-
PDF
- Split View
-
Views
-
Cite
Cite
Kevin R. Kozak, John S. Moody, Giant cell glioblastoma: A glioblastoma subtype with distinct epidemiology and superior prognosis, Neuro-Oncology, Volume 11, Issue 6, December 2009, Pages 833–841, https://doi.org/10.1215/15228517-2008-123
Close - Share Icon Share
Abstract
Giant cell glioblastoma (GC) is an uncommon subtype of glioblastoma multiforme (GBM). Consequently, the epidemiology, natural history, and factors associated with outcome are not well defined. Patients diagnosed with GC from 1988 through 2004 were identified in the Surveillance, Epidemiology, and End Results (SEER) database. Outcomes were examined with Kaplan-Meier survival analysis and Cox models. For comparison, similar analyses were conducted for patients diagnosed with GBM. GC was identified in 1% of 16,430 patients diagnosed with either GC or GBM. Compared with GBM, GC showed similar gender and racial distributions. Likewise, tumor size and location were not significantly different between the two histologies. GC tended to occur in younger patients with a median age at diagnosis of 51 years, compared with 62 years for GBM. Additionally, patients with GC were more likely to undergo complete resection compared with patients with GBM. For both histologies, young age, tumor size, extent of resection, and the use of adjuvant radiation therapy (RT) were associated with improved survival. Cox modeling suggests the prognosis for GC is significantly superior to that for GBM (hazard ratio = 0.76; 95% confidence interval, 0.59–0.97) even after adjustment for factors affecting survival. GC is an uncommon GBM subtype that tends to occur in younger patients. Prospective data defining optimal treatment for GC are unavailable; however, these retrospective findings suggest that resection, as opposed to biopsy only, and adjuvant RT may improve survival. The prognosis of GC is superior to that of GBM, and long-term survival is possible, suggesting aggressive therapy is warranted.
Giant cell glioblastoma (GC) is a rare neoplasm characterized by a predominance of bizarre multinucleated giant cells with abundant eosinophilic cytoplasm.1 This histology, also known as monstrocellular brain tumor, was first described by Schmincke in the early twentieth century and first called “giant-celled glioma” by Meyer in 1913.2,3 Significant efforts to characterize this unusual malignancy have established a glial origin, and it is now considered a subtype of glioblastoma multiforme (GBM).4–9
GC has been reported to represent between 2% and 5% of GBM cases but may be more common in the pediatric population.10–12 Due to the rarity of this malignancy, our knowledge about GC is limited to small retrospective case series and case reports. In general, the epidemiology and natural history appear to differ between GC and GBM. GC tends to occur in younger patients and has been variably reported to show a male predominance and a predilection for the temporal lobe.5,6,10,12–16 Importantly, several small series and case reports have suggested that the prognosis of GC is significantly better than that observed for GBM.4,5,9,11,17–23
Unfortunately, owing to small patient numbers, reported GC case series are not sufficiently powered to precisely characterize this neoplasm. For example, it remains unclear whether the improved prognosis observed for GC is a function of true biologic differences between this histology and GBM, or if it is a consequence of younger age at presentation or even misclassification of the similar, but significantly more indolent, pleomorphic xanthoastrocytoma (PXA). Furthermore, modest but clinically meaningful differences between GC and GBM may not be appreciated until a large cohort of GC patients is examined. To refine our understanding of GC, we used the National Cancer Institute's Surveillance, Epidemiology, and End Results (SEER) database to identify and analyze 171 GC patients and compared them with more than 16,000 GBM patients.
Materials and Methods
Study Population
Data were obtained from the SEER database using the SEER 17 Registries Database for 1973–2006.24 Patients with GC and GBM diagnosed between January 1988 and December 2004 were identified. Histologic classification was based on the International Classification of Diseases for Oncology code (GC, 9441; GBM, 9440).25 Patients were excluded if this was not their first malignancy or if pathologic confirmation was not obtained. Only intracranial tumors were considered. A total of 171 GC and 16,259 GBM patients met the criteria for inclusion.
Statistical Analysis
GC and GBM patient populations were evaluated with respect to multiple patient, tumor, and treatment characteristics (age, sex, race, tumor location, tumor size, extent of surgery, and use of adjuvant radiation therapy [RT]). Unadjusted associations of categoric variables of interest were evaluated using Pearson's chi square test. The two-sample t-test was used to compare the means of variables from different populations of interest. The primary end point in this study was overall survival. Patients were censored either at death or at date of last follow-up. Kaplan-Meier survival analysis was used to assess overall survival. The Mantel-Cox log-rank test was used to assess differences between survival curves. Univariate and multivariate Cox proportional hazard models were developed to calculate hazard ratios (HRs) and 95% confidence intervals (CIs) to quantify the impact of individual variables on overall survival. For multivariate models, forward stepwise procedures were chosen in an a priori fashion wherein variables were entered into the model with p < 0.05 and removed if the significance of that variable subsequently exceeded p = 0.10.
SEER*Stat version 6.3.5 (Surveillance Research Program, National Cancer Institute, Bethesda, MD, USA) was used to extract case-level data from the SEER public-use databases. All analyses were conducted using the Statistical Package for the Social Sciences (SPSS, version 14.0; SPSS, Inc., Chicago, IL, USA).
Results
GC accounted for 171, or 1%, of the 16,430 patients diagnosed with GC or GBM. Patient, tumor, and treatment characteristics are displayed in Table 1. GC and GBM share several characteristics. Both display a 1.4- to 1.5-fold male predominance. Racial distribution is comparable. Tumor size and location do not differ between GC and GBM; there is no apparent GC proclivity for the temporal lobe. In contrast, GC patients present earlier than do GBM patients: the median age at diagnosis differs by more than a decade, and 3.5 times more GC patients than GBM patients present prior to 40 years of age. Additionally, GC patients tend to receive more aggressive surgical resection.
Patient, tumor, and treatment characteristics
| Characteristic . | GC (n = 171) . | GBM (n = 16,259) . | p-Value . |
|---|---|---|---|
| Patient characteristics | |||
| Median age, years (range) | 51 (2–87) | 62 (0–96) | |
| Age, years | < 0.001 | ||
| 0–20 | 9.4% | 1.4% | |
| 21–39 | 18.1% | 6.4% | |
| 40–49 | 21.1% | 13.0% | |
| 50–59 | 16.4% | 22.5% | |
| 60–69 | 15.8% | 26.0% | |
| ≥70 | 19.3% | 30.7% | |
| Gender | 0.718 | ||
| Male | 59.6% | 58.3% | |
| Female | 40.4% | 41.7% | |
| Race | 0.372 | ||
| White | 87.7% | 90.9% | |
| Black | 5.8% | 4.7% | |
| American Indian/Alaska Native | 0.0% | 0.2% | |
| Asian/Pacific Islander | 6.4% | 3.9% | |
| Other unspecified | 0.0% | 0.3% | |
| Tumor characteristics | |||
| Location | 0.273 | ||
| Frontal | 28.1% | 24.1% | |
| Temporal | 26.3% | 23.1% | |
| Parietal | 18.7% | 17.2% | |
| Occipital | 4.1% | 4.2% | |
| Ventricle | 1.2% | 0.5% | |
| Cerebellum | 0.6% | 0.7% | |
| Brainstem | 0.6% | 0.7% | |
| Overlapping/NOS | 20.5% | 29.6% | |
| Median tumor size, cm (range)a | 4.2 (1.8–10.0) | 4.5 (0.1 to ≥10.0) | 0.699 |
| Treatment characteristics | |||
| Extent of resection | 0.004 | ||
| No cancer-directed surgery | 15.2% | 24.0% | |
| Local tumor destruction | 0.0% | 0.4% | |
| Subtotal tumor excision | 12.3% | 18.8% | |
| Gross total tumor excision | 19.3% | 14.1% | |
| Partial excision of primary site (i.e., partial lobectomy) | 21.6% | 20.7% | |
| Total excision of primary site (i.e., total lobectomy) | 29.8% | 20.6% | |
| Surgery NOS | 1.8% | 1.1% | |
| Unknown | 0.0% | 0.2% | |
| Radiation therapy | 0.069 | ||
| Yes | 79.5% | 73.4% | |
| No | 19.9% | 23.4% | |
| Unknown | 0.6% | 3.2% |
| Characteristic . | GC (n = 171) . | GBM (n = 16,259) . | p-Value . |
|---|---|---|---|
| Patient characteristics | |||
| Median age, years (range) | 51 (2–87) | 62 (0–96) | |
| Age, years | < 0.001 | ||
| 0–20 | 9.4% | 1.4% | |
| 21–39 | 18.1% | 6.4% | |
| 40–49 | 21.1% | 13.0% | |
| 50–59 | 16.4% | 22.5% | |
| 60–69 | 15.8% | 26.0% | |
| ≥70 | 19.3% | 30.7% | |
| Gender | 0.718 | ||
| Male | 59.6% | 58.3% | |
| Female | 40.4% | 41.7% | |
| Race | 0.372 | ||
| White | 87.7% | 90.9% | |
| Black | 5.8% | 4.7% | |
| American Indian/Alaska Native | 0.0% | 0.2% | |
| Asian/Pacific Islander | 6.4% | 3.9% | |
| Other unspecified | 0.0% | 0.3% | |
| Tumor characteristics | |||
| Location | 0.273 | ||
| Frontal | 28.1% | 24.1% | |
| Temporal | 26.3% | 23.1% | |
| Parietal | 18.7% | 17.2% | |
| Occipital | 4.1% | 4.2% | |
| Ventricle | 1.2% | 0.5% | |
| Cerebellum | 0.6% | 0.7% | |
| Brainstem | 0.6% | 0.7% | |
| Overlapping/NOS | 20.5% | 29.6% | |
| Median tumor size, cm (range)a | 4.2 (1.8–10.0) | 4.5 (0.1 to ≥10.0) | 0.699 |
| Treatment characteristics | |||
| Extent of resection | 0.004 | ||
| No cancer-directed surgery | 15.2% | 24.0% | |
| Local tumor destruction | 0.0% | 0.4% | |
| Subtotal tumor excision | 12.3% | 18.8% | |
| Gross total tumor excision | 19.3% | 14.1% | |
| Partial excision of primary site (i.e., partial lobectomy) | 21.6% | 20.7% | |
| Total excision of primary site (i.e., total lobectomy) | 29.8% | 20.6% | |
| Surgery NOS | 1.8% | 1.1% | |
| Unknown | 0.0% | 0.2% | |
| Radiation therapy | 0.069 | ||
| Yes | 79.5% | 73.4% | |
| No | 19.9% | 23.4% | |
| Unknown | 0.6% | 3.2% |
Abbreviations: GC, giant cell glioblastoma; GBM, glioblastoma; NOS, not otherwise specified
Tumor size was known for 112 patients with GC and 10,201 patients with GBM
Patient, tumor, and treatment characteristics
| Characteristic . | GC (n = 171) . | GBM (n = 16,259) . | p-Value . |
|---|---|---|---|
| Patient characteristics | |||
| Median age, years (range) | 51 (2–87) | 62 (0–96) | |
| Age, years | < 0.001 | ||
| 0–20 | 9.4% | 1.4% | |
| 21–39 | 18.1% | 6.4% | |
| 40–49 | 21.1% | 13.0% | |
| 50–59 | 16.4% | 22.5% | |
| 60–69 | 15.8% | 26.0% | |
| ≥70 | 19.3% | 30.7% | |
| Gender | 0.718 | ||
| Male | 59.6% | 58.3% | |
| Female | 40.4% | 41.7% | |
| Race | 0.372 | ||
| White | 87.7% | 90.9% | |
| Black | 5.8% | 4.7% | |
| American Indian/Alaska Native | 0.0% | 0.2% | |
| Asian/Pacific Islander | 6.4% | 3.9% | |
| Other unspecified | 0.0% | 0.3% | |
| Tumor characteristics | |||
| Location | 0.273 | ||
| Frontal | 28.1% | 24.1% | |
| Temporal | 26.3% | 23.1% | |
| Parietal | 18.7% | 17.2% | |
| Occipital | 4.1% | 4.2% | |
| Ventricle | 1.2% | 0.5% | |
| Cerebellum | 0.6% | 0.7% | |
| Brainstem | 0.6% | 0.7% | |
| Overlapping/NOS | 20.5% | 29.6% | |
| Median tumor size, cm (range)a | 4.2 (1.8–10.0) | 4.5 (0.1 to ≥10.0) | 0.699 |
| Treatment characteristics | |||
| Extent of resection | 0.004 | ||
| No cancer-directed surgery | 15.2% | 24.0% | |
| Local tumor destruction | 0.0% | 0.4% | |
| Subtotal tumor excision | 12.3% | 18.8% | |
| Gross total tumor excision | 19.3% | 14.1% | |
| Partial excision of primary site (i.e., partial lobectomy) | 21.6% | 20.7% | |
| Total excision of primary site (i.e., total lobectomy) | 29.8% | 20.6% | |
| Surgery NOS | 1.8% | 1.1% | |
| Unknown | 0.0% | 0.2% | |
| Radiation therapy | 0.069 | ||
| Yes | 79.5% | 73.4% | |
| No | 19.9% | 23.4% | |
| Unknown | 0.6% | 3.2% |
| Characteristic . | GC (n = 171) . | GBM (n = 16,259) . | p-Value . |
|---|---|---|---|
| Patient characteristics | |||
| Median age, years (range) | 51 (2–87) | 62 (0–96) | |
| Age, years | < 0.001 | ||
| 0–20 | 9.4% | 1.4% | |
| 21–39 | 18.1% | 6.4% | |
| 40–49 | 21.1% | 13.0% | |
| 50–59 | 16.4% | 22.5% | |
| 60–69 | 15.8% | 26.0% | |
| ≥70 | 19.3% | 30.7% | |
| Gender | 0.718 | ||
| Male | 59.6% | 58.3% | |
| Female | 40.4% | 41.7% | |
| Race | 0.372 | ||
| White | 87.7% | 90.9% | |
| Black | 5.8% | 4.7% | |
| American Indian/Alaska Native | 0.0% | 0.2% | |
| Asian/Pacific Islander | 6.4% | 3.9% | |
| Other unspecified | 0.0% | 0.3% | |
| Tumor characteristics | |||
| Location | 0.273 | ||
| Frontal | 28.1% | 24.1% | |
| Temporal | 26.3% | 23.1% | |
| Parietal | 18.7% | 17.2% | |
| Occipital | 4.1% | 4.2% | |
| Ventricle | 1.2% | 0.5% | |
| Cerebellum | 0.6% | 0.7% | |
| Brainstem | 0.6% | 0.7% | |
| Overlapping/NOS | 20.5% | 29.6% | |
| Median tumor size, cm (range)a | 4.2 (1.8–10.0) | 4.5 (0.1 to ≥10.0) | 0.699 |
| Treatment characteristics | |||
| Extent of resection | 0.004 | ||
| No cancer-directed surgery | 15.2% | 24.0% | |
| Local tumor destruction | 0.0% | 0.4% | |
| Subtotal tumor excision | 12.3% | 18.8% | |
| Gross total tumor excision | 19.3% | 14.1% | |
| Partial excision of primary site (i.e., partial lobectomy) | 21.6% | 20.7% | |
| Total excision of primary site (i.e., total lobectomy) | 29.8% | 20.6% | |
| Surgery NOS | 1.8% | 1.1% | |
| Unknown | 0.0% | 0.2% | |
| Radiation therapy | 0.069 | ||
| Yes | 79.5% | 73.4% | |
| No | 19.9% | 23.4% | |
| Unknown | 0.6% | 3.2% |
Abbreviations: GC, giant cell glioblastoma; GBM, glioblastoma; NOS, not otherwise specified
Tumor size was known for 112 patients with GC and 10,201 patients with GBM
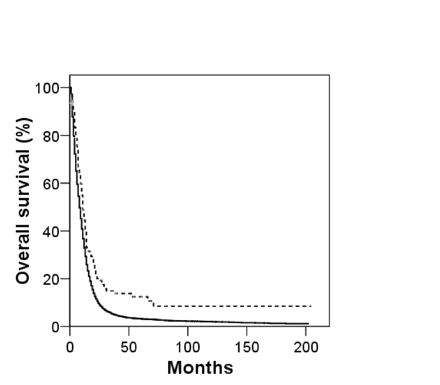
Overall survival for both GC and GBM patients is shown in Fig. 1. Although the prognosis for both GC and GBM is poor, with median survivals of 11 and 8 months, respectively, overall survival of patients with GC is superior to patients with GBM. Furthermore, prolonged survival (i.e., 5 years) is rare for GBM patients, whereas it is observed in more than 10% of GC patients (overall 5-year survival: GC, 12.3%; GBM, 3.4%). Factors affecting overall survival for both cohorts were examined, and the univariate analysis is shown in Table 2. As expected, age at presentation, extent of resection, and adjuvant RT were significantly associated with GBM survival. Gender, race, tumor location, and tumor size also had small, but significant, associations with GBM overall survival, which in part reflects the very large population examined. For GC patients, factors influencing survival included age at presentation, tumor size, extent of resection, and adjuvant RT use. Tumor location also affected GC survival, with atypical locations (i.e., brainstem, ventricle, or cerebellum) generally faring worse. Gender and race did not correlate with GC survival. Multivariate Cox proportional hazard models for GC and GBM are shown in Table 3. Age at presentation, tumor size, cancer-directed surgery, and adjuvant RT use remained significant predictors of GC overall survival. For GBM, tumor location was also significantly associated with survival. Gender and race were not significantly associated with overall survival for either histology. To investigate the prognostic significance of GC histology, a multivariate Cox model was constructed using the entire cohort and histology as an additional variable. GC histology was associated with prolonged survival (HR = 0.76; 95% CI, 0.59–0.97). To ensure this finding was not biased by potential misclassifications of PXA as GC, the analysis was repeated excluding patients younger than 40 years of age. GC histology remained significantly associated with overall survival (HR = 0.71; 95% CI, 0.53–0.95).
Univariate analysis of the impact of patient, tumor, and treatment factors on overall survival (hazard ratio [95% confidence interval])
| Characteristic . | GC . | GBM . |
|---|---|---|
| Patient characteristics | ||
| Age, years | p < 0.001 | p < 0.001 |
| 0-20a | 1.00 | 1.00 |
| 21-39 | 1.08 (0.51-2.31) | 0.82 (0.69-0.97) |
| 40-49 | 1.03 (0.49-2.16) | 1.30 (1.11-1.53) |
| 50-59 | 1.49 (0.70-3.19) | 1.65 (1.41-1.93) |
| 60-69 | 2.32 (1.10-4.91) | 2.36 (2.02-2.76) |
| ≥70 | 3.84 (1.85-7.98) | 3.70 (3.17-4.32) |
| Gender | p = 0.464 | p = 0.004 |
| Malea | 1.00 | 1.00 |
| Female | 0.88 (0.62-1.25) | 1.05 (1.02-1.08) |
| Race | p = 0.409 | p < 0.001 |
| Whitea | 1.00 | 1.00 |
| Black | 1.36 (0.66-2.79) | 1.02 (0.94-1.10) |
| American Indian/Alaska Native | — | 0.81 (0.58-1.14) |
| Asian/Pacific Islander | 0.72 (0.38-1.39) | 0.82 (0.76-0.90) |
| Other unspecified | — | 0.62 (0.43-0.88) |
| Tumor characteristics | ||
| Location | p < 0.001 | p < 0.001 |
| Frontala | 1.00 | 1.00 |
| Temporal | 1.11 (0.69-1.78) | 1.01 (0.96-1.06) |
| Parietal | 0.90 (0.53-1.54) | 1.10 (1.04-1.15) |
| Occipital | 0.40 (0.10-1.69) | 1.03 (0.94-1.12) |
| Ventricle | 6.20 (1.46-26.32) | 1.51 (1.19-1.91) |
| Cerebellum | 4.60 (0.62-34.27) | 0.94 (0.76-1.15) |
| Brainstem | 72.14 (7.34-709.20) | 1.25 (1.02-1.53) |
| Overlapping/NOS | 1.99 (1.22-3.25) | 1.30 (1.24-1.36) |
| Tumor sizeb | p = 0.016 | p < 0.001 |
| <Median sizea | 1.00 | 1.00 |
| ≥Median size | 1.71 (1.10-2.65) | 1.08 (1.04-1.13) |
| Treatment characteristics | ||
| Extent of resectionc | p < 0.001 | p < 0.001 |
| No cancer-directed surgerya | 1.00 | 1.00 |
| Local tumor destruction | — | 0.63 (0.49-0.81) |
| Subtotal tumor excision | 0.31 (0.16-0.58) | 0.54 (0.51-0.56) |
| Gross total tumor excision | 0.48 (0.28-0.82) | 0.50 (0.48-0.53) |
| Partial excision of primary site (i.e., partial lobectomy) | 0.45 (0.26-0.78) | 0.58 (0.55-0.61) |
| Total excision of primary site (i.e., total lobectomy) | 0.33 (0.19-0.57) | 0.40 (0.38-0.42) |
| Surgery NOS | 1.42 (0.33-6.07) | 0.49 (0.42-0.57) |
| Radiation therapyd | p < 0.001 | p < 0.001 |
| Yesa | 1.00 | 1.00 |
| No | 4.33 (2.83-6.62) | 2.66 (2.56-2.76) |
| Characteristic . | GC . | GBM . |
|---|---|---|
| Patient characteristics | ||
| Age, years | p < 0.001 | p < 0.001 |
| 0-20a | 1.00 | 1.00 |
| 21-39 | 1.08 (0.51-2.31) | 0.82 (0.69-0.97) |
| 40-49 | 1.03 (0.49-2.16) | 1.30 (1.11-1.53) |
| 50-59 | 1.49 (0.70-3.19) | 1.65 (1.41-1.93) |
| 60-69 | 2.32 (1.10-4.91) | 2.36 (2.02-2.76) |
| ≥70 | 3.84 (1.85-7.98) | 3.70 (3.17-4.32) |
| Gender | p = 0.464 | p = 0.004 |
| Malea | 1.00 | 1.00 |
| Female | 0.88 (0.62-1.25) | 1.05 (1.02-1.08) |
| Race | p = 0.409 | p < 0.001 |
| Whitea | 1.00 | 1.00 |
| Black | 1.36 (0.66-2.79) | 1.02 (0.94-1.10) |
| American Indian/Alaska Native | — | 0.81 (0.58-1.14) |
| Asian/Pacific Islander | 0.72 (0.38-1.39) | 0.82 (0.76-0.90) |
| Other unspecified | — | 0.62 (0.43-0.88) |
| Tumor characteristics | ||
| Location | p < 0.001 | p < 0.001 |
| Frontala | 1.00 | 1.00 |
| Temporal | 1.11 (0.69-1.78) | 1.01 (0.96-1.06) |
| Parietal | 0.90 (0.53-1.54) | 1.10 (1.04-1.15) |
| Occipital | 0.40 (0.10-1.69) | 1.03 (0.94-1.12) |
| Ventricle | 6.20 (1.46-26.32) | 1.51 (1.19-1.91) |
| Cerebellum | 4.60 (0.62-34.27) | 0.94 (0.76-1.15) |
| Brainstem | 72.14 (7.34-709.20) | 1.25 (1.02-1.53) |
| Overlapping/NOS | 1.99 (1.22-3.25) | 1.30 (1.24-1.36) |
| Tumor sizeb | p = 0.016 | p < 0.001 |
| <Median sizea | 1.00 | 1.00 |
| ≥Median size | 1.71 (1.10-2.65) | 1.08 (1.04-1.13) |
| Treatment characteristics | ||
| Extent of resectionc | p < 0.001 | p < 0.001 |
| No cancer-directed surgerya | 1.00 | 1.00 |
| Local tumor destruction | — | 0.63 (0.49-0.81) |
| Subtotal tumor excision | 0.31 (0.16-0.58) | 0.54 (0.51-0.56) |
| Gross total tumor excision | 0.48 (0.28-0.82) | 0.50 (0.48-0.53) |
| Partial excision of primary site (i.e., partial lobectomy) | 0.45 (0.26-0.78) | 0.58 (0.55-0.61) |
| Total excision of primary site (i.e., total lobectomy) | 0.33 (0.19-0.57) | 0.40 (0.38-0.42) |
| Surgery NOS | 1.42 (0.33-6.07) | 0.49 (0.42-0.57) |
| Radiation therapyd | p < 0.001 | p < 0.001 |
| Yesa | 1.00 | 1.00 |
| No | 4.33 (2.83-6.62) | 2.66 (2.56-2.76) |
Abbreviations: GC, giant cell glioblastoma; GBM, glioblastoma; NOS, not otherwise specified
Reference category
Only tumors with known size are included in the analysis (n = 112 GC patients, n = 10,201 GBM patients)
Patients with unknown extent of surgery were excluded (n = 31 GBM patients)
Patients with unknown use of radiation therapy were excluded (n = 1 GC patient, n = 513 GBM patients)
Univariate analysis of the impact of patient, tumor, and treatment factors on overall survival (hazard ratio [95% confidence interval])
| Characteristic . | GC . | GBM . |
|---|---|---|
| Patient characteristics | ||
| Age, years | p < 0.001 | p < 0.001 |
| 0-20a | 1.00 | 1.00 |
| 21-39 | 1.08 (0.51-2.31) | 0.82 (0.69-0.97) |
| 40-49 | 1.03 (0.49-2.16) | 1.30 (1.11-1.53) |
| 50-59 | 1.49 (0.70-3.19) | 1.65 (1.41-1.93) |
| 60-69 | 2.32 (1.10-4.91) | 2.36 (2.02-2.76) |
| ≥70 | 3.84 (1.85-7.98) | 3.70 (3.17-4.32) |
| Gender | p = 0.464 | p = 0.004 |
| Malea | 1.00 | 1.00 |
| Female | 0.88 (0.62-1.25) | 1.05 (1.02-1.08) |
| Race | p = 0.409 | p < 0.001 |
| Whitea | 1.00 | 1.00 |
| Black | 1.36 (0.66-2.79) | 1.02 (0.94-1.10) |
| American Indian/Alaska Native | — | 0.81 (0.58-1.14) |
| Asian/Pacific Islander | 0.72 (0.38-1.39) | 0.82 (0.76-0.90) |
| Other unspecified | — | 0.62 (0.43-0.88) |
| Tumor characteristics | ||
| Location | p < 0.001 | p < 0.001 |
| Frontala | 1.00 | 1.00 |
| Temporal | 1.11 (0.69-1.78) | 1.01 (0.96-1.06) |
| Parietal | 0.90 (0.53-1.54) | 1.10 (1.04-1.15) |
| Occipital | 0.40 (0.10-1.69) | 1.03 (0.94-1.12) |
| Ventricle | 6.20 (1.46-26.32) | 1.51 (1.19-1.91) |
| Cerebellum | 4.60 (0.62-34.27) | 0.94 (0.76-1.15) |
| Brainstem | 72.14 (7.34-709.20) | 1.25 (1.02-1.53) |
| Overlapping/NOS | 1.99 (1.22-3.25) | 1.30 (1.24-1.36) |
| Tumor sizeb | p = 0.016 | p < 0.001 |
| <Median sizea | 1.00 | 1.00 |
| ≥Median size | 1.71 (1.10-2.65) | 1.08 (1.04-1.13) |
| Treatment characteristics | ||
| Extent of resectionc | p < 0.001 | p < 0.001 |
| No cancer-directed surgerya | 1.00 | 1.00 |
| Local tumor destruction | — | 0.63 (0.49-0.81) |
| Subtotal tumor excision | 0.31 (0.16-0.58) | 0.54 (0.51-0.56) |
| Gross total tumor excision | 0.48 (0.28-0.82) | 0.50 (0.48-0.53) |
| Partial excision of primary site (i.e., partial lobectomy) | 0.45 (0.26-0.78) | 0.58 (0.55-0.61) |
| Total excision of primary site (i.e., total lobectomy) | 0.33 (0.19-0.57) | 0.40 (0.38-0.42) |
| Surgery NOS | 1.42 (0.33-6.07) | 0.49 (0.42-0.57) |
| Radiation therapyd | p < 0.001 | p < 0.001 |
| Yesa | 1.00 | 1.00 |
| No | 4.33 (2.83-6.62) | 2.66 (2.56-2.76) |
| Characteristic . | GC . | GBM . |
|---|---|---|
| Patient characteristics | ||
| Age, years | p < 0.001 | p < 0.001 |
| 0-20a | 1.00 | 1.00 |
| 21-39 | 1.08 (0.51-2.31) | 0.82 (0.69-0.97) |
| 40-49 | 1.03 (0.49-2.16) | 1.30 (1.11-1.53) |
| 50-59 | 1.49 (0.70-3.19) | 1.65 (1.41-1.93) |
| 60-69 | 2.32 (1.10-4.91) | 2.36 (2.02-2.76) |
| ≥70 | 3.84 (1.85-7.98) | 3.70 (3.17-4.32) |
| Gender | p = 0.464 | p = 0.004 |
| Malea | 1.00 | 1.00 |
| Female | 0.88 (0.62-1.25) | 1.05 (1.02-1.08) |
| Race | p = 0.409 | p < 0.001 |
| Whitea | 1.00 | 1.00 |
| Black | 1.36 (0.66-2.79) | 1.02 (0.94-1.10) |
| American Indian/Alaska Native | — | 0.81 (0.58-1.14) |
| Asian/Pacific Islander | 0.72 (0.38-1.39) | 0.82 (0.76-0.90) |
| Other unspecified | — | 0.62 (0.43-0.88) |
| Tumor characteristics | ||
| Location | p < 0.001 | p < 0.001 |
| Frontala | 1.00 | 1.00 |
| Temporal | 1.11 (0.69-1.78) | 1.01 (0.96-1.06) |
| Parietal | 0.90 (0.53-1.54) | 1.10 (1.04-1.15) |
| Occipital | 0.40 (0.10-1.69) | 1.03 (0.94-1.12) |
| Ventricle | 6.20 (1.46-26.32) | 1.51 (1.19-1.91) |
| Cerebellum | 4.60 (0.62-34.27) | 0.94 (0.76-1.15) |
| Brainstem | 72.14 (7.34-709.20) | 1.25 (1.02-1.53) |
| Overlapping/NOS | 1.99 (1.22-3.25) | 1.30 (1.24-1.36) |
| Tumor sizeb | p = 0.016 | p < 0.001 |
| <Median sizea | 1.00 | 1.00 |
| ≥Median size | 1.71 (1.10-2.65) | 1.08 (1.04-1.13) |
| Treatment characteristics | ||
| Extent of resectionc | p < 0.001 | p < 0.001 |
| No cancer-directed surgerya | 1.00 | 1.00 |
| Local tumor destruction | — | 0.63 (0.49-0.81) |
| Subtotal tumor excision | 0.31 (0.16-0.58) | 0.54 (0.51-0.56) |
| Gross total tumor excision | 0.48 (0.28-0.82) | 0.50 (0.48-0.53) |
| Partial excision of primary site (i.e., partial lobectomy) | 0.45 (0.26-0.78) | 0.58 (0.55-0.61) |
| Total excision of primary site (i.e., total lobectomy) | 0.33 (0.19-0.57) | 0.40 (0.38-0.42) |
| Surgery NOS | 1.42 (0.33-6.07) | 0.49 (0.42-0.57) |
| Radiation therapyd | p < 0.001 | p < 0.001 |
| Yesa | 1.00 | 1.00 |
| No | 4.33 (2.83-6.62) | 2.66 (2.56-2.76) |
Abbreviations: GC, giant cell glioblastoma; GBM, glioblastoma; NOS, not otherwise specified
Reference category
Only tumors with known size are included in the analysis (n = 112 GC patients, n = 10,201 GBM patients)
Patients with unknown extent of surgery were excluded (n = 31 GBM patients)
Patients with unknown use of radiation therapy were excluded (n = 1 GC patient, n = 513 GBM patients)
Multivariate analysis of the impact of patient, tumor, and treatment factors on overall survival (hazard ratio [95% confidence interval])a
| Characteristic . | GC . | GBM . |
|---|---|---|
| Patient characteristics | ||
| Age, years | p < 0.001 | p < 0.001 |
| <40b | 1.00 | 1.00 |
| 40-59 | 0.79 (0.38-1.68) | 2.03 (1.80-2.29) |
| ≥60 | 3.68 (1.86-7.28) | 3.69 (3.28-4.15) |
| Tumor characteristics | ||
| Location | p = 0.009 | |
| Frontalb | 1.00 | |
| Temporal | 0.98 (0.92-1.04) | |
| Parietal | 1.09 (1.02-1.17) | |
| Occipital | 1.01 (0.90-1.13) | |
| Tumor size | p = 0.028 | p < 0.001 |
| <Median sizeb | 1.00 | 1.00 |
| ≥Median size | 1.84 (1.07-3.18) | 1.15 (1.10-1.22) |
| Treatment characteristics | ||
| Extent of resection | p = 0.004 | p < 0.001 |
| No cancer-directed surgeryb | 1.00 | 1.00 |
| Cancer-directed surgery | 0.30 (0.13-0.68) | 0.55 (0.51-0.59) |
| Radiation therapy | p < 0.001 | p < 0.001 |
| Yesb | 1.00 | 1.00 |
| No | 4.51 (2.27-8.96) | 2.65 (2.48-2.82) |
| Characteristic . | GC . | GBM . |
|---|---|---|
| Patient characteristics | ||
| Age, years | p < 0.001 | p < 0.001 |
| <40b | 1.00 | 1.00 |
| 40-59 | 0.79 (0.38-1.68) | 2.03 (1.80-2.29) |
| ≥60 | 3.68 (1.86-7.28) | 3.69 (3.28-4.15) |
| Tumor characteristics | ||
| Location | p = 0.009 | |
| Frontalb | 1.00 | |
| Temporal | 0.98 (0.92-1.04) | |
| Parietal | 1.09 (1.02-1.17) | |
| Occipital | 1.01 (0.90-1.13) | |
| Tumor size | p = 0.028 | p < 0.001 |
| <Median sizeb | 1.00 | 1.00 |
| ≥Median size | 1.84 (1.07-3.18) | 1.15 (1.10-1.22) |
| Treatment characteristics | ||
| Extent of resection | p = 0.004 | p < 0.001 |
| No cancer-directed surgeryb | 1.00 | 1.00 |
| Cancer-directed surgery | 0.30 (0.13-0.68) | 0.55 (0.51-0.59) |
| Radiation therapy | p < 0.001 | p < 0.001 |
| Yesb | 1.00 | 1.00 |
| No | 4.51 (2.27-8.96) | 2.65 (2.48-2.82) |
Abbreviations: GC, giant cell glioblastoma; GBM, glioblastoma; NOS, not otherwise specified
Age, gender, race, tumor location, tumor size, surgery, and radiation therapy were used to construct a forward-conditional Cox model of overall survival for both GC and GBM patients. Only patients with known age, gender, race, tumor size, surgical extent, and radiation therapy use were included in the analysis; furthermore, only patients with cerebral tumor locations were included (n = 90 GC patients, n = 6,897 GBM patients)
Reference category
Multivariate analysis of the impact of patient, tumor, and treatment factors on overall survival (hazard ratio [95% confidence interval])a
| Characteristic . | GC . | GBM . |
|---|---|---|
| Patient characteristics | ||
| Age, years | p < 0.001 | p < 0.001 |
| <40b | 1.00 | 1.00 |
| 40-59 | 0.79 (0.38-1.68) | 2.03 (1.80-2.29) |
| ≥60 | 3.68 (1.86-7.28) | 3.69 (3.28-4.15) |
| Tumor characteristics | ||
| Location | p = 0.009 | |
| Frontalb | 1.00 | |
| Temporal | 0.98 (0.92-1.04) | |
| Parietal | 1.09 (1.02-1.17) | |
| Occipital | 1.01 (0.90-1.13) | |
| Tumor size | p = 0.028 | p < 0.001 |
| <Median sizeb | 1.00 | 1.00 |
| ≥Median size | 1.84 (1.07-3.18) | 1.15 (1.10-1.22) |
| Treatment characteristics | ||
| Extent of resection | p = 0.004 | p < 0.001 |
| No cancer-directed surgeryb | 1.00 | 1.00 |
| Cancer-directed surgery | 0.30 (0.13-0.68) | 0.55 (0.51-0.59) |
| Radiation therapy | p < 0.001 | p < 0.001 |
| Yesb | 1.00 | 1.00 |
| No | 4.51 (2.27-8.96) | 2.65 (2.48-2.82) |
| Characteristic . | GC . | GBM . |
|---|---|---|
| Patient characteristics | ||
| Age, years | p < 0.001 | p < 0.001 |
| <40b | 1.00 | 1.00 |
| 40-59 | 0.79 (0.38-1.68) | 2.03 (1.80-2.29) |
| ≥60 | 3.68 (1.86-7.28) | 3.69 (3.28-4.15) |
| Tumor characteristics | ||
| Location | p = 0.009 | |
| Frontalb | 1.00 | |
| Temporal | 0.98 (0.92-1.04) | |
| Parietal | 1.09 (1.02-1.17) | |
| Occipital | 1.01 (0.90-1.13) | |
| Tumor size | p = 0.028 | p < 0.001 |
| <Median sizeb | 1.00 | 1.00 |
| ≥Median size | 1.84 (1.07-3.18) | 1.15 (1.10-1.22) |
| Treatment characteristics | ||
| Extent of resection | p = 0.004 | p < 0.001 |
| No cancer-directed surgeryb | 1.00 | 1.00 |
| Cancer-directed surgery | 0.30 (0.13-0.68) | 0.55 (0.51-0.59) |
| Radiation therapy | p < 0.001 | p < 0.001 |
| Yesb | 1.00 | 1.00 |
| No | 4.51 (2.27-8.96) | 2.65 (2.48-2.82) |
Abbreviations: GC, giant cell glioblastoma; GBM, glioblastoma; NOS, not otherwise specified
Age, gender, race, tumor location, tumor size, surgery, and radiation therapy were used to construct a forward-conditional Cox model of overall survival for both GC and GBM patients. Only patients with known age, gender, race, tumor size, surgical extent, and radiation therapy use were included in the analysis; furthermore, only patients with cerebral tumor locations were included (n = 90 GC patients, n = 6,897 GBM patients)
Reference category
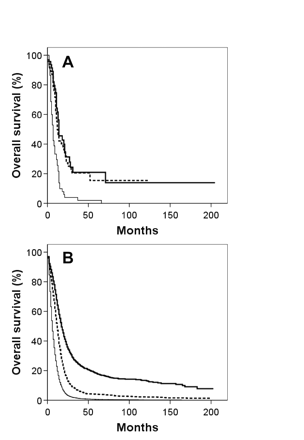
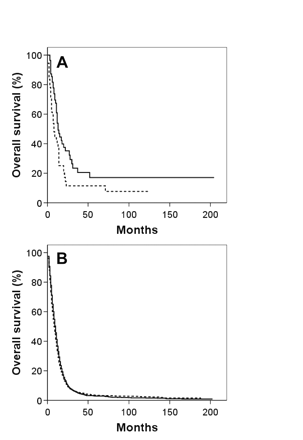
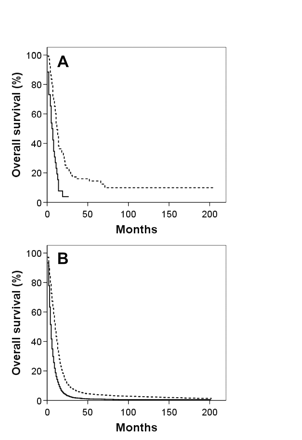
Kaplan-Meier overall survival curves for both GBM and GC patients segregated by the four variables associated with survival based on multivariate analysis for both histologies—age, tumor size, extent of resection, and adjuvant RT use—are shown in Figs. 2, 3, 4, 5. To correct for postoperative mortality, the impact of adjuvant RT was reanalyzed with the exclusion of patients who survived less than 2 months after diagnosis (Fig. 5C, D). Analyses using alternate exclusion time points (1 and 3 months) yielded nearly identical results (data not shown).
Kaplan-Meier overall survival curves for giant cell glioblastoma (dashed line) and glioblastoma multiforme (solid line) patients.
Discussion
Unambiguous characterization of GC is hampered by the rarity of this malignancy. For example, Palma and colleagues reviewed more than 5,000 brain tumors resected between 1952 and 1984 at the Neurosurgical Clinic of Rome Medical School and identified 42 cases of GC.10 Shinojima and colleagues reviewed 113 patients enrolled on one of two phase III trials for GBM and identified three patients with GC.11 Interestingly, these three patients represented half of all patients who survived at least 5 years. Similarly, Burger and Vollmer reviewed 184 cases of malignant gliomas from the National Brain Tumor Study Group, identifying 12 patients with GC neoplasms.17 Importantly, this study suggested that the presence of GC histology portends improved survival. Additional small series and case reports have been published. However, scarcely more than 100 cases have appeared in the world literature.1 Consequently, a comprehensive understanding of GC has remained elusive. Using the SEER database, we have attempted to identify a large patient cohort to define the epidemiology, natural history, and factors associated with the outcome of GC.
In contrast to the study by Palma et al.10 that found GC to represent 1% of all brain tumors and 5% of all GBM tumors, our results suggest that GC represents approximately 1% of all GBM tumors. Consistent with prior reports, our data indicate that GC tends to present at an earlier age compared with GBM.5,6,10,11,13–15 We found no difference in the male predominance between GC and GBM, with males affected approximately 1.5 times more frequently than females. This observation is consistent with the male predominance of GC reported by several investigators5,8,10,15,17 but conflicts with the gender neutrality or female predominance reported by others.6,11,13,14 Although the racial distribution of GC has not previously been reported, we found similar racial distributions for both GC and GBM. We also found no statistically significant differences between GC and GBM regarding tumor size or location. A modest temporal lobe predilection has been reported by some authors;5,16 however, consistent with our observations, others have found no such propensity for temporal lobe involvement.6,10 An interesting difference identified between GC and GBM relates to the extent of surgical resection undertaken; patients with GC tended to undergo more aggressive surgical therapy than those with GBM. Given the absence of anatomic and tumor size differences, these factors do not likely explain the observed disparity. Two potential factors may contribute to more aggressive surgical management of GC patients. First, GC patients tend to be younger, and age at presentation has clearly been demonstrated to affect the surgical patterns of care for GBM patients. For example, Barnholtz-Sloan and colleagues reported that GBM patients 75 or more years of age were approximately 1.6-fold more likely to undergo biopsy, as opposed to surgical resection, compared with patients 66–74 years of age.26 Second, GC tumors have been reported to be better circumscribed than GBM tumors, potentially increasing resectability.1,2,5
Our survival analyses confirm reports that GC survival is superior to that observed with GBM.4–6,9–12,17– 21,23 The median survival among all GC patients was 11 months, compared with 8 months for GBM. Importantly, long-term survival was significantly more common with the GC histology, leading to a marked difference in mean survival durations (GC, 32 months [95% CI, 20–41 months]; GBM, 15 months [95% CI, 14–15 months]). Several hypotheses have been offered to explain the improved prognosis of GC. Our results support the suggestion that the younger age of GC patients favorably affects survival compared with GBM patients.27 However, in multivariate analyses including age, tumor histology remained significantly associated with outcome. Thus, the younger presenting age for GC does not entirely explain the superior prognosis. Others have suggested that the more circumscribed radiologic and histopathologic appearance of GC may permit more complete resection and thus improve prognosis compared with GBM.1,5 Our data indirectly support this hypothesis, because more aggressive resection was both more likely for GC patients and associated with improved outcome. However, survival differences are not entirely attributable to differences in surgical management; histology remained an important predictor of survival when extent of resection was included in multivariate models. Several authors have suggested that prolonged survival for GC patients may be affected by the erroneous inclusion of PXA in case series describing GC natural history.6,20,27 PXA is a WHO grade II glioma that shares several radiographic and histopathologic features with GC, leading to potential challenges in distinguishing these two histologies. PXA characteristically affects adolescents and young adults and is associated with favorable patient outcomes. However, in our study, the impact of GC histology on survival was unchanged when we excluded patients younger than 40 years, which indicates that misclassification of PXA as GC was not a significant confounding factor in our series. Finally, emerging evidence suggests that fundamental genetic differences may underlie the different natural histories of GC and GBM. GC tumors have been shown to harbor p53 mutations more commonly than do GBM tumors. However, compared with GBM tumors, p16 deletion and EGFR, MDM2, and CDK4 amplification are rare in GC tumors.13–15,28 Our data suggest that the improved outcomes observed with GC are not entirely attributable to known clinical prognostic factors (e.g., age, surgical management, RT), and it is tempting to speculate that identified, and other yet undefined, genetic differences may contribute to the improved survival of GC patients.
Kaplan-Meier overall survival curves for giant cell glioblastoma (A) and glioblastoma multiforme (B) patients segregated by age: thick solid line, <40 years; dashed line, 40–59 years; thin solid line, ≥60 years.
Kaplan-Meier overall survival curves for giant cell glioblastoma (A) and glioblastoma multiforme (B) patients segregated by tumor size: solid line, tumor size < median; dashed line, tumor size ≥ median.
Kaplan-Meier overall survival curves for giant cell glioblastoma (A) and glioblastoma multiforme (B) patients segregated by extent of tumor resection: solid line, no cancer-directed surgery; dashed line, cancer-directed surgery (i.e., more than biopsy).
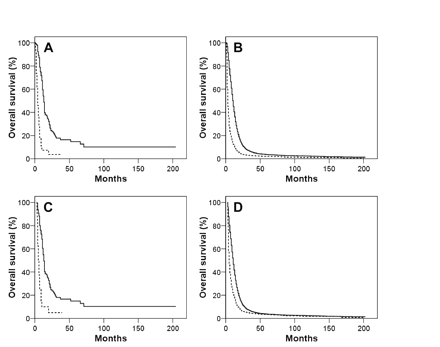
The optimal management of GC is imperfectly defined due to the rarity of this neoplasm. Our data confirm the potential prognostic importance of tumor resection in GBM outcome and extend this observation to GC.29–32 Median survival of GC and GBM patients undergoing biopsy only was found to be 6 and 5 months, respectively. Median survival doubled to 12 months and 10 months for GC and GBM, respectively, when some form of cancer-directed surgery was undertaken. Furthermore, consistent with the observation of Palma and colleagues,10 we found that adjuvant RT improved survival of GC patients. Among GC patients surviving at least 2 months postoperatively, the addition of adjuvant RT was associated with an improvement in median survival from 5 to 13 months. This improvement closely paralleled the benefits of adjuvant RT in GBM patients: median survival increased from 4 to 11 months. Taken together, our data suggest that GC patients, like GBM patients, are likely to benefit from maximal safe resection followed by adjuvant RT.
(A and B) Kaplan-Meier overall survival curves for giant cell glioblastoma (GC; A) and glioblastoma multiforme (GBM; B) patients who received (solid line) and who did not receive (dashed line) adjuvant radiation therapy. (C and D) comparable Kaplan-Meier overall survival curves for GC (C) and GBM (D) patients who received (solid line) and who did not receive (dashed line) adjuvant radiation therapy, with the exclusion of patients who did not survive a minimum of 2 months following diagnosis.
This study has a number of limitations. The SEER database provides exceptionally large patient numbers and thus offers sufficient statistical power to identify factors influencing outcomes of rare malignancies. However, several clinically relevant factors are not captured in the database, including RT details, the use of chemotherapy, patient performance status, and presenting symptom severity and duration. These factors influence GBM outcome and may affect GC patient overall survival.29,33 Furthermore, this study requires all of the important caveats of other retrospective analyses.
Despite these limitations, this study represents the most comprehensive analysis of GC and provides clinically meaningful prognostic and therapeutic insights. With the exception of the younger age at presentation, GC presents similarly to GBM. Despite a superior prognosis compared with GBM, GC outcomes remain poor, with median survival of about 1 year. However, long-term survival is possible, and tumor resection, as opposed to biopsy only, and adjuvant RT appear to improve outcome. Further improvements in GC management may be possible as the unique features of this rare malignancy continue to be explored.
References
Ohgaki H, Peraud A, Nakazato Y, Watanabe K, von Deimling A. Giant cell glioblastoma. In: Kleihues P, cavenee WK, eds.
Schmincke A. Beitrag zur lehre der ganglioneurome: ein ganglioneurom des gehirns.
Becker DP, Benyo R, Roessmann U. Glial origin of monstrocellular tumor: case report of prolonged survival.
Margetts JC, Kalyan-Raman UP. Giant-celled glioblastoma of brain: a clinico-pathological and radiological study of ten cases (including immunohistochemistry and ultrastructure).
Katoh M, Aida T, Sugimoto S, et al. Immunohistochemical analysis of giant cell glioblastoma.
Hadfield MG, Silverberg SG. Light and electron microscopy of giant-cell glioblastoma.
Kawano H, Kubota T, Sato K, Goya T, Arikawa S. Wakisaka S. Immunohistochemical study of giant cell in glioblastoma.
Akslen LA, Mork SJ, Larsen JL, Myrseth E. Giant cell glioblastoma: a work-up of 2 cases with long survival.
Palma L, Celli P, Maleci A, Di Lorenzo N, cantore G. Malignant monstrocellular brain tumors: a study of 42 surgically treated cases.
Shinojima N, Kochi M, Hamada J-I, et al. The influence of sex and the presence of giant cells on postoperative long-term survival in adult patients with supratentorial glioblastoma multiforme.
Artico M, Cervoni L, celli P, Salvati M, Palma L. Supratentorial glioblastoma in children: a series of 27 surgically treated cases.
Meyer-Puttlitz B, Hayashi Y, Waha A, et al. Molecular genetic analysis of giant cell glioblastomas.
Martinez R, Roggendorf W, Baretton G, et al. Cytogenetic and molecular genetic analyses of giant cell glioblastoma multiforme reveal distinct profiles in giant cell and non-giant cell subpopulations.
Peraud A, Watanabe K, Schwechheimer K, Yonekawa Y, Kleihues P, Ohgaki H. Genetic profile of the giant cell glioblastoma.
De Prada I, cordobes F, Azorin D, Contra T, Colmenero I, Glez-Mediero I. Pediatric giant cell glioblastoma: a case report and review of the literature.
Burger PC, Vollmer RT. Histologic factors of prognostic significance in the glioblastoma multiforme.
Klein R, Molenkamp G, Sorensen N, Roggendorf W. Favorable outcome of giant cell glioblastoma in a child: report of an 11-year survival period.
Sabel M, Reifenberger J, Weber RG, Reifenberger G, Schmitt HP. Long-term survival of a patient with giant cell glioblastoma: case report.
Kroh H, Matyja E, Marchel A, Bojarski P. Heavily lipidized, calcified giant cell glioblastoma in an 8-year-old patient, associated with neurofibromatosis type 1 (NF1): report of a case with long-term survival.
Deb P, Sharma MC, Chander B, Mahapatra AK, Sarkar C. Giant cell glioblastoma multiforme: report of a case with prolonged survival and transformation to gliosarcoma.
Chang C-C, Kuwana N, Ito S, Kitamura H. Spinal leptomeningeal metastases of giant cell glioblastoma associated with subarachnoid haemorrhage: case report.
Gullotta F, Casentini L, Neumann J. Giant cell gliomas of the temporal lobe.
Piercy C, Holt VV, Muir C.
Barnholtz-Sloan JS, Williams VL, Maldonado JL, et al. Patterns of care and outcomes among elderly individuals with primary malignant astrocytoma.
Martinez-Diaz H, Kleinschmidt-DeMasters BK, Powell SZ, Yachnis AT. Giant cell glioblastoma and pleomorphic xanthoastrocytoma show different immunohistochemical profiles for neuronal antigens and p53 but share reactivity for class III β-tubulin.
Peraud A, Watanabe K, Plate KH, Yonekawa Y, Kleihues P, Ohgaki H. p53 mutations versus EGF receptor expression in giant cell glioblastomas.
Curran WJ, Scott CB, Horton J, et al. Recursive partitioning analysis of prognostic factors in three Radiation Therapy Oncology Group malignant glioma trials.
Quigley MR, Maroon JC. The relationship between survival and the extent of the resection in patients with supratentorial malignant gliomas.
Devaux BC, O'Fallon JR, Kelly PJ. Resection, biopsy, and survival in malignant glial neoplasms. A retrospective study of clinical parameters, therapy, and outcome.
Laws ER, Parney IF, Huang W, et al. Survival following surgery and prognostic factors for recently diagnosed malignant glioma: data from the Glioma Outcomes Project.