Published online Aug 16, 2014. doi: 10.12998/wjcc.v2.i8.362
Revised: June 25, 2014
Accepted: July 12, 2014
Published online: August 16, 2014
AIM: To investigate the outcomes of trauma patients with traumatic brain injury (TBI) on Dabigatran Etexilate (DE).
METHODS: Following IRB approval, all patients taking DE who were admitted to our level 1 trauma service were enrolled in the study. Injury complexity, length of stay (LOS), intensive care length of stay, operative intervention, therapeutic interventions and outcomes were analyzed retrospectively.
RESULTS: Twenty-eight of 4310 admissions were taking DE. Eleven patients were excluded on concurrent antiplatelet therapy. Average age was 77.14 years (64-94 years), and average LOS was 4.7 d (1-35 d). Thirty-two percent were admitted with intracranial hemorrhage. Eighteen percent received factor VII, and 22% received dialysis in attempts to correct coagulopathy. Mortality was 21%.
CONCLUSION: The low incidence, absence of reversal agents, and lack of practice guidelines makes managing patients with TBI taking DE frustrating and provider specific. Local practice guidelines may be helpful in managing such patients.
Core tip: Dabigatran Etexilate (DE) and other novel anticoagulants that lack reversal agents complicate the care of trauma patients. Current practice guidelines should be available to aid in managing patients with traumatic brain injury on DE.
- Citation: Pakraftar S, Atencio D, English J, Corcos A, Altschuler EM, Stahlfeld K. Dabigatran etixilate and traumatic brain injury: Evolving anticoagulants require evolving care plans. World J Clin Cases 2014; 2(8): 362-366
- URL: https://www.wjgnet.com/2307-8960/full/v2/i8/362.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v2.i8.362
Arterial and venous thromboembolism (VTE) is a significant cause of mortality and morbidity. Direct and indirect inhibitors of coagulation are being increasingly utilized for prophylaxis and treatment of myocardial infarction, valvular disease, deep venous thrombosis, pulmonary embolism, atrial fibrillation, and stroke[1]. Compliance rates for VTE prophylaxis are being used in pay for performance by third party payors and have been included as an independent new core measure by the Center for Medicare and Medicaid Services[2].
Many anticoagulants are available to the clinician: antiplatelet agents, thromboxane A2 receptor antagonists, Adenosine Diphosphate (ADP) receptor antagonists, Protease Activated Receptor (PAR)-1 antagonists, inhibitors of initiation or propagation of coagulation, Factor IX-directed antibodies, direct and indirect Factor Xa inhibitors, factor Va and VIIIa inhibitors, inhibitors of fibrin formation, and medications than enhance fibrinolysis[3]. Due to the complication rate, volume of distribution, delayed onset, prolonged effect, unpredictable pharmacokinetics, food and medication interactions, and frequent monitoring associated with warfarin usage, industry has focused on developing oral thrombin and Factor Xa inhibitors for patients who require long-term anticoagulation.
Dabigatran Etexilate (DE) (Pradaxa®) 150 mg twice daily is the first orally available FDA approved direct thrombin inhibitor (DTI) in the United States. Due to predictable pharmacokinetics and pharmacodynamics, limited drug-drug interaction or effect of food, and no need for coagulation monitoring, DE was introduced enthusiastically and approved for treatment of non-vavlular atrial fibrillation (AF) with a class 1 recommendation[4]. Head to head trials with warfarin showed that DE reduced the risk of stroke by more than one-half and that mortality from intracranial hemorrhage was not increased (1B)[5]. Patients on DE had a significantly higher rate of gastrointestinal bleeding and trended toward an increased number of adjudicated coronary events[5]. As no reversal agent or accurate method of measuring the clinical effect of DE exists, recommendations for patients undergoing elective surgery currently taking DE are to stop the DE 1-5 d prior to the procedure, depending on the complexity of the surgery and the patient’s creatinine clearance (CrCL)[3,6,7].
Trauma patients and those with acute surgical issues frequently do not have the luxury of waiting 1-5 d for the pharmacologic effects of DE to subside. After several frustrating patient interactions that essentially involved supportive care, we hypothesized that patients taking DE admitted with traumatic injuries would have poor outcomes due to the lack of a reversal agent. We herein report our series of patients admitted to our trauma and acute care surgery service on DE, focusing on patients with traumatic brain injury (TBI), and comment on potential treatment strategies available.
After receiving institutional board approval, all patients between October 2011 and September 2012 admitted through the emergency room to one health system’s two Level 1 trauma centers were prospectively evaluated to include all patients who were actively taking DE on admission. Only patients over the age of 18 with vital signs on arrival were included in the study.
Patient management was directed by the trauma and acute care surgeon in conjunction with subspecialized physicians. Presence of traumatic brain injury on computed tomography (CT) was verified by a board certified radiologist, and demographic data, admission laboratory data including hemoglobin, prothrombin time (PT/INR), and partial thromboplastin time (PTT), patient acuity, therapeutic interventions, transfusion requirements, and patient outcomes were evaluated retrospectively.
Statistical analysis was performed using Microsoft Excel Analysis ToolPak (Student t-Test, χ2 Test, Anova).
Of the 4310 admissions to the trauma and acute care surgery service over the twelve month period, 31 (0.7%) patients taking DE were identified. Nine of the 1259 admissions with CT evidence of TBI were taking DE. Three of the 31 patients on DE were excluded because no significant surgical pathology was present. Of the remaining 28, the average age (SD) was 77.14 (10.5), median admission INR/PTT was 1.45/50.3, 11 were on concurrent anti-platelet medications. 6 received DE directed dialysis and 6 received factor VIIa. Mortality was 21% (6/28). Results for the subgroups of patients with TBI, injury without TBI, and acute care surgery are displayed in Table 1.
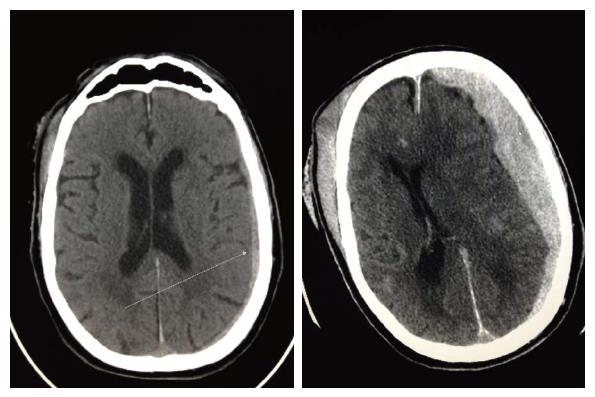
The individual data for the nine TBI injured patients on DE are listed in Table 2. Eight patients (89%) were taking DE for stroke prophylaxis and one for treatment of a prior pulmonary embolism. Recorded dosage was 150mg BID for all 9 subjects. Eight of nine patients had an elevated INR (mean = 1.68) and PTT (mean = 54). Four patients were taking antiplatelet medications concomitantly. Types of intracranial hemorrhage observed in these patients were sub-arachnoid (4), sub-dural (2), combined (2), and intraparenchymal (1). Two of the three patients who received no intervention died: one presented with a non-survivable injury and the second initially appeared to have a minor injury that within hours progressed clinically and radiographically (Figure 1).
| Case | Age, yr | LOS | iLOS | INR | PTT | Anti-Platelet | CrCl | HD | VIIa | FFP | Anti-Platelet | Mortality |
| 1 | 94 | 4 | 3 | 1.6 | 61.3 | Y | 24.1 | N | N | Y | Y | N |
| 2 | 83 | 1 | 1 | 2.5 | 69 | Y | 45.6 | N | N | N | N | Y |
| 3 | 79 | 2 | 0 | 1.6 | 54.3 | N | 49.8 | N | N | N | N | N |
| 4 | 89 | 6 | 3 | 1.4 | 35.7 | N | 40.2 | N | N | Y | N | N |
| 5 | 86 | 35 | 5 | 1.8 | 50.7 | Y | 32.4 | Y | Y | Y | Y | N |
| 6 | 64 | 3 | 2 | 1.7 | 57 | Y | 99 | N | N | Y | N | N |
| 7 | 88 | 1 | 1 | 1.8 | 61 | N | 61.3 | N | N | N | N | Y |
| 8 | 86 | 2 | 1 | 0.9 | 20 | N | 46 | N | N | Y | N | N |
| 9 | 82 | 4 | 2 | 1.9 | 82 | Y | 46.4 | Y | Y | Y | Y | N |
Coagulopathy and associated bleeding remain significant issues in the trauma population. Coagulopathy due to blood loss is addressed by controlling the ongoing bleeding, keeping the patient warm and perfused, and using accepted protocols to replace blood and blood products. Pharmacologically induced coagulopathy poses a similar risk and is becoming more prevalent, with approximately 1.5 million Americans taking a vitamin K antagonist daily[8]. Treatment of these patients is fairly straightforward as the effect of vitamin K is easily measured and the deficient clotting factors can be replaced.
With the introduction of DE, and subsequent FDA approval of direct factor Xa inhibitors rivaroxaban (Xarelto®) and apixaban (Eliquis®), the trauma surgeon faces a unique challenge in patients with ongoing bleeding who may or may not require surgery. DE is an orally available direct thrombin inhibitor that is rapidly converted to dabigatran and binds to free and clot bound thrombin. Time to maximum concentration is 2 h, half-life is 12-17 h, limited protein binding suggest DE may be dialyzed, and over 80% of the drug is excreted by the kidneys[3,7,9]. The FDA-approved indication is to reduce the risk of stroke and systemic embolism in patients with non-valvular atrial fibrillation[10].
Advantages of DE include the significant risk reduction of stroke and systemic embolization, predictable pharmacokinetics requiring no coagulation monitoring, a fast onset and offset of action, a relatively short half-life, and limited drug-drug interaction[3,7,11]. Drug cost compared to monitoring with warfarin is revenue neutral. Worldwide there have been at least 260 episodes of post-marketing bleeds resulting in death in patients on DE[12,13]. Our study documents the institutional complication rates of patients on DE and not the effectiveness of DE vs oral vitamin K antagonist. The dilemma facing the trauma surgeon is that there is no accepted laboratory test to measure the effect of DE nor are there recommended reversal agents[3,6,7,14]. Both of these factors are especially relevant in the patient with a TBI. The anticoagulant effects have attempted to be quantified in normal human subjects, laboratory animals, and in vitro by adding DE to human serum. Assays evaluated include PT, aPTT, factors II, VIII, IX, X, and XI, quantitative D-dimer, reptilase time, von Willebrand factor antigen , antithrombin, plasminogen, thrombin clotting time, protein C activity, ecarin clotting time, and activated protein C resistance[1,11]. Although analytes may be elevated with various concentrations of DE, most notably the aPTT and thrombin clotting time, reported levels frequently are factitiously elevated or low, display incomplete correction, do not correlate with serum levels leading to misdiagnosis and mismanagement, or are insensitive or oversensitive, making virtually any result unreliable[11]. The best determinate of DE effect is knowing the timing of administration, as peak effect is usually two hours after ingestion, the dosage and the patient’s renal function (CrCl > 50 provides normal excretion)[9].
Treatment can be simplistic and futile as no known DE counteracting agent exists, so any form of intervention in patients with life-threatening bleeding is empirical. What makes this even more frustrating is the individual trauma surgeon most likely treats a patient taking DE once every several months, has no recommended guidelines, and may be unfamiliar with the intricacies and pharmacokinetics of the most recently approved oral anticoagulant. Considering that not intervening when a patient is actively bleeding is difficult for the treating surgeon, we will discuss the rationale behind several available treatment strategies although all lack even level 3 evidence.
Excluding direct compression, topical thrombin, and simple surgical procedures to obtain hemostasis, viable options to treat extensively injured, TBI, and complex surgical patients taking DE include oral charcoal, activated prothrombin complex concentrates (aPCC), recombinant factor VIIa, concentrates of coagulation factors II, IX, and X, and dialysis.
Oral charcoal can be used within two hours of ingestion as charcoal significantly inhibits absorption of DE[6,7]. Kcentra (CSL Behring LLC) is the only four factor prothrombin complex concentrate available in the United States, has not been shown to correct the aPTT in healthy volunteers taking DE, but high doses have been shown to limit intracranial bleeding in rats[3,14]. In a patient with life-threatening bleeding with limited therapeutic options, an INR based dose of 25-50 IU/kg may be justified[6]. Recombinant VIIa has not demonstrated any alteration in the coagulation profile or outcomes in healthy volunteers or laboratory animals taking DE and has documented higher arterial thromboembolic events[15]. Subsequently, salvage therapy with rVIIa should be used cautiously, although a case report suggests high dose therapy (7.2 mg × 2) may be beneficial[16]. Activated PCC has been shown to correct the anticoagulant effect of DE in animal models and reduces clot initiation time in humans in vitro. Siegal suggests using aPCC (80 U/kg) over PCC in patients taking DE, but reverses the recommendation for patients taking rivaroxaban (XareltoR) or apixaban (Eliquis), acknowledging that any such recommendation is based on limited data[17]. Kcentra has been shown to partially reverse the effects of factor Xa inhibitors[14].
Dialysis is an attractive option as DE is not plasma bound and excreted renally, but this is the most invasive option and use may be limited due to injury severity. In patients with end-stage renal disease, dialysis removed 62% of circulating DE within two hours, although due to the volume of distribution serum levels rebounded quickly upon cessation of dialysis[9]. Selective case reports suggest that prolonged dialysis (6 h) with flow rates of 700 mL/min improve outcome[16].
Maintaining adequate diuresis is important for all patients, but should not be overlooked as DE is excreted renally. Currently no role exists for desmopressin, protamine sulfate, tranexamic acid, or vitamin K, or fresh frozen plasma[3,6,7]. Platelet concentrates should only be used in cases with thrombocytopenia or concurrent antiplatelet therapies. Although not yet available, a monoclonal antibody directed against DE is under development[17].
In our experience, less than one percent of trauma and acute surgical admissions were taking DE and each surgeon averaged fewer than two patients per year. The percentage of patients with TBI is remarkably similar. With such limited numbers, and reviewing the largest industry sponsored trial (18113 patients) reporting outcomes of 22 patients with TBI, level 1 management recommendations are unlikely[18].
Subsequently, we developed an in-house protocol for patients admitted taking DE, where we obtain baseline clotting studies, a stat hematology consult for major or life-threatening hemorrhage, a nephrology consult for initiation of hemodialysis, and the option of giving a 40 mcg/kg IV dose of rfactor VIIa or Kcentra.
Our study is limited by the small sample size and retrospective collection of the data. Additionally, recommendations extrapolated from the literature combine data from multiple laboratories and include human, animal, and in-vitro studies. Finally, treatment is individualized and up to the discretion of the surgeon.
In a conclusion, DE is a cost-neutral highly effective oral direct thrombin inhibitor approved recently along with two factor Xa inhibitors, rivaroxaban and apixaban. Management of the traumatic brain injury patient taking DE poses unique and confounding issues as the effect of DE is not measurable and no reversal agents are currently recommended. Trauma surgeons manage patients on DE infrequently and such encounters may be frustrating. For patients taking DE, strategies for non-operative management of bleeding are discretionary and institution dependent and include oral charcoal, maintaining adequate diuresis, PCC, aPCC, and dialysis.
Seventy million Americans will be over the age of 65 by 2030 and five percent of these patients have atrial fibrillation and are candidates for anticoagulation. In 2010, the ACC Foundation and the AHA added Dabigatran Etexilate (DE) to their treatment guidelines with a class 1 recommendation for non-valvular atrial fibrillation. DE is an attractive alternative to warfarin (WF) due to improved outcomes and the lack of need for serial monitoring. However, it poses a risk to the trauma population because of an extended half-life and the lack of a reversal agent. Therefore it was our aim to review the outcomes of patients with TBI on DE.
DE and other novel anticoagulants that lack a true reversal agent post a unique dilemma for trauma surgeons. Local care plans should be initiated until dose specific reversal agents are commercial available.
Current practice guidelines should be available to aid in managing patients with traumatic brain injury on DE. Therapeutic options include: oral charcoal, maintaining adequate diuresis, prothrombin complex concentrates, activated prothrombin complex concentrates, and dialysis.
Dabigatran Etexilate is a new oral anticoagulant that works by directly inhibiting thrombin in the clotting cascade.
This is a single institution observation study and it is limited as such. Future research goals will be multi institution collaborations on not just DE but other novel agents in the hopes of developing nationwide guidelines for treatment of novel agents until industry specific antidotes are commercially available.
P- Reviewer: Kim D S- Editor: Wen LL L- Editor: A E- Editor: Lu YJ
| 1. | Weitz JI, Hirsh J, Samama MM. New antithrombotic drugs: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest. 2008;133:234S-256S. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 179] [Cited by in F6Publishing: 190] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 2. | Amin AN, Deitelzweig SB. Optimizing the prevention of venous thromboembolism: recent quality initiatives and strategies to drive improvement. Jt Comm J Qual Patient Saf. 2009;35:558-564. [PubMed] [Cited in This Article: ] |
| 3. | Ageno W, Gallus AS, Wittkowsky A, Crowther M, Hylek EM, Palareti G. Oral anticoagulant therapy: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141:e44S-e88S. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1106] [Cited by in F6Publishing: 1016] [Article Influence: 84.7] [Reference Citation Analysis (0)] |
| 4. | Wann LS, Curtis AB, Ellenbogen KA, Estes NA, Ezekowitz MD, Jackman WM, January CT, Lowe JE, Page RL, Slotwiner DJ. 2011 ACCF/AHA/HRS focused update on the management of patients with atrial fibrillation (update on dabigatran). A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Heart Rhythm. 2011;8:e1-e8. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 155] [Cited by in F6Publishing: 169] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 5. | Connolly SJ, Ezekowitz MD, Yusuf S, Eikelboom J, Oldgren J, Parekh A, Pogue J, Reilly PA, Themeles E, Varrone J. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361:1139-1151. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7917] [Cited by in F6Publishing: 7742] [Article Influence: 516.1] [Reference Citation Analysis (0)] |
| 6. | Siegal DM, Crowther MA. Acute management of bleeding in patients on novel oral anticoagulants. Eur Heart J. 2013;34:489-498b. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 117] [Cited by in F6Publishing: 131] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 7. | van Ryn J, Stangier J, Haertter S, Liesenfeld KH, Wienen W, Feuring M, Clemens A. Dabigatran etexilate--a novel, reversible, oral direct thrombin inhibitor: interpretation of coagulation assays and reversal of anticoagulant activity. Thromb Haemost. 2010;103:1116-1127. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1046] [Cited by in F6Publishing: 1091] [Article Influence: 77.9] [Reference Citation Analysis (0)] |
| 8. | Gage BF, Fihn SD, White RH. Management and dosing of warfarin therapy. Am J Med. 2000;109:481-488. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 102] [Cited by in F6Publishing: 90] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 9. | Chang DN, Dager WE, Chin AI. Removal of dabigatran by hemodialysis. Am J Kidney Dis. 2013;61:487-489. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 125] [Cited by in F6Publishing: 129] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 10. | FDA Drug Safety Communication. Safety review of post-market reports of serious bleeding events with the anticoagulant Pradaxa (Dabigatran Etexilate Mesylate) 2012. Available from: http: //wwwfdagov/Drugs/DrugSafety/ucm282724htm#data. [Cited in This Article: ] |
| 11. | Adcock DM, Gosselin R, Kitchen S, Dwyre DM. The effect of dabigatran on select specialty coagulation assays. Am J Clin Pathol. 2013;139:102-109. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 58] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 12. | Huang GS, Chance EA. When dabigatran and trauma collide. Am Surg. 2013;79:113-114. [PubMed] [Cited in This Article: ] |
| 13. | Wood S. Dabigatran: 260 fatal bleeds since approval worldwide. 2011; Medscape Multispeciality. Available from: http: //www.medscape.com/viewarticle/753816. [Cited in This Article: ] |
| 14. | Eerenberg ES, Kamphuisen PW, Sijpkens MK, Meijers JC, Buller HR, Levi M. Reversal of rivaroxaban and dabigatran by prothrombin complex concentrate: a randomized, placebo-controlled, crossover study in healthy subjects. Circulation. 2011;124:1573-1579. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1065] [Cited by in F6Publishing: 991] [Article Influence: 76.2] [Reference Citation Analysis (0)] |
| 15. | Mayer SA, Brun NC, Begtrup K, Broderick J, Davis S, Diringer MN, Skolnick BE, Steiner T. Efficacy and safety of recombinant activated factor VII for acute intracerebral hemorrhage. N Engl J Med. 2008;358:2127-2137. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 942] [Cited by in F6Publishing: 847] [Article Influence: 52.9] [Reference Citation Analysis (0)] |
| 16. | Warkentin TE, Margetts P, Connolly SJ, Lamy A, Ricci C, Eikelboom JW. Recombinant factor VIIa (rFVIIa) and hemodialysis to manage massive dabigatran-associated postcardiac surgery bleeding. Blood. 2012;119:2172-2174. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 176] [Cited by in F6Publishing: 185] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 17. | Siegal DM, Cuker A. Reversal of novel oral anticoagulants in patients with major bleeding. J Thromb Thrombolysis. 2013;35:391-398. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 68] [Cited by in F6Publishing: 62] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 18. | Hart RG, Diener HC, Yang S, Connolly SJ, Wallentin L, Reilly PA, Ezekowitz MD, Yusuf S. Intracranial hemorrhage in atrial fibrillation patients during anticoagulation with warfarin or dabigatran: the RE-LY trial. Stroke. 2012;43:1511-1517. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 309] [Cited by in F6Publishing: 308] [Article Influence: 25.7] [Reference Citation Analysis (0)] |