 39 |
| 14 patients also received adjuvant TMZ | ||||||
| D'Amico et al. (2006)57 | 10 | GBM | 50 mg/m2 days 1–7 and 15–21 q28d cycle 1, then escalation in 25 mg/m2 increments to 150 mg/m2 vs. 150 mg/m2 days 1–5 q28d (cycle 1), then 200 mg/m2 days 1–5 q28d | NA | NA | 21 |
| 10 | 14 | |||||
| Donfrancesco et al. (2005)58 | 17 (pediatric) | Neuroblastoma | 180 or 215 mg/m2 days 1–5 q28d | 3.4 | NA | 8 |
| Jauch et al. (2007)59 | 45 | GBM (n = 23) | Rechallenged with one of following: 150–200 mg/m2 days 1–5 q28d (n = 35) 150 mg/m2 days 1–7 q28d (n = 7) 75 mg/m2 days 1–21 q28d (n = 4) 50 mg/m2/day (n = 7) | NA | 41% (GBM) | NA |
| AG (n = 22) | 43% (AG) | |||||
| Khan et al. (2002)60 | 35 | 28 GBM, 3 AA, 2 AO, 2 oligodendroglioma | 75 mg/m2 days 1–42 q70d | 2.5 | NA | 8.7 |
| Neyns et al. (2008)61 | 19 | AA and AO | 100 mg/m2 days 1–21 q28d | NA | 56% | 12.9 |
| Strik et al. (2007)62 | 13 | GBM (n = 11) | First-line: RT + TMZ 200 mg/m2 days 1–5 q28d | NA | 55% (GBM) | |
| AA, AO | Second-line: 8 of 13 patients switched to dose-dense regimen (75–130 mg/m2 days 1–21 q28d) individually tailored to stay below target hematologic toxicity | 44% (AA, AO) | 18 | |||
| Wen et al. (2005)63 | 29 | Glioma | 75 mg/m2 days 1–49 q77d | > 16 | NA | NA |
| Wick et al. (2007)30 | 90 | GBM (n = 64) | 150 mg/m2/day days 1–7 and 15–21 q28d | 6 | 44% (GBM) | 9.5 from progression |
| AG (n = 11) | ||||||
| Low-grade (n = 9) | ||||||
| Other (n = 6) | ||||||
| Wick et al. (2004)65 | 21 | Recurrent GBM | 150 mg/m2 days 1–7 and 15–21 q28d | 5 | 48% | NA |
Abbreviations: PFS, progression-free survival; OS, overall survival; qXd, every X days; NA, not available; Dx, diagnosed; GBM, glioblastoma; AA, anaplastic astrocytoma; AO, anaplastic oligoastrocytoma; q12h, every 12 h; RT, radiotherapy; TMZ, temozolomide; AG, anaplastic glioma
21/28-Day Regimen. Several studies have tested the 21/28-day schedule at doses ranging from 75 to 100 mg/m2/day. An Italian phase II cooperative group study reported by Brandes et al.55,56 investigated the safety and efficacy of this regimen at a dose of 75 mg/m2/day in 33 chemotherapy-naive patients with recurrent GBM. The regimen was fairly well tolerated: only 18% of patients developed grade 3 lymphopenia, no patients developed grade 3/4 thrombocytopenia, and no Pneumocystis jiroveci pneumonia or other opportunistic infections were reported. However, lymphopenia was cumulative, and nearly all patients who received more than nine cycles developed lymphopenia. They reported a 6-month PFS rate of 30%. Similarly, a small Belgian phase II study investigated the 21/28-day schedule at a dose of 100 mg/m2/day in 19 patients at first recurrence of AA or anaplastic oligoastrocytoma, and none of these patients had been previously treated with temozolomide.61 These investigators observed a high incidence of cumulative grade 3/4 lymphopenia (100%), and three patients developed profound and prolonged pancytopenia during the first cycle, suggesting that this dose may be too high for patients who are intrinsically susceptible because of low MGMT levels in normal tissue.61 Therefore, the authors suggested a starting dose of 75 mg/m2/day with escalation to 100 mg/m2/day if well tolerated; regular monitoring of WBC counts and prophylaxis against opportunistic infections also may be warranted. Although this was a small study,61 56% of patients were free of progression at 6 months, which is encouraging considering that none of the patients enrolled had loss of chromosome 1p or 19q in their tumor (a positive prognostic factor).
Other studies have examined the activity of the 21/28-day regimen in patients previously exposed to temozolomide. For example, Jauch et al.59 reported a retrospective analysis of 45 patients with recurrent GBM (n = 23) or AA (n = 22) who had all progressed during or after completing first-line temozolomide. The majority were retreated with the standard 5-day regimen, but seven received the 7-days-on/7-days-off regimen at 150 mg/m2/day, four received the 21/28-day regimen at 75 mg/m2/day, and seven received continuous daily temozolomide at 50 mg/m2/day. They reported that 8 of 12 patients who progressed during first-line temozolomide and who were subsequently treated with one of these alternative regimens had an objective response or stable disease. Six-month PFS was 41% and 43% for patients with GBM and AA, respectively. The approach of rechallenge with continuous low-dose temozolomide at 50 mg/m2/day has been taken further by a large multi center Canadian trial (RESCUE), which has completed accrual and will be reported soon.64 This and other studies suggest that rechallenge with a dose-dense temozolomide regimen can result in good responses, but there have not been any direct prospective comparisons of different regimens in this setting.
Seven-Days-On/Seven-Days-Off Regimen. It has been suggested that intermittent dosing may reduce the frequency and severity of hematologic toxicity compared with more extended dosing schedules such as the 21/28-day or extended daily schedules. Several studies have tested the 7-days-on/7-days-off regimen in the recurrent setting. Our experience with this regimen includes a phase II study in 21 patients with recurrent GBM65 and a larger phase II study in 90 patients with recurrent glioma (the majority of whom had not been previously treated with temozolomide).30 In both of these studies, we found that this regimen was well tolerated at a dose of 150 mg/m2/day. With appropriate dose reductions guided by WBC counts, there was no cumulative lymphopenia, and the overall incidence of grade 4 lymphopenia was 12%.30,65,66 Moreover, no patient developed an opportunistic infection.30 In the small phase II study in patients with recurrent GBM, this regimen produced a response rate of 9.5%, a 6-month PFS rate of 48%, and a median PFS of 21 weeks (∼5 months).65 In our subsequent phase II trial in a larger group of patients that included GBM (n = 64), anaplastic glioma (n = 11), low-grade glioma (n = 9), and other brain tumors (n = 6), the same regimen was applied with individual dose adjustments according to hematologic toxicity.30 Grade 4 lymphopenia occurred in 12% of patients. Among patients with GBM, median PFS was 6 months, and the 6-month PFS rate was 44%. Analyses of MGMT promoter methylation were also performed on available tumor tissue from 36 patients with GBM; 17 patients had a methylated promoter, and 19 had an unmethylated promoter.30 Median PFS was not significantly different between patients with a methylated or unmethylated MGMT promoter (27 vs. 19 weeks; p = 0.22). Moreover, median survival from diagnosis was similar in these two subgroups. Although the sample size was small, this is the first evidence to suggest that a dose-dense temozolomide regimen may improve clinical outcomes in patients with an unmethylated MGMT promoter, potentially by depleting MGMT in tumor tissue. For comparison, among newly diagnosed GBM patients in the EORTC/NCIC trial who were treated with temozolomide, Hegi et al.14 observed a median survival of 22 months for patients with a methylated MGMT promoter and 13 months for patients with an unmethylated promoter.
The only study that directly compared the 7-days-on/7-days-off regimen with the standard 5-day regimen in the recurrent setting was reported by D'Amico et al.57 Unfortunately, this was a very small study involving only 20 patients with GBM who progressed after surgery and RT. The dose used in the 7-days-on/7-days-off regimen was initiated at 50 mg/m2/day and escalated up to 150 mg/m2/day, and the investigators reported that this was well tolerated. Median survival was 21 months for patients treated with the 7-days-on/7-days-off regimen versus 14 months for patients treated with the standard 5-day regimen.57 Although this was a small study, it suggests that the 7-days-on/7-days-off regimen may be more effective in this setting.
Discussion
Resistance to alkylating agents remains a critical barrier to the effective treatment of malignant glioma. This has been most clearly demonstrated by studies showing that patients with an unmethylated MGMT promoter or higher levels of MGMT protein in their tumors are less likely to respond to alkylating agents and have shorter survival compared with patients with a methylated MGMT promoter or lower levels of MGMT protein. These clinical observations have spawned a concerted research effort to better understand the mechanisms of resistance to alkylating agents and to simultaneously design strategies to overcome chemoresistance in malignant glioma. In particular, the emergence of temozolomide as a highly effective agent for the treatment of high-grade glioma has provided opportunities to exploit the flexibility of this oral agent and to design dosing regimens with the potential to deplete MGMT and increase the cytotoxic activity of the drug. The depletion of MGMT by prolonged exposure to temozolomide appears to be a promising strategy to overcome resistance, and several alternative dose-dense temozolomide regimens have been shown to effectively deplete MGMT in PBMCs. However, it remains unclear how effectively these regimens can deplete MGMT activity in tumor tissue, and dose-limiting hematologic toxicity remains an issue. A variety of alternative temozolomide dosing regimens have been investigated for the treatment of glioma in both the front-line and recurrent settings. These regimens appear to be effective and may improve clinical outcomes; however, there is a paucity of prospective studies comparing these alternative regimens with the standard 5-day regimen, and there are as yet few data to suggest that they can overcome MGMT-mediated resistance. Given the impact of MGMT promoter methylation on outcome in these trials, it is critical to include MGMT promoter methylation analysis in all future studies. Some studies will include MGMT promoter methylation status as an inclusion criteria and hence stratify the study population based on this molecular marker (e.g., EORTC 26071/22072 and 26082/22081). Large-scale trials are under way that may provide some answers to a variety of unanswered questions.
Financial support for medical editorial assistance was provided by Schering-Plough International. We thank Jeff Riegel of ProEd Communications, Inc. for his medical editorial assistance with the manuscript.
References
Yung WK, Albright RE, Olson J, et al. A phase II study of temozolomide vs. procarbazine in patients with glioblastoma multiforme at first relapse.
Yung WK, Prados MD, Yaya-Tur R, et al. Multicenter phase II trial of temozolomide in patients with anaplastic astrocytoma or anaplastic oligoastrocytoma at first relapse.
Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma.
Stevens MFG, Hickman JA, Langdon SP, et al. Antitumor activity and pharmacokinetics in mice of 8-carbamoyl-3-methyl-imidazo[5,1-d]-1,2,3,5-tetrazin-4(3H)-one (CCRG 81045; M & B 39831), a novel drug with potential as an alternative to dacarbazine.
Newlands ES, blackledge GR, Slack JA, et al. Phase I trial of temozolomide (CCRG 81045: M&B 39831: NSC 362856).
Newlands ES, Stevens MFG, Wedge SR, Wheelhouse RT, Brock C. Temozolomide: a review of its discovery, chemical properties, preclinical development and clinical trials.
Brock CS, Newlands ES, Wedge SR, et al. Phase I trial of temozolomide using an extended continuous oral schedule.
Liu L, Taverna P, Whitacre CM, Chatterjee S, Gerson SL. Pharmacologic disruption of base excision repair sensitizes mismatch repair-deficient and -proficient colon cancer cells to methylating agents.
Trivedi RN, Almeida KH, Fornsaglio JL, Schamus S, Sobol RW. The role of base excision repair in the sensitivity and resistance to temozolomide-mediated cell death.
Tentori L, Graziani G. Pharmacological strategies to increase the anti-tumor activity of methylating agents.
Bignami M, O'Driscoll M, Aquilina G, Karran P. Unmasking a killer: DNA O(6)-methylguanine and the cytotoxicity of methylating agents.
Roos WP, Batista LF, Naumann SC, et al. Apoptosis in malignant glioma cells triggered by the temozolomide-induced DNA lesion O6-methylguanine.
Stupp R, Dietrich P-Y, Ostermann Kraljevic S, et al. Promising survival for patients with newly diagnosed glioblastoma multiforme treated with concomitant radiation plus temozolomide followed by adjuvant temozolomide.
Hegi ME, Diserens A-C, Gorlia T, et al. MGMT gene silencing and benefit from temozolomide in glioblastoma.
Esteller M, Garcia-Foncillas J, Andion E, et al. Inactivation of the DNA-repair gene MGMT and the clinical response of gliomas to alkylating agents.
Kamiryo T, Tada K, Shiraishi S, Shinojima N, Kochi M, Ushio Y. Correlation between promoter hypermethylation of the O6-methylguaninedeoxyribonucleic acid methyltransferase gene and prognosis in patients with high-grade astrocytic tumors treated with surgery, radiotherapy, and 1-(4-amino-2-methyl-5-pyrimidinyl)methyl-3-(2-chloroethyl)-3-nitrosourea-based chemotherapy.
Pegg AE, Dolan ME, Moschel RC. Structure, function, and inhibition of O6-alkylguanine-DNA alkyltransferase.
Tano K, Shiota S, Collier J, Foote RS, Mitra S. Isolation and structural characterization of a cDNA clone encoding the human DNA repair protein for O6-alkylguanine.
Hegi ME, Liu L, Herman JG, et al. Correlation of O6-methylguanine methyltransferase (MGMT) promoter methylation with clinical outcomes in glioblastoma and clinical strategies to modulate MGMT activity.
Esteller M, Hamilton SR, Burger PC, Baylin SB, Herman JG. Inactivation of the DNA repair gene O6-methylguanine-DNA methyltransferase by promoter hypermethylation is a common event in primary human neoplasia.
Watts GS, Pieper RO, Costello JF, Peng Y-M, Dalton WS, Futscher BW. Methylation of discrete regions of the O 6-methylguanine DNA methyltransferase (MGMT) CpG island is associated with heterochromatinization of the MGMT transcription start site and silencing of the gene.
Blanc JL, Wager M, Guilhot J, et al. Correlation of clinical features and methylation status of MGMT gene promoter in glioblastomas.
Gonzalez-Gomez P, Bello MJ, Arjona D, et al. Promoter hypermethylation of multiple genes in astrocytic gliomas.
Hotta T, Saito Y, Fujita H, et al. O6-alkylguanine-DNA alkyltransferase activity of human malignant glioma and its clinical implications.
Stupp R, Hegi ME, van den Bent MJ, et al. Changing paradigms—an update on the multidisciplinary management of malignant glioma.
Spiro TP, Gerson SL, Liu L, et al. O6-benzylguanine: a clinical trial establishing the biochemical modulatory dose in tumor tissue for alkyltransferase-directed DNA repair.
Spiro TP, Liu L, Majka S, Haaga J, Willson JK, Gerson SL. Temozolomide: the effect of once- and twice-a-day dosing on tumor tissue levels of the DNA repair protein O 6-alkylguanine-DNA-alkyltransferase.
Lee SM, Thatcher N, Crowther D, Margison GP. Inactivation of O6-alkylguanine-DNA alkyltransferase in human peripheral blood mononuclear cells by temozolomide.
Schold SC Jr, Kokkinakis DM, Chang SM, et al. O 6-benzylguanine suppression of O 6-alkylguanine-DNA alkyltransferase in anaplastic gliomas.
Wick A, Felsberg J, Steinbach JP, et al. Efficacy and tolerability of temozolomide in an alternating weekly regimen in patients with recurrent glioma.
Middleton MR, Lee SM, Arance A, Wood M, Thatcher N, Margison GP. O6-methylguanine formation, repair protein depletion and clinical outcome with a 4 hr schedule of temozolomide in the treatment of advanced melanoma: results of a phase II study.
Su YB, Sohn S, Krown SE, et al. Selective CD4+ lymphopenia in melanoma patients treated with temozolomide: a toxicity with therapeutic implications.
Tolcher AW, Gerson SL, Denis L, et al. Marked inactivation of O 6-alkylguanine-DNA alkyltransferase activity with protracted temozolomide schedules.
Athanassiou H, Synodinou M, Maragoudakis E, et al. Randomized phase II study of temozolomide and radiotherapy compared with radiotherapy alone in newly diagnosed glioblastoma multiforme.
Buttolo L, Giunta F, Ferrari VD, Grisanti S, Marini G, Mortini P. Alternative schedules of adjuvant temozolomide in glioblastoma multiforme: a 6-year experience [abstract 1511].
Caroli M, Locatelli M, Campanella R, et al. Temozolomide in glioblastoma: results of administration at first relapse and in newly diagnosed cases. Is still proposable an alternative schedule to concomitant protocol?
Chinot OL, Barrie M, Fuentes S, et al. Correlation between O6-methylguanine-DNA methyltransferase and survival in inoperable newly diagnosed glioblastoma patients treated with neoadjuvant temozolomide.
Combs SE, Gutwein S, Schulz-Ertner D, et al. Temozolomide combined with irradiation as postoperative treatment of primary glioblastoma multiforme. Phase I/II study.
Combs SE, Schulz-Ertner D, Welzel T, Bischof M, Debus J. Re-irradiation using high precision radiotherapy and concomitant temozolomide in patients with recurrent glioma: re-challenge with radio-chemotherapy [abstract 12517].
D'Agostino G, Balducci M, Anile C, et al. An analysis of two different schedules of radiochemotherapy with concomitant temozolomide in high grade gliomas [abstract 2035].
Montemaggi P, Guerrieri P, Mortellaro G, Piazza D, Rizzo S. Temozolomide (TMZ) in radio-chemotherapy combined schedule for treatment of newly-diagnosed high-grade gliomas(HGG) [abstract 1578].
Neurooncological Working Group of the German Cancer Society.
Sul J, Panageas KS, Lassman AB, et al. A randomized phase II trial of concurrent temozolomide (TMZ) and radiotherapy (RT) followed by dose dense compared to metronomic TMZ and maintenance cisretinoic acid for patients with newly diagnosed glioblastoma multiforme (GBM) [abstract 2031].
Zorlu F, Sari S, Özyigit G, et al.
Chinot O, Barrié M, Cournède A, et al. Phase II study of temozolomide (TMZ) administered on a 7 days on-7days off regimen as primary treatment before radiotherapy (RT) in inoperable newly diagnosed glioblastoma multiforme (GBM) [abstract 1523].
Curran WJ Jr, Scott CB, Horton J, et al. Recursive partitioning analysis of prognostic factors in three Radiation Therapy Oncology Group malignant glioma trials.
Brada M, Ashley S, Dowe A, et al. Neoadjuvant phase II multicentre study of new agents in patients with malignant glioma after minimal surgery. Report of a cohort of 187 patients treated with temozolomide.
Friedman HS, McLendon RE, Kerby T, et al. DNA mismatch repair and O6-alkylguanine-DNA alkyltransferase analysis and response to Temodal in newly diagnosed malignant glioma.
Gilbert MR, Friedman HS, Kuttesch JF, et al. A phase II study of temozolomide in patients with newly diagnosed supratentorial malignant glioma before radiation therapy.
Clarke J, Sul J, DeAngelis L, et al. A randomized phase II trial of concurrent temozolomide (TMZ) and radiotherapy (RT) followed by dose-dense compared to metronomic TMZ and maintenance cis-retinoic acid for patients with newly diagnosed glioblastoma multiforme (GBM) [Abstract MA-557].
European Organisation for Research and Treatment of Cancer.
National Cancer Institute.
Balmaceda C, Peereboom D, Pannullo S, et al. Multi-institutional phase II study of temozolomide administered twice daily in the treatment of recurrent high-grade gliomas.
Berrocal A, Perez-Segura P, Gil M, et al. Phase II study of extended schedule temozolomide in refractory gliomas [abstract 1516].
Brandes AA, Tosoni A, Cavallo G, et al. Temozolomide 3 weeks on and 1 week off as first-line therapy for recurrent glioblastoma: phase II study from Gruppo Italiano Cooperativo di Neuro-oncologia (GICNO).
Brandes AA, Cavallo G, Tosoni A, et al. Temozolomide (TMZ) for progressive primitive brain tumors: safety at 75 mg/m2 a day for 21 days every 28: a GICNO (Italian Neuro-Oncology Group) study [abstract 1541].
D'Amico A, Gabbani M, Dall'oglio S, et al. Protracted administration of low doses of temozolomide (TMZ) in the treatment of relapse glioblastoma (GBM) enhances the antitumor activity of this agent [abstract 1572].
Donfrancesco A, Milano GM, Jenkner A, et al. Temozolomide in resistant or relapsed neuroblastoma [abstract 8522].
Jauch T, Hau P, Bogdahn U. Re-challenge with temozolomide (TMZ) at recurrence in high-grade gliomas (HGG) [abstract 2034].
Khan RB, Raizer JJ, Malkin MG, Bazylewicz KA, Abrey LE. A phase II study of extended low-dose temozolomide in recurrent malignant gliomas.
Neyns B, Chaskis C, Joosens E, et al. A multicenter cohort study of dose-dense temozolomide (21 of 28 days) for the treatment of recurrent anaplastic astrocytoma or oligoastrocytoma.
Strik HM, Buhk JH, Bock HC, Nitsche M, Giese A, Baehr M. Rechallenge of malignant gliomas with temozolomide—can it be effective? [abstract 2072].
Wen PY, Schiff D, Doherty L, et al. A phase II study of prolonged daily temozolomide for low-grade glioma [abstract 1522].
Perry JR, Mason WP, Belanger K, et al. The temozolomide RESCUE study: A phase II trial of continuous (28/28) dose-intensive temozolomide (TMZ) after progression on conventional 5/28 day TMZ in patients with recurrent malignant glioma [abstr. 2010].
Wick W, Steinbach JP, Kuker WM, Dichgans J, Bamberg M, Weller M. One week on/one week off: a novel active regimen of temozolomide for recurrent glioblastoma.