-
PDF
- Split View
-
Views
-
Cite
Cite
Terri S. Armstrong, Yumei Cao, Michael E. Scheurer, Elizabeth Vera-Bolaños, Rochelle Manning, Mehmet F. Okcu, Melissa Bondy, Renke Zhou, Mark R. Gilbert, Risk analysis of severe myelotoxicity with temozolomide: The effects of clinical and genetic factors, Neuro-Oncology, Volume 11, Issue 6, December 2009, Pages 825–832, https://doi.org/10.1215/15228517-2008-120
Close - Share Icon Share
Abstract
A benefit of temozolomide (TMZ) is that myelotoxicity is uncommon. Recently, several small series have reported significant myelotoxicity resulting in treatment delays or death. The ability to predict risk of myelotoxicity may influence patient care. We retrospectively reviewed 680 malignant glioma patients and developed a clinical risk formula for myelotoxicity for each gender by logistic regression. The variables that remained were assigned a score of 1 and added together for a final risk score. Women experienced more myelotoxicity than did men (p = 0.015). For males, risk factors included body surface area (BSA) ⩾ 2 m2 (odds ratio [OR] = 2.712, p = 0.04), not on steroids (OR = 2.214, p = 0.06), and on bowel medication (OR = 3.955, p = 0.008). For females, final factors included no prior chemotherapy (OR = 3.727, p = 0.001), creatinine ⩾ 1 mg/dl (OR = 6.08, p = 0.002), platelets < 270,000/mm3 (OR = 2.438, p = 0.03), BSA < 2 m2 (OR = 4.178, p = 0.04), not on medication for gastroesophageal reflux disease (OR = 2.942, p = 0.01), and on analgesics (OR = 2.169, p = 0.05). Age was included because of observable trends. Risk of developing myelotoxicity ranged from 0% to 33% (male) and from 0% to 100% (females). Polymorphisms in NQO1 (NAD(P)H dehydrogenase, quinone 1), MGMT (O6-methylguanine-DNA methyltransferase), and GSTP1 (glutathione S-transferase pi 1) were related to risk of developing myelotoxicity in a subset of patients. Myelotoxicity with TMZ is a significant clinical issue for those at risk. Use of a clinical model to predict risk and evaluation of identified genetic polymorphisms related to myelotoxicity may allow for individualized dosing, optimizing patient management.
Gliomas are the most frequently occurring primary brain tumors in adults. The prognosis remains poor, particularly for patients diagnosed with glioblastoma multiforme (GBM), where the median survival is reported to be 12–14 months. Standard treatment approaches include the use of surgery, radiation therapy, and chemotherapy. The most commonly prescribed chemotherapy is temozolomide (TMZ), an oral alkylating agent with demonstrated safety and efficacy in patients with either recurrent or newly diagnosed malignant gliomas.1,2
TMZ is rapidly and completely absorbed after oral administration. Peak plasma concentrations occur in 1 h, and the rate and extent of absorption are reduced by food. TMZ metabolism has not been shown to be affected by mild to moderate renal or hepatic dysfunction. In addition, population analysis did not demonstrate any influence on drug clearance with coadministration of dexamethasone, prochlorperazine, phenytoin, carbamazepine, ondansetron, H2-receptor antagonists, or phenobarbital. These findings suggest that cytochrome P450 enzymes play only a minimal role in the metabolism of TMZ. TMZ is spontaneously hydrolyzed at physiologic pH to 3-methyl-(triazen-1-yl) imidazole-4-carboxamide (MTIC), the probable active moiety, and to a TMZ acid metabolite. MTIC is further hydrolyzed to 5-aminoimidazole-4-carboxamide and to methylhydrazine. Thirty-eight percent of administered TMZ total radioactive dose is recovered over 7 days, with 37.7% in the urine and 0.8% in the feces.3
Although myelosuppression is a dose-limiting toxicity of most cytotoxic chemotherapies, a reported benefit of TMZ is that myelosuppression is relatively uncommon. Most studies report an overall incidence of 5%–8% for grade 3/4 myelotoxicity. In the initial trial of 445 cancer patients treated with TMZ, a higher incidence (11%) of significant myelotoxicity was reported in females. Older female patients who received higher doses had a greater chance of developing both neutropenia and thrombocytopenia. However, the reported low incidence and severity of myelotoxicity in early trials of TMZ have led to health care providers considering the agent well tolerated by patients.
Recently, several case studies and small series have reported significant myelotoxicity with TMZ, including thrombocytopenia, neutropenia, and rare occurrences of aplastic anemia.4–8 In our practice, we recognized a trend toward female brain tumor patients having a higher incidence of clinically significant myelotoxicity and related complications during the first course of chemotherapy with conventional adjuvant dosing of TMZ. Therefore, we reviewed the hematologic toxicity in 18 consecutive patients after their first course of treatment with standard-dose TMZ (200 mg/m2 days 1–5 of a 28-day cycle).9 A marked difference in the incidence of clinically significant myelosuppression (grade 3 or 4) was noted between male and female patients (14% vs. 46%). Furthermore, four women required hospitalization for febrile neutropenia, whereas none of the male patients developed this complication.
These preliminary findings, generated from observations in our clinical practice and in the recent series of case reports, prompted concern that myelotoxicity may be an important consideration in select patients undergoing glioma treatment. For the majority of patients, significant toxicity is rare, which may reflect underdosing in these patients. For the minority who develop significant myelotoxicity, treatment delays, complications such as infection and hospitalizations, and even death may result. Determining possible risk factors for development of serious myelotoxicity would affect patient care and potentially alter treatment practice for these select patients. We therefore conducted a retrospective chart review of all adult patients at The University of Texas M. D. Anderson Cancer Center with primary malignant gliomas who received TMZ between December 1997 and December 2004. We evaluated the incidence and grade of myelotoxicity that occurred with the first course of TMZ, identified associated factors, and calculated a risk model that can be applied in the clinical setting to evaluate the probability of developing serious myelotoxicity. Furthermore, we examined a preliminary set of candidate single nucleotide polymorphisms (SNPs) to determine if genetic factors may also play a role in the risk of developing myelotoxicity in these patients.
Materials and Methods
This study was approved by the Institutional Review Board of M. D. Anderson Cancer Center. Using the Brain and Spine Center longitudinal database, we identified patients who fulfilled the following requirements: tumor histology of glioma (astrocytoma, oligodendroglioma, anaplastic astrocytoma, anaplastic oligodendroglioma, mixed anaplastic glioma) or GBM (glioblastoma or gliosarcoma); treatment with standard-dose TMZ (150–200 mg/m2 days 1–5 of a 28-day cycle); and age ⩾ 18 years; 1,283 patients met these criteria. We then reviewed the clinical records for the following information: patient demographics (age, gender, ethnicity, and smoking status); clinical characteristics, including histologic diagnosis, number and type of prior chemotherapies, height, weight, body surface area (BSA), ideal body weight; concurrent medications; and laboratory results, comprising complete blood count (CBC), blood urea nitrogen, serum creatinine performed within 4 weeks of initiation of TMZ, and CBC performed within 5 weeks of receiving TMZ.
Data were then analyzed to assess the grade of myelotoxicity using the National Cancer Institute Common Terminology Criteria for Adverse Events version 3.0.10 For those patients experiencing grade 3 or grade 4 myelotoxicity, analyses were carried out to evaluate factors associated with its incidence. To increase the clinical utility of identified risk factors for the individual patient, we developed a model to predict risk of myelosuppression separately for each gender. Those variables that had potential associations with risk of developing toxicity suggested by univariate logistic regression analysis (p ⩽ 0.15) were considered for building multivariable logistic regression models. A backward elimination strategy was used, and a threshold p-value level of 0.15 for the likelihood ratio test was used as a cutoff to select the covariates retained in the final multivariable model. All of the covariates retained in the model were categorical. The level with the highest odds ratio (OR) was defined as the risk group compared to the remaining level(s) for each covariate. The risk group of each covariate was then assigned a statistical weight of 1, which was added in the final formula. A final risk score was then assigned to each patient by adding the covariate risk scores together. Based on percentage of patients who experienced myelotoxicity with each score, groups of patients were then classified by risk of myelotoxicity as “no risk,” “low risk,” “intermediate risk,” or “high risk.” These groupings were based on both the American Society of Clinical Oncology guidelines for the management of neutropenia and use of colony stimulating factors and the National Comprehensive Cancer Network (NCCN) Practice Guidelines in Oncology (version 1.2007).11,12 Both of these sources designate chemotherapy regimens with high risk of febrile neutropenia as >20% incidence, intermediate risk as 10%–20% incidence, and low risk as <10%.
For the genetic analysis, we identified glioma patients from those with complete clinical data who had been previously genotyped for SNPs in the DNA repair and xenobiotic metabolism pathways of interest. These SNPs were chosen based on their putative functional effects on protein levels or function reported in the scientific literature. Those who developed severe (grade 3 or 4) myelotoxicity after their first course of TMZ were considered cases, and those who did not were considered controls. Controls were frequency matched to cases based on gender and age at treatment. Logistic regression was used to calculate the odds ratio of severe myelotoxicity for the genes of interest, adjusting for gender, age at treatment, and clinical risk score. These analyses were performed to gather some preliminary information about the potential susceptibility of these patients to the development of myelotoxicity after TMZ administration.
For the genotyping, genomic DNA was extracted from white blood cell fractions using the Qiagen QIAamp DNA Blood Mini Kit (Qiagen Inc., Valencia, CA, USA). Nonsynonymous coding SNPs and SNPs previously reported to be associated with DNA repair, inflammation, and xenobiotic metabolism were selected for genotyping using the MassARRAY iPLEX platform (Sequenom, San Diego, CA, USA), following the manufacturer's protocol (http://www.sequenom.com/seq.genotyping.html). Quality control analysis included genotyping internal positive control samples, no template controls, and replicates for 10% of the samples.
Results
Of 1,283 treated patients identified, 680 had complete data and were used as part of this analysis. Of the 603 patients with incomplete data, 434 were missing at least one baseline or nadir laboratory study, preventing an accurate analysis of myelotoxicity. The remaining 169 patients were missing other variables used in this analysis, such as concurrent medications, and therefore were not included. Table 1 outlines the demographic and clinic characteristics of the final patient population. As anticipated by the known epidemiology of gliomas, the sample included more men than women. The majority of patients were diagnosed with GBM, and most were treated with TMZ at the time of recurrence. Use of TMZ during radiation was not the standard of care during the time these patients were treated; therefore, none of the patients had been treated with concurrent chemoradiation. A marked difference in the incidence of clinically significant neutropenia (grade 3 or 4) was noted between male (6.25%) and female patients (12.36%). When any type of myelotoxicity (leukopenia, neutropenia, anemia, or thrombocytopenia) was considered, women were even more likely to experience grade 3 or 4 myelotoxicity than were men (women, 43 of 265, 16%; men, 30 of 415, 7%; p = 0.015). Eleven percent of all treated patients (72 of 680) required hospitalization during the first course of chemotherapy. Twenty percent of these patients (14 of 72) were admitted because of febrile neutropenia. Six patients died during the first course of therapy. Autopsies were not performed, but three of these patients died while hospitalized for febrile neutropenia.
Patient and clinical characteristics
| Patient Characteristic . | All Patients (n = 680) . | Females (n = 265) . | Males (n = 415) . |
|---|---|---|---|
| Age, years | |||
| Median | 47 | 47 | 48 |
| Range | 18-81 | 18-81 | 18-79 |
| Ethnicity, n (%) | |||
| White | 615 (90%) | 236 (89%) | 379 (91%) |
| Hispanic | 41 (6%) | 17 (6%) | 24 (6%) |
| Black | 18 (3%) | 10 (4%) | 8 (2%) |
| Asian | 3 (0.5%) | 1 (0.5%) | 2 (0.5%) |
| Other | 3 (0.5%) | 1 (0.5%) | 2 (0.5%) |
| Smoking status, n (%) | |||
| Nonsmoker | 470 (77%) | 185 (78%) | 285 (77%) |
| Smoker | 79 (13%) | 32 (13%) | 47 (13%) |
| Previous smoker | 60 (10%) | 21 (9%) | 39 (11%) |
| Anxiety medication, n (%) | |||
| No | 609 (91%) | 230 (89%) | 379 (93%) |
| Yes | 60 (9%) | 30 (11%) | 30 (7%) |
| Bowel medication, n (%) | |||
| No | 609 (91%) | 241 (93%) | 368 (90%) |
| Yes | 60 (9%) | 19 (7%) | 41 (10%) |
| Gastroesophageal reflux disease medication, n (%) | |||
| No | 352 (53 %) | 156 (60%) | 196 (48%) |
| Yes | 317 (47%) | 104 (40%) | 213 (52%) |
| Pain medication, n (%) | |||
| No | 462 (69%) | 180 (69%) | 282 (69%) |
| Yes | 207 (31%) | 80 (31%) | 126 (31%) |
| Corticosteroid medication, n (%) | |||
| No | 311 (47%) | 128 (49%) | 183 (45%) |
| Yes | 358 (53%) | 132 (51%) | 226 (55%) |
| Temozolomide dose, n (%) | |||
| 150 mg/m2 | 370 (54%) | 132 (50%) | 238 (57%) |
| 200 mg/m2 | 310 (46%) | 133 (50%) | 177 (43%) |
| Pretreatment creatinine level ⩾ 1 mg/dl, n (%) | |||
| No | 500 (75%) | 239 (92%) | 261 (64%) |
| Yes | 196 (25%) | 20 (8%) | 145 (36%) |
| Pretreatment platelet count <270,000/mm3, n (%) | |||
| No | 171 (25%) | 89 (34%) | 90 (20%) |
| Yes | 507 (75%) | 175 (66%) | 174 (80%) |
| Body surface area ⩾ 2 m2, n (%) | |||
| No | 348 (52%) | 216 (83%) | 132 (33%) |
| Yes | 319 (48%) | 45 (17%) | 274 (68%) |
| Pretreatment white blood cell count <6,500/mm3, n (%) | |||
| No | 342 (50%) | 132 (50%) | 210 (51%) |
| Yes | 337 (50%) | 132 (50%) | 205 (49%) |
| Tumor type, n (%) | |||
| Glioblastoma | 428 (63%) | 153 (58%) | 275 (66%) |
| Astrocytoma, anaplastic | 100 (15%) | 44 (17%) | 56 (13%) |
| Oligodendroglioma, anaplastic | 55 (8%) | 22 (8%) | 33 (8%) |
| Oligoastrocytoma, anaplastic | 23 (3%) | 12 (5%) | 11 (3%) |
| Oligodendroglioma | 17 (3%) | 8 (3%) | 9 (2%) |
| Glioma, not otherwise specified | 16 (2%) | 6 (2%) | 10 (2%) |
| Astrocytoma | 15 (2%) | 9 (3%) | 6 (1%) |
| Other primary brain tumors | 26 (4%) | 11 (4%) | 15 (4%) |
| Prior chemotherapy, n (%) | |||
| Yes | 373 (55%) | 133 (50%) | 240 (58%) |
| No | 307 (45%) | 132 (50%) | 175 (42%) |
| Patient Characteristic . | All Patients (n = 680) . | Females (n = 265) . | Males (n = 415) . |
|---|---|---|---|
| Age, years | |||
| Median | 47 | 47 | 48 |
| Range | 18-81 | 18-81 | 18-79 |
| Ethnicity, n (%) | |||
| White | 615 (90%) | 236 (89%) | 379 (91%) |
| Hispanic | 41 (6%) | 17 (6%) | 24 (6%) |
| Black | 18 (3%) | 10 (4%) | 8 (2%) |
| Asian | 3 (0.5%) | 1 (0.5%) | 2 (0.5%) |
| Other | 3 (0.5%) | 1 (0.5%) | 2 (0.5%) |
| Smoking status, n (%) | |||
| Nonsmoker | 470 (77%) | 185 (78%) | 285 (77%) |
| Smoker | 79 (13%) | 32 (13%) | 47 (13%) |
| Previous smoker | 60 (10%) | 21 (9%) | 39 (11%) |
| Anxiety medication, n (%) | |||
| No | 609 (91%) | 230 (89%) | 379 (93%) |
| Yes | 60 (9%) | 30 (11%) | 30 (7%) |
| Bowel medication, n (%) | |||
| No | 609 (91%) | 241 (93%) | 368 (90%) |
| Yes | 60 (9%) | 19 (7%) | 41 (10%) |
| Gastroesophageal reflux disease medication, n (%) | |||
| No | 352 (53 %) | 156 (60%) | 196 (48%) |
| Yes | 317 (47%) | 104 (40%) | 213 (52%) |
| Pain medication, n (%) | |||
| No | 462 (69%) | 180 (69%) | 282 (69%) |
| Yes | 207 (31%) | 80 (31%) | 126 (31%) |
| Corticosteroid medication, n (%) | |||
| No | 311 (47%) | 128 (49%) | 183 (45%) |
| Yes | 358 (53%) | 132 (51%) | 226 (55%) |
| Temozolomide dose, n (%) | |||
| 150 mg/m2 | 370 (54%) | 132 (50%) | 238 (57%) |
| 200 mg/m2 | 310 (46%) | 133 (50%) | 177 (43%) |
| Pretreatment creatinine level ⩾ 1 mg/dl, n (%) | |||
| No | 500 (75%) | 239 (92%) | 261 (64%) |
| Yes | 196 (25%) | 20 (8%) | 145 (36%) |
| Pretreatment platelet count <270,000/mm3, n (%) | |||
| No | 171 (25%) | 89 (34%) | 90 (20%) |
| Yes | 507 (75%) | 175 (66%) | 174 (80%) |
| Body surface area ⩾ 2 m2, n (%) | |||
| No | 348 (52%) | 216 (83%) | 132 (33%) |
| Yes | 319 (48%) | 45 (17%) | 274 (68%) |
| Pretreatment white blood cell count <6,500/mm3, n (%) | |||
| No | 342 (50%) | 132 (50%) | 210 (51%) |
| Yes | 337 (50%) | 132 (50%) | 205 (49%) |
| Tumor type, n (%) | |||
| Glioblastoma | 428 (63%) | 153 (58%) | 275 (66%) |
| Astrocytoma, anaplastic | 100 (15%) | 44 (17%) | 56 (13%) |
| Oligodendroglioma, anaplastic | 55 (8%) | 22 (8%) | 33 (8%) |
| Oligoastrocytoma, anaplastic | 23 (3%) | 12 (5%) | 11 (3%) |
| Oligodendroglioma | 17 (3%) | 8 (3%) | 9 (2%) |
| Glioma, not otherwise specified | 16 (2%) | 6 (2%) | 10 (2%) |
| Astrocytoma | 15 (2%) | 9 (3%) | 6 (1%) |
| Other primary brain tumors | 26 (4%) | 11 (4%) | 15 (4%) |
| Prior chemotherapy, n (%) | |||
| Yes | 373 (55%) | 133 (50%) | 240 (58%) |
| No | 307 (45%) | 132 (50%) | 175 (42%) |
Patient and clinical characteristics
| Patient Characteristic . | All Patients (n = 680) . | Females (n = 265) . | Males (n = 415) . |
|---|---|---|---|
| Age, years | |||
| Median | 47 | 47 | 48 |
| Range | 18-81 | 18-81 | 18-79 |
| Ethnicity, n (%) | |||
| White | 615 (90%) | 236 (89%) | 379 (91%) |
| Hispanic | 41 (6%) | 17 (6%) | 24 (6%) |
| Black | 18 (3%) | 10 (4%) | 8 (2%) |
| Asian | 3 (0.5%) | 1 (0.5%) | 2 (0.5%) |
| Other | 3 (0.5%) | 1 (0.5%) | 2 (0.5%) |
| Smoking status, n (%) | |||
| Nonsmoker | 470 (77%) | 185 (78%) | 285 (77%) |
| Smoker | 79 (13%) | 32 (13%) | 47 (13%) |
| Previous smoker | 60 (10%) | 21 (9%) | 39 (11%) |
| Anxiety medication, n (%) | |||
| No | 609 (91%) | 230 (89%) | 379 (93%) |
| Yes | 60 (9%) | 30 (11%) | 30 (7%) |
| Bowel medication, n (%) | |||
| No | 609 (91%) | 241 (93%) | 368 (90%) |
| Yes | 60 (9%) | 19 (7%) | 41 (10%) |
| Gastroesophageal reflux disease medication, n (%) | |||
| No | 352 (53 %) | 156 (60%) | 196 (48%) |
| Yes | 317 (47%) | 104 (40%) | 213 (52%) |
| Pain medication, n (%) | |||
| No | 462 (69%) | 180 (69%) | 282 (69%) |
| Yes | 207 (31%) | 80 (31%) | 126 (31%) |
| Corticosteroid medication, n (%) | |||
| No | 311 (47%) | 128 (49%) | 183 (45%) |
| Yes | 358 (53%) | 132 (51%) | 226 (55%) |
| Temozolomide dose, n (%) | |||
| 150 mg/m2 | 370 (54%) | 132 (50%) | 238 (57%) |
| 200 mg/m2 | 310 (46%) | 133 (50%) | 177 (43%) |
| Pretreatment creatinine level ⩾ 1 mg/dl, n (%) | |||
| No | 500 (75%) | 239 (92%) | 261 (64%) |
| Yes | 196 (25%) | 20 (8%) | 145 (36%) |
| Pretreatment platelet count <270,000/mm3, n (%) | |||
| No | 171 (25%) | 89 (34%) | 90 (20%) |
| Yes | 507 (75%) | 175 (66%) | 174 (80%) |
| Body surface area ⩾ 2 m2, n (%) | |||
| No | 348 (52%) | 216 (83%) | 132 (33%) |
| Yes | 319 (48%) | 45 (17%) | 274 (68%) |
| Pretreatment white blood cell count <6,500/mm3, n (%) | |||
| No | 342 (50%) | 132 (50%) | 210 (51%) |
| Yes | 337 (50%) | 132 (50%) | 205 (49%) |
| Tumor type, n (%) | |||
| Glioblastoma | 428 (63%) | 153 (58%) | 275 (66%) |
| Astrocytoma, anaplastic | 100 (15%) | 44 (17%) | 56 (13%) |
| Oligodendroglioma, anaplastic | 55 (8%) | 22 (8%) | 33 (8%) |
| Oligoastrocytoma, anaplastic | 23 (3%) | 12 (5%) | 11 (3%) |
| Oligodendroglioma | 17 (3%) | 8 (3%) | 9 (2%) |
| Glioma, not otherwise specified | 16 (2%) | 6 (2%) | 10 (2%) |
| Astrocytoma | 15 (2%) | 9 (3%) | 6 (1%) |
| Other primary brain tumors | 26 (4%) | 11 (4%) | 15 (4%) |
| Prior chemotherapy, n (%) | |||
| Yes | 373 (55%) | 133 (50%) | 240 (58%) |
| No | 307 (45%) | 132 (50%) | 175 (42%) |
| Patient Characteristic . | All Patients (n = 680) . | Females (n = 265) . | Males (n = 415) . |
|---|---|---|---|
| Age, years | |||
| Median | 47 | 47 | 48 |
| Range | 18-81 | 18-81 | 18-79 |
| Ethnicity, n (%) | |||
| White | 615 (90%) | 236 (89%) | 379 (91%) |
| Hispanic | 41 (6%) | 17 (6%) | 24 (6%) |
| Black | 18 (3%) | 10 (4%) | 8 (2%) |
| Asian | 3 (0.5%) | 1 (0.5%) | 2 (0.5%) |
| Other | 3 (0.5%) | 1 (0.5%) | 2 (0.5%) |
| Smoking status, n (%) | |||
| Nonsmoker | 470 (77%) | 185 (78%) | 285 (77%) |
| Smoker | 79 (13%) | 32 (13%) | 47 (13%) |
| Previous smoker | 60 (10%) | 21 (9%) | 39 (11%) |
| Anxiety medication, n (%) | |||
| No | 609 (91%) | 230 (89%) | 379 (93%) |
| Yes | 60 (9%) | 30 (11%) | 30 (7%) |
| Bowel medication, n (%) | |||
| No | 609 (91%) | 241 (93%) | 368 (90%) |
| Yes | 60 (9%) | 19 (7%) | 41 (10%) |
| Gastroesophageal reflux disease medication, n (%) | |||
| No | 352 (53 %) | 156 (60%) | 196 (48%) |
| Yes | 317 (47%) | 104 (40%) | 213 (52%) |
| Pain medication, n (%) | |||
| No | 462 (69%) | 180 (69%) | 282 (69%) |
| Yes | 207 (31%) | 80 (31%) | 126 (31%) |
| Corticosteroid medication, n (%) | |||
| No | 311 (47%) | 128 (49%) | 183 (45%) |
| Yes | 358 (53%) | 132 (51%) | 226 (55%) |
| Temozolomide dose, n (%) | |||
| 150 mg/m2 | 370 (54%) | 132 (50%) | 238 (57%) |
| 200 mg/m2 | 310 (46%) | 133 (50%) | 177 (43%) |
| Pretreatment creatinine level ⩾ 1 mg/dl, n (%) | |||
| No | 500 (75%) | 239 (92%) | 261 (64%) |
| Yes | 196 (25%) | 20 (8%) | 145 (36%) |
| Pretreatment platelet count <270,000/mm3, n (%) | |||
| No | 171 (25%) | 89 (34%) | 90 (20%) |
| Yes | 507 (75%) | 175 (66%) | 174 (80%) |
| Body surface area ⩾ 2 m2, n (%) | |||
| No | 348 (52%) | 216 (83%) | 132 (33%) |
| Yes | 319 (48%) | 45 (17%) | 274 (68%) |
| Pretreatment white blood cell count <6,500/mm3, n (%) | |||
| No | 342 (50%) | 132 (50%) | 210 (51%) |
| Yes | 337 (50%) | 132 (50%) | 205 (49%) |
| Tumor type, n (%) | |||
| Glioblastoma | 428 (63%) | 153 (58%) | 275 (66%) |
| Astrocytoma, anaplastic | 100 (15%) | 44 (17%) | 56 (13%) |
| Oligodendroglioma, anaplastic | 55 (8%) | 22 (8%) | 33 (8%) |
| Oligoastrocytoma, anaplastic | 23 (3%) | 12 (5%) | 11 (3%) |
| Oligodendroglioma | 17 (3%) | 8 (3%) | 9 (2%) |
| Glioma, not otherwise specified | 16 (2%) | 6 (2%) | 10 (2%) |
| Astrocytoma | 15 (2%) | 9 (3%) | 6 (1%) |
| Other primary brain tumors | 26 (4%) | 11 (4%) | 15 (4%) |
| Prior chemotherapy, n (%) | |||
| Yes | 373 (55%) | 133 (50%) | 240 (58%) |
| No | 307 (45%) | 132 (50%) | 175 (42%) |
The next step was to identify factors by univariate analyses that were associated with increased risk of severe (grade 3 or 4) neutropenia, thrombocytopenia, or myelotoxicity of any type. We performed the analyses for each gender separately. Table 2 provides the covariates that remained in the final analysis for males and females. For males, BSA > 2 m2, not being on steroids, and taking bowel medication were associated with an increased risk of myelotoxicity and remained in the final model. For females, the following were associated with an increased risk of myelotoxicity and remained in the final risk model: no prior chemotherapy, baseline creatinine ⩾ 1 mg/dl, baseline platelet count < 270,000/mm3, BSA < 2 m2, being on analgesics, and not being on medication for gastroesophageal reflux disease. In addition, we did note a significant trend for older men to have an increased risk of myelotoxicity. For women, there was a trend for an increased risk in those 31–40 years of age, and again for those older than 60 years. Because of the observed trends and the recognized clinical significance, age was included in the final model. However, removing age did not affect the inclusion of the other variables or the final risk scores for this group of patients.
Covariates associated with myelotoxicity for males and females in the final multivariable model
| Effect . | Odds Ratio . | 95% Wald Confidence Limits . | . | p-Value . | Assigned Score . | |
|---|---|---|---|---|---|---|
| Males | ||||||
| Age at treatment (years) | 0.7935 | |||||
| ⩽30 | 1 | 0 | ||||
| 31-40 | 1.394 | 0.254 | 7.639 | 0 | ||
| >40 | 1.644 | 0.360 | 7.499 | 1 | ||
| On bowel medication | 0.0087 | |||||
| No | 1 | 0 | ||||
| Yes | 3.955 | 1.416 | 11.047 | 1 | ||
| On corticosteroids | 0.0609 | |||||
| No | 2.214 | 0.964 | 5.085 | 1 | ||
| Yes | 1 | 0 | ||||
| Body surface area (m2) | 0.0494 | |||||
| <2 | 1 | 0 | ||||
| ⩾2 | 2.712 | 1.002 | 7.335 | 1 | ||
| Females | ||||||
| Age at treatment (years) | 0.5482 | |||||
| ⩽30 | 1 | 0 | ||||
| 31-40 | 2.727 | 0.636 | 11.688 | 1 | ||
| 41-60 | 1.799 | 0.435 | 7.438 | 0 | ||
| >60 | 2.082 | 0.412 | 10.512 | 0 | ||
| Prior chemotherapy | 0.0016 | |||||
| No | 3.727 | 1.644 | 8.447 | 1 | ||
| Yes | 1 | 0 | ||||
| On gastroesophageal reflux disease medication | 0.0147 | |||||
| No | 2.942 | 1.236 | 7.002 | 1 | ||
| Yes | 1 | 0 | ||||
| On pain medication | 0.0530 | |||||
| No | 1 | 0 | ||||
| Yes | 2.169 | 0.990 | 4.753 | 1 | ||
| Body surface area (m2) | 0.0440 | |||||
| <2 | 4.178 | 1.039 | 16.797 | 1 | ||
| ⩾2 | 1 | 0 | ||||
| Pretreatment creatinine level | 0.0023 | |||||
| <1 | 1 | 0 | ||||
| ⩾1 | 6.080 | 1.907 | 19.391 | 1 | ||
| Pretreatment platelet count | 0.0368 | |||||
| <270 | 2.438 | 1.056 | 5.627 | 1 | ||
| ⩾270 | 1 | 0 | ||||
| Effect . | Odds Ratio . | 95% Wald Confidence Limits . | . | p-Value . | Assigned Score . | |
|---|---|---|---|---|---|---|
| Males | ||||||
| Age at treatment (years) | 0.7935 | |||||
| ⩽30 | 1 | 0 | ||||
| 31-40 | 1.394 | 0.254 | 7.639 | 0 | ||
| >40 | 1.644 | 0.360 | 7.499 | 1 | ||
| On bowel medication | 0.0087 | |||||
| No | 1 | 0 | ||||
| Yes | 3.955 | 1.416 | 11.047 | 1 | ||
| On corticosteroids | 0.0609 | |||||
| No | 2.214 | 0.964 | 5.085 | 1 | ||
| Yes | 1 | 0 | ||||
| Body surface area (m2) | 0.0494 | |||||
| <2 | 1 | 0 | ||||
| ⩾2 | 2.712 | 1.002 | 7.335 | 1 | ||
| Females | ||||||
| Age at treatment (years) | 0.5482 | |||||
| ⩽30 | 1 | 0 | ||||
| 31-40 | 2.727 | 0.636 | 11.688 | 1 | ||
| 41-60 | 1.799 | 0.435 | 7.438 | 0 | ||
| >60 | 2.082 | 0.412 | 10.512 | 0 | ||
| Prior chemotherapy | 0.0016 | |||||
| No | 3.727 | 1.644 | 8.447 | 1 | ||
| Yes | 1 | 0 | ||||
| On gastroesophageal reflux disease medication | 0.0147 | |||||
| No | 2.942 | 1.236 | 7.002 | 1 | ||
| Yes | 1 | 0 | ||||
| On pain medication | 0.0530 | |||||
| No | 1 | 0 | ||||
| Yes | 2.169 | 0.990 | 4.753 | 1 | ||
| Body surface area (m2) | 0.0440 | |||||
| <2 | 4.178 | 1.039 | 16.797 | 1 | ||
| ⩾2 | 1 | 0 | ||||
| Pretreatment creatinine level | 0.0023 | |||||
| <1 | 1 | 0 | ||||
| ⩾1 | 6.080 | 1.907 | 19.391 | 1 | ||
| Pretreatment platelet count | 0.0368 | |||||
| <270 | 2.438 | 1.056 | 5.627 | 1 | ||
| ⩾270 | 1 | 0 | ||||
Covariates associated with myelotoxicity for males and females in the final multivariable model
| Effect . | Odds Ratio . | 95% Wald Confidence Limits . | . | p-Value . | Assigned Score . | |
|---|---|---|---|---|---|---|
| Males | ||||||
| Age at treatment (years) | 0.7935 | |||||
| ⩽30 | 1 | 0 | ||||
| 31-40 | 1.394 | 0.254 | 7.639 | 0 | ||
| >40 | 1.644 | 0.360 | 7.499 | 1 | ||
| On bowel medication | 0.0087 | |||||
| No | 1 | 0 | ||||
| Yes | 3.955 | 1.416 | 11.047 | 1 | ||
| On corticosteroids | 0.0609 | |||||
| No | 2.214 | 0.964 | 5.085 | 1 | ||
| Yes | 1 | 0 | ||||
| Body surface area (m2) | 0.0494 | |||||
| <2 | 1 | 0 | ||||
| ⩾2 | 2.712 | 1.002 | 7.335 | 1 | ||
| Females | ||||||
| Age at treatment (years) | 0.5482 | |||||
| ⩽30 | 1 | 0 | ||||
| 31-40 | 2.727 | 0.636 | 11.688 | 1 | ||
| 41-60 | 1.799 | 0.435 | 7.438 | 0 | ||
| >60 | 2.082 | 0.412 | 10.512 | 0 | ||
| Prior chemotherapy | 0.0016 | |||||
| No | 3.727 | 1.644 | 8.447 | 1 | ||
| Yes | 1 | 0 | ||||
| On gastroesophageal reflux disease medication | 0.0147 | |||||
| No | 2.942 | 1.236 | 7.002 | 1 | ||
| Yes | 1 | 0 | ||||
| On pain medication | 0.0530 | |||||
| No | 1 | 0 | ||||
| Yes | 2.169 | 0.990 | 4.753 | 1 | ||
| Body surface area (m2) | 0.0440 | |||||
| <2 | 4.178 | 1.039 | 16.797 | 1 | ||
| ⩾2 | 1 | 0 | ||||
| Pretreatment creatinine level | 0.0023 | |||||
| <1 | 1 | 0 | ||||
| ⩾1 | 6.080 | 1.907 | 19.391 | 1 | ||
| Pretreatment platelet count | 0.0368 | |||||
| <270 | 2.438 | 1.056 | 5.627 | 1 | ||
| ⩾270 | 1 | 0 | ||||
| Effect . | Odds Ratio . | 95% Wald Confidence Limits . | . | p-Value . | Assigned Score . | |
|---|---|---|---|---|---|---|
| Males | ||||||
| Age at treatment (years) | 0.7935 | |||||
| ⩽30 | 1 | 0 | ||||
| 31-40 | 1.394 | 0.254 | 7.639 | 0 | ||
| >40 | 1.644 | 0.360 | 7.499 | 1 | ||
| On bowel medication | 0.0087 | |||||
| No | 1 | 0 | ||||
| Yes | 3.955 | 1.416 | 11.047 | 1 | ||
| On corticosteroids | 0.0609 | |||||
| No | 2.214 | 0.964 | 5.085 | 1 | ||
| Yes | 1 | 0 | ||||
| Body surface area (m2) | 0.0494 | |||||
| <2 | 1 | 0 | ||||
| ⩾2 | 2.712 | 1.002 | 7.335 | 1 | ||
| Females | ||||||
| Age at treatment (years) | 0.5482 | |||||
| ⩽30 | 1 | 0 | ||||
| 31-40 | 2.727 | 0.636 | 11.688 | 1 | ||
| 41-60 | 1.799 | 0.435 | 7.438 | 0 | ||
| >60 | 2.082 | 0.412 | 10.512 | 0 | ||
| Prior chemotherapy | 0.0016 | |||||
| No | 3.727 | 1.644 | 8.447 | 1 | ||
| Yes | 1 | 0 | ||||
| On gastroesophageal reflux disease medication | 0.0147 | |||||
| No | 2.942 | 1.236 | 7.002 | 1 | ||
| Yes | 1 | 0 | ||||
| On pain medication | 0.0530 | |||||
| No | 1 | 0 | ||||
| Yes | 2.169 | 0.990 | 4.753 | 1 | ||
| Body surface area (m2) | 0.0440 | |||||
| <2 | 4.178 | 1.039 | 16.797 | 1 | ||
| ⩾2 | 1 | 0 | ||||
| Pretreatment creatinine level | 0.0023 | |||||
| <1 | 1 | 0 | ||||
| ⩾1 | 6.080 | 1.907 | 19.391 | 1 | ||
| Pretreatment platelet count | 0.0368 | |||||
| <270 | 2.438 | 1.056 | 5.627 | 1 | ||
| ⩾270 | 1 | 0 | ||||
The model is a summation of each of the risk components. If present, each covariate added one (+1) to the formula, and a final “risk” number was generated. Overall risk varied, based on scores from 0% (zero risk) to 33% in men and from 0% to 100% for women. Table 3 provides the scores with associated risk for both genders. We then classified the risk score in terms of clinical risk, as groups with no risk, low risk, intermediate risk, and high risk. These results, by gender, are provided in Table 4. The objective was to provide a clinical tool for oncologists to estimate percent risk of myelotoxicity in patients prior to treatment with TMZ. For example, if a patient has no risk of myelotoxicity based on the risk model, standard dosing at the recommended full dose or treatment with a more dose-dense regimen may be used. For a patient with significant clinical risk, lower TMZ dosing, use of prophylactic colony-stimulating factors, or more frequent monitoring may be better options for treatment.
Risk score and percentage risk
| Risk Score . | No Toxicity . | Toxicity . | Total . |
|---|---|---|---|
| Males | |||
| 0 | 16 | 0 (0%) | 16 |
| 1 | 99 | 3 (2.94%) | 102 |
| 2 | 170 | 14 (7.61%) | 184 |
| 3 | 82 | 12 (12.77%) | 94 |
| 4 | 2 | 1 (33.33%) | 3 |
| Total | 369 | 30 | 399 |
| Females | |||
| 0 | 1 | 0 | 1 |
| 1 | 9 | 0 (0%) | 9 |
| 2 | 57 | 0 (0%) | 57 |
| 3 | 78 | 16 (17.02%) | 94 |
| 4 | 46 | 12 (20.69%) | 58 |
| 5 | 17 | 12 (41.38%) | 29 |
| 6 | 0 | 3 (100%) | 3 |
| Total | 208 | 43 | 251 |
| Risk Score . | No Toxicity . | Toxicity . | Total . |
|---|---|---|---|
| Males | |||
| 0 | 16 | 0 (0%) | 16 |
| 1 | 99 | 3 (2.94%) | 102 |
| 2 | 170 | 14 (7.61%) | 184 |
| 3 | 82 | 12 (12.77%) | 94 |
| 4 | 2 | 1 (33.33%) | 3 |
| Total | 369 | 30 | 399 |
| Females | |||
| 0 | 1 | 0 | 1 |
| 1 | 9 | 0 (0%) | 9 |
| 2 | 57 | 0 (0%) | 57 |
| 3 | 78 | 16 (17.02%) | 94 |
| 4 | 46 | 12 (20.69%) | 58 |
| 5 | 17 | 12 (41.38%) | 29 |
| 6 | 0 | 3 (100%) | 3 |
| Total | 208 | 43 | 251 |
Risk score for males = age > 40 years + BSA > 2 m2 + not on corticosteroids + on bowel medications. Risk score for females = age 31-40 years + no prior chemotherapy + pretreatment creatinine level ⩾ 1 mg/dl + pretreatment platelet count < 270,000/mm3 + BSA < 2 m2 + not on medications for gastroesophageal reflux disease + on analgesics. Final factors for males included BSA ⩾ 2 (OR = 2.712, p = 0.05), not on steroids (OR = 2.214, p = 0.06), on bowel medication (OR = 3.955, p = 0.009), and age > 40 years (OR = 1.644, p = 0.80). Final factors for females included no prior chemotherapy (OR = 3.727, p = 0.002), creatinine level ⩾ 1 (OR = 6.080, p = 0.002), platelet count < 270,000/mm3 (OR = 2.438, p = 0.04), BSA < 2 m2 (OR = 4.178, p = 0.04), not on gastroesophageal reflux disease medication (OR = 2.942, p = 0.01), on pain medication (OR = 2.169, p = 0.05), and age at treatment 31-40 years (OR = 2.727, p = 0.55)
Risk score and percentage risk
| Risk Score . | No Toxicity . | Toxicity . | Total . |
|---|---|---|---|
| Males | |||
| 0 | 16 | 0 (0%) | 16 |
| 1 | 99 | 3 (2.94%) | 102 |
| 2 | 170 | 14 (7.61%) | 184 |
| 3 | 82 | 12 (12.77%) | 94 |
| 4 | 2 | 1 (33.33%) | 3 |
| Total | 369 | 30 | 399 |
| Females | |||
| 0 | 1 | 0 | 1 |
| 1 | 9 | 0 (0%) | 9 |
| 2 | 57 | 0 (0%) | 57 |
| 3 | 78 | 16 (17.02%) | 94 |
| 4 | 46 | 12 (20.69%) | 58 |
| 5 | 17 | 12 (41.38%) | 29 |
| 6 | 0 | 3 (100%) | 3 |
| Total | 208 | 43 | 251 |
| Risk Score . | No Toxicity . | Toxicity . | Total . |
|---|---|---|---|
| Males | |||
| 0 | 16 | 0 (0%) | 16 |
| 1 | 99 | 3 (2.94%) | 102 |
| 2 | 170 | 14 (7.61%) | 184 |
| 3 | 82 | 12 (12.77%) | 94 |
| 4 | 2 | 1 (33.33%) | 3 |
| Total | 369 | 30 | 399 |
| Females | |||
| 0 | 1 | 0 | 1 |
| 1 | 9 | 0 (0%) | 9 |
| 2 | 57 | 0 (0%) | 57 |
| 3 | 78 | 16 (17.02%) | 94 |
| 4 | 46 | 12 (20.69%) | 58 |
| 5 | 17 | 12 (41.38%) | 29 |
| 6 | 0 | 3 (100%) | 3 |
| Total | 208 | 43 | 251 |
Risk score for males = age > 40 years + BSA > 2 m2 + not on corticosteroids + on bowel medications. Risk score for females = age 31-40 years + no prior chemotherapy + pretreatment creatinine level ⩾ 1 mg/dl + pretreatment platelet count < 270,000/mm3 + BSA < 2 m2 + not on medications for gastroesophageal reflux disease + on analgesics. Final factors for males included BSA ⩾ 2 (OR = 2.712, p = 0.05), not on steroids (OR = 2.214, p = 0.06), on bowel medication (OR = 3.955, p = 0.009), and age > 40 years (OR = 1.644, p = 0.80). Final factors for females included no prior chemotherapy (OR = 3.727, p = 0.002), creatinine level ⩾ 1 (OR = 6.080, p = 0.002), platelet count < 270,000/mm3 (OR = 2.438, p = 0.04), BSA < 2 m2 (OR = 4.178, p = 0.04), not on gastroesophageal reflux disease medication (OR = 2.942, p = 0.01), on pain medication (OR = 2.169, p = 0.05), and age at treatment 31-40 years (OR = 2.727, p = 0.55)
Clinical divisions of risk
| Division . | Grouped Risk Score . | Percent with Toxicity . |
|---|---|---|
| Male | ||
| No risk | 0 | 0 |
| Low risk | 1/2 | 2.94-7.6 |
| Intermediate risk | 3 | 12.77 |
| High risk | 4 | 33.3 |
| Female | ||
| No risk | 0/1/2 | 0 |
| Intermediate risk | 3/4 | 17.02-20.69 |
| High risk | 5/6 | 41.38-100 |
| Division . | Grouped Risk Score . | Percent with Toxicity . |
|---|---|---|
| Male | ||
| No risk | 0 | 0 |
| Low risk | 1/2 | 2.94-7.6 |
| Intermediate risk | 3 | 12.77 |
| High risk | 4 | 33.3 |
| Female | ||
| No risk | 0/1/2 | 0 |
| Intermediate risk | 3/4 | 17.02-20.69 |
| High risk | 5/6 | 41.38-100 |
Clinical divisions of risk
| Division . | Grouped Risk Score . | Percent with Toxicity . |
|---|---|---|
| Male | ||
| No risk | 0 | 0 |
| Low risk | 1/2 | 2.94-7.6 |
| Intermediate risk | 3 | 12.77 |
| High risk | 4 | 33.3 |
| Female | ||
| No risk | 0/1/2 | 0 |
| Intermediate risk | 3/4 | 17.02-20.69 |
| High risk | 5/6 | 41.38-100 |
| Division . | Grouped Risk Score . | Percent with Toxicity . |
|---|---|---|
| Male | ||
| No risk | 0 | 0 |
| Low risk | 1/2 | 2.94-7.6 |
| Intermediate risk | 3 | 12.77 |
| High risk | 4 | 33.3 |
| Female | ||
| No risk | 0/1/2 | 0 |
| Intermediate risk | 3/4 | 17.02-20.69 |
| High risk | 5/6 | 41.38-100 |
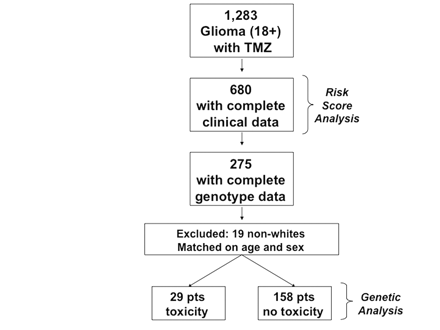
For the polymorphism analysis, 187 patients from the 680 with complete clinical data had available genotype data for inclusion in this part of the analysis (see Fig. 1). In a case-control analysis, after matching on gender and age at treatment, 29 “cases” developed severe (grade 3 or 4) myelotoxicity after their first course of TMZ and 158 “controls” did not (Table 5). Polymorphisms in the genes NAD(P)H dehydrogenase, quinone 1 (NQO1), O6-methylguanine-DNA methyltransferase (MGMT), and glutathione S-transferase pi 1 (GSTP1) showed significant differences in risk of severe myelotoxicity. Presence of the A allele of NQO1 (rs1800566) resulted in a 70% decrease in risk of toxicity (95% confidence interval [CI], 0.11–0.85). Additionally, patients carrying the G allele of the GSTP1 105 (rs1695) polymorphism experienced a 72% reduction in risk of toxicity (95% CI, 0.10–0.75). Patients carrying the G allele of MGMT (rs2308327) exhibited a 240% increase in risk of toxicity (95% CI, 0.99–5.84).
Preliminary results from genetic polymorphisms analysis
| . | Toxicity . | . | . | . | |
|---|---|---|---|---|---|
| Allele . | Yes . | No . | ORa . | 95% CI . | |
| NQO1 (rs1800566) | |||||
| GG | 22 | 90 | Reference | ||
| AA/AG | 7 | 68 | 0.30 | 0.11-0.85 | |
| MGMT (rs2308321) | |||||
| AA | 17 | 119 | Reference | ||
| GG/GA | 12 | 38 | 2.32 | 0.95-5.62 | |
| MGMT (rs2308327) | |||||
| AA | 17 | 120 | Reference | ||
| GG/GA | 12 | 37 | 2.40 | 0.99-5.84 | |
| MGMT (rs12917) | |||||
| CC | 24 | 126 | Reference | ||
| TT/TC | 5 | 31 | 0.84 | 0.28-2.55 | |
| XRCC3 (rs861539) | |||||
| CC | 10 | 64 | Reference | ||
| TT/TC | 18 | 91 | 1.14 | 0.47-2.78 | |
| GSTP105 (rs1695) | |||||
| AA | 9 | 69 | Reference | ||
| AG/GG | 16 | 44 | 0.28 | 0.10-0.75 | |
| . | Toxicity . | . | . | . | |
|---|---|---|---|---|---|
| Allele . | Yes . | No . | ORa . | 95% CI . | |
| NQO1 (rs1800566) | |||||
| GG | 22 | 90 | Reference | ||
| AA/AG | 7 | 68 | 0.30 | 0.11-0.85 | |
| MGMT (rs2308321) | |||||
| AA | 17 | 119 | Reference | ||
| GG/GA | 12 | 38 | 2.32 | 0.95-5.62 | |
| MGMT (rs2308327) | |||||
| AA | 17 | 120 | Reference | ||
| GG/GA | 12 | 37 | 2.40 | 0.99-5.84 | |
| MGMT (rs12917) | |||||
| CC | 24 | 126 | Reference | ||
| TT/TC | 5 | 31 | 0.84 | 0.28-2.55 | |
| XRCC3 (rs861539) | |||||
| CC | 10 | 64 | Reference | ||
| TT/TC | 18 | 91 | 1.14 | 0.47-2.78 | |
| GSTP105 (rs1695) | |||||
| AA | 9 | 69 | Reference | ||
| AG/GG | 16 | 44 | 0.28 | 0.10-0.75 | |
Abbreviations: OR, odds ratio; CI, confidence interval; NQO1, NAD(P)H dehydrogenase, quinone 1; MGMT, O6-methylguanine-DNA methyltransferase; XRCC3, x-ray repair complementing defective repair in Chinese hamster cells 3; GSTP1, glutathione S-transferase pi 1
Adjusted for age, sex, and clinical risk score
Preliminary results from genetic polymorphisms analysis
| . | Toxicity . | . | . | . | |
|---|---|---|---|---|---|
| Allele . | Yes . | No . | ORa . | 95% CI . | |
| NQO1 (rs1800566) | |||||
| GG | 22 | 90 | Reference | ||
| AA/AG | 7 | 68 | 0.30 | 0.11-0.85 | |
| MGMT (rs2308321) | |||||
| AA | 17 | 119 | Reference | ||
| GG/GA | 12 | 38 | 2.32 | 0.95-5.62 | |
| MGMT (rs2308327) | |||||
| AA | 17 | 120 | Reference | ||
| GG/GA | 12 | 37 | 2.40 | 0.99-5.84 | |
| MGMT (rs12917) | |||||
| CC | 24 | 126 | Reference | ||
| TT/TC | 5 | 31 | 0.84 | 0.28-2.55 | |
| XRCC3 (rs861539) | |||||
| CC | 10 | 64 | Reference | ||
| TT/TC | 18 | 91 | 1.14 | 0.47-2.78 | |
| GSTP105 (rs1695) | |||||
| AA | 9 | 69 | Reference | ||
| AG/GG | 16 | 44 | 0.28 | 0.10-0.75 | |
| . | Toxicity . | . | . | . | |
|---|---|---|---|---|---|
| Allele . | Yes . | No . | ORa . | 95% CI . | |
| NQO1 (rs1800566) | |||||
| GG | 22 | 90 | Reference | ||
| AA/AG | 7 | 68 | 0.30 | 0.11-0.85 | |
| MGMT (rs2308321) | |||||
| AA | 17 | 119 | Reference | ||
| GG/GA | 12 | 38 | 2.32 | 0.95-5.62 | |
| MGMT (rs2308327) | |||||
| AA | 17 | 120 | Reference | ||
| GG/GA | 12 | 37 | 2.40 | 0.99-5.84 | |
| MGMT (rs12917) | |||||
| CC | 24 | 126 | Reference | ||
| TT/TC | 5 | 31 | 0.84 | 0.28-2.55 | |
| XRCC3 (rs861539) | |||||
| CC | 10 | 64 | Reference | ||
| TT/TC | 18 | 91 | 1.14 | 0.47-2.78 | |
| GSTP105 (rs1695) | |||||
| AA | 9 | 69 | Reference | ||
| AG/GG | 16 | 44 | 0.28 | 0.10-0.75 | |
Abbreviations: OR, odds ratio; CI, confidence interval; NQO1, NAD(P)H dehydrogenase, quinone 1; MGMT, O6-methylguanine-DNA methyltransferase; XRCC3, x-ray repair complementing defective repair in Chinese hamster cells 3; GSTP1, glutathione S-transferase pi 1
Adjusted for age, sex, and clinical risk score
Discussion
This study has revealed clinical factors that may predict the occurrence of myelotoxicity resulting from the first course of TMZ chemotherapy. Overall, as has been previously reported, women had a higher risk of myelotoxicity than did men. This association with female gender has also been reported with other chemotherapeutic agents and with a variety of cancers.13 Gender differences in pharmacologic response and clinically relevant adverse drug reactions have been increasingly recognized.14 Female sex has been shown to be associated with a 1.5- to 1.7-fold greater risk of adverse drug reactions.15,16 This increased risk is due to sex differences in pharmacokinetics and in pharmacodynamics. Both the volume of distribution and clearance determine drug concentrations.14 Females have a higher percentage of body fat, which may affect volume of distribution, and lower glomerular filtration rate, which may affect clearance of the drug.14 In addition, sex differences have been demonstrated in the activity of hepatic enzymes, drug transporters, and renal excretion.17,18 In this study, clinical risk factors for females included lower BSA and higher creatinine, which may reflect differences in absorption and elimination. Medication groups associated with risk by gender may also indicate underlying differences or may augment differences that are seen.
Inclusion of patients in the analysis. Of the 1,283 patients identified for chart review, 680 of them with complete clinical data were included in the risk score analysis. Of those, 275 with genotyping data were included in the preliminary polymorphism analysis. We excluded 19 nonwhite patients, as there were insufficient numbers to adequately look for differences based on ethnicity, and matched for age and sex. This left 29 patients with toxicity and 158 patients without toxicity for the genetic analysis.
Previously, an increased risk of myelotoxicity was thought to be associated with prior cytotoxic chemotherapy and with patients with obesity. However, our results indicate a higher risk in women who are chemotherapy naive. Although intriguing, this result should be interpreted with caution because it may reflect that women who had prior chemotherapy and developed significant myelotoxicity were prevented from being considered for additional cytotoxic chemotherapy, thereby precluding treatment with TMZ. Recent analysis has shown that obesity was not a risk factor for patients with breast cancer.19 In the literature, the data on age are conflicting and may be more related to concurrent organ dysfunction in elderly cancer patients.20 Our results also support the contention that obesity and age are not as important as pretreatment blood counts and concomitant medications. In fact, contrary to prior speculations, in women a low BSA was associated with a higher risk of myelotoxicity.
It is becoming increasingly recognized that dose adaptation based only on BSA does not take into account interpatient variability.13,21 As a result of the use of BSA alone, clinically significant myelosuppression can occur with significant morbidity and mortality. In our study, 11% of patients were admitted to the hospital during their first course of therapy for myelotoxicity-related issues, and six patients died; autopsies were not performed on these patients. Several anecdotal reports of serious complications associated with myelotoxicity in patients with malignant glioma, including inability to continue treatment and even death, have recently been published.4–8 Gerber et al.5 recently reported severe thrombocytopenia in 15%–20% of patients treated with concurrent low-dose TMZ and radiation. Although the study was too small to evaluate potential risk factors, they also reported significant morbidity and the potential for delayed recovery affecting tolerance of further therapy.
The pathophysiologic basis for the impact of the concurrent use of other medications is not known. Potentially, differences in drug metabolism from either drug-drug interactions or genetic differences due to single SNPs in genes coding for drug-metabolizing enzymes may account for these findings. A subgroup of patients from this analysis was evaluated for genetic polymorphisms that may be associated with alterations in drug metabolism and risk of toxicity. In this small pilot study, we found three SNPs associated with increased (MGMT) and decreased (NQO1, GSTP1) risk for severe myelotoxicity. High levels of MGMT in the tumor are known to be associated with TMZ resistance and shorter overall survival.22 The G allele of MGMT, identified here as a risk factor, results in lower DNA repair and increased DNA damage,23 which could render leukocytes sensitive to the alkylating effects of the drug. In addition, reduced levels of NQO1 could lead to reduced activation of TMZ, thereby reducing the effects of the drug on normal cells, such as leukocytes. There is very little evidence in the literature to support the activation of TMZ by NQO1, but it is involved in the activation of similar chemotherapeutic agents.24 Finally, the effects of GSTP1 on reduced risk of myelotoxicity need further study, because the effects of the polymorphism are substrate specific and a relationship with TMZ has not been reported. We are currently planning a larger prospective study to confirm these preliminary results and to examine a more comprehensive panel of SNPs that may be important for risk of myelotoxicity. Furthermore, an analysis of concurrent clinical factors may provide a method to screen for patients at high risk of myelotoxicity who might need further genetic analyses. This genetic association, coupled with the calculation of clinical risk, has the potential to allow screening of patients for risk and for need for pharmacogenomic evaluation.
Conclusions
This retrospective analysis of a large database has led to the development of a risk model that may eventually be useful clinically to predict risk of myelotoxicity. If the group of patients at risk for clinically significant myelotoxicity could be identified, dose adjustments for these patients may reduce their risk. For those patients who are not clinically at risk, the potential exists for dose intensification with less concern for serious toxicity. A prospective analysis of the clinical risk model in a separate sample of patients is under way. Furthermore, an expansion of the SNP analysis is planned to help further define those patients at significant risk of myelotoxicity.
This study was supported in part by a grant from Schering Plough Oncology and an NCI grant R01CA070917 (M.B.).
References
Stupp R, Hegi ME, van den Bent MJ, et al. Changing paradigms— an update on the multidisciplinary management of malignant glioma.
Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma.
Schering-Plough.
Doyle TJ, Middelsen T, Croteau D. Fatal hematologic toxicity with prolonged continuous administration of temozolomide (TMZ) during radiation therapy (RT) in the treatment of newly-diagnosed glioblastoma multiforme (GBM): Report of a phase II trial [abstract].
Gerber DE, Grossman SA, Zeltzman M, Parisi MA, Kleinberg L. The impact of thrombocytopenia from temozolomide and radiation in newly diagnosed adults with high-grade gliomas.
Jalali R, Singh P, Menon H, Gujral S. Unexpected case of aplastic anemia in a patient with glioblastoma multiforme treated with temozolomide.
Noronha V, Berliner N, Ballen KK, et al. Treatment-related myelodysplasia/AML in a patient with a history of breast cancer and an oligodendroglioma treated with temozolomide: case study and review of the literature.
Singhal N, Selva-Nayagam S, Brown MP. Prolonged and severe myelosuppression in two patients after low-dose temozolomide treatment— case study and review of literature.
Armstrong TS, et al.
National Cancer Institute.
Smith TJ, et al. 2006 update of recommendations for the use of white blood cell growth factors: an evidence-based clinical practice guideline.
National Comprehensive Cancer Network.
Kloft C, Wallin J, Henningsson A, Chatelut E, Karlsson MO. Population pharmacokinetic-pharmacodynamic model for neutropenia with patient subgroup identification: comparison across anticancer drugs.
Fattinger K, Roos M, Vergères P, et al. Epidemiology of drug exposure and adverse drug reactions in two Swiss departments of internal medicine.
Tran C, Knowles SR, Liu BA, Shear NH. Gender differences in adverse drug reactions.
Wrighton SA, Stevens JC. The human hepatic cytochromes P450 involved in drug metabolism.
Burchell B, Brierley CH, Rance D. Specificity of human UDP-glucuronosyltransferases and xenobiotic glucuronidation.
Jenkins P, Elyan S, Freeman S. Obesity is not associated with increased myelosuppression in patients receiving chemotherapy for breast cancer.
Hurria A, Lichtman SM. Pharmacokinetics of chemotherapy in the older patient.
Grochow LB, Baraldi C, Noe D. Is dose normalization to weight or body surface area useful in adults?
Friedman HS. DNA mismatch repair and 06-alkylguanine-DNA methyltransferase analysis and response to Temodal in newly diagnosed malignant glioma.
Margison GP, Heighway J, Pearson S, et al. Quantitative trait locus analysis reveals two intragenic sites that influence O6-alkylguanine-DNA alkyltransferase activity in peripheral blood mononuclear cells.
- analgesics
- polymorphism
- chemotherapy regimen
- creatinine
- gastroesophageal reflux disease
- glutathione
- body surface area
- dna
- intestines
- methyltransferase
- o(6)-methylguanine-dna methyltransferase
- oxidoreductase
- quinones
- risk assessment
- steroids
- transferase
- genetic aspects
- gender
- temozolomide
- glioma, malignant