Published online Mar 26, 2020. doi: 10.12998/wjcc.v8.i6.1002
Peer-review started: December 10, 2019
First decision: January 13, 2020
Revised: February 25, 2020
Accepted: March 9, 2020
Article in press: March 9, 2020
Published online: March 26, 2020
The intestinal tract (i.e., the gut), is where the body’s nutrients are absorbed, and is simultaneously inhabited by numerous microbes. An increasing body of literature suggests a crucial role for the gut microbiome in modulating systemic inflammatory disease. Psoriasis is a chronic systemic inflammatory disease and its pathogenesis is related to the interaction between genetic susceptibility, immune response and environmental triggers. The omics era has allowed physicians to assess different aspects of psoriasis pathogenesis such as the microbiome, infectome, and autoinfectome. Furthermore, diet appears to play an important role in modulating disease activity, perhaps by influencing gut microbes. Given these observations, we aimed to summarize the current knowledge regarding skin-microbiome-gut-nutrients and psoriasis.
Core tip: Psoriasis is a chronic systemic inflammatory disease and its pathogenesis is related to the interaction between genetic susceptibility, immune response and environmental triggers. The omics era has allowed physicians to study psoriasis pathogenesis from different perspectives such as the microbiome, infectome, and autoinfectome. Furthermore, diet appears to play an important role in modulating disease activity. Given these observations, this review aimed to summarize the current knowledge on skin-microbiome-gut-nutrients and psoriasis.
- Citation: Damiani G, Bragazzi NL, McCormick TS, Pigatto PDM, Leone S, Pacifico A, Tiodorovic D, Di Franco S, Alfieri A, Fiore M. Gut microbiota and nutrient interactions with skin in psoriasis: A comprehensive review of animal and human studies. World J Clin Cases 2020; 8(6): 1002-1012
- URL: https://www.wjgnet.com/2307-8960/full/v8/i6/1002.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v8.i6.1002
Psoriasis is alternatively regarded as an inflammatory[1], pruritic[2], autoimmune[3,4], or even autoinflammatory[5] systemic chronic disease affecting not only the skin but the whole body, providing a potential explanation for the numerous comorbidities discovered in relation to this disease[6-12]. The biomarker research performed in the past decades identified substantial inadequacies in describing the wide spectrum of psoriasis[13-15], and failed to explain the complexity of psoriasis endotypes[16]. The genetic background of psoriasis patients reveals that deficiency of the interleukin 1 (IL-1) receptor antagonist and deficiency of the IL-36 receptor antagonist are the only recognized mutations[17], conversely other psoriatic forms are considered complex diseases, due to the intricate interaction between inherited susceptibility alleles and environmental triggers[18]. In addition, epigenetic modifications seem to play a pivotal role in developing psoriasis, establishing environmental triggers as potential modulatory factors[19] as well as in response to anti-psoriatic therapies[20]. These new findings provide a rationale for observed treatment loss-of-response and biologically justify switching to alternative treatments in patient management[21-24]. Furthermore, an increased body of evidence suggests a crucial role for the gut microbiome in modulating psoriasis; linking the skin and gut microbiome[25]. The gut is the site where nutrients are absorbed and at the same time is inhabited by nutrient-modifying microbes. The Skin-Microbiome-Gut-Nutrient interaction is still only partially understood; therefore, this review aimed to summarize the current knowledge in this field.
Psoriasis is associated with metabolic and cardiovascular disease[6,26,27]. It has been widely demonstrated that genetic and environmental factors including nutrition, may strongly influence psoriatic pathogenesis and disease progression[28,29]. Increased body mass and a high fat diet may trigger as well as exacerbate psoriasis[29]. Moderate to severe psoriasis is frequently associated with numerous metabolic disorders including obesity, diabetes, dyslipidemia, metabolic syndrome and non-alcoholic fatty liver disease[30]. Fatty acids are increased in obese patients, leading to augmented inflammation and insulin resistance. Furthermore, obesity may also affect drug pharmacokinetics and pharmacodynamics[29]. The option to treat psoriasis patients with phototherapy is affected by body mass index; obese patients require an excessive amount of photosensitizing drug which may lead to toxicities. Obesity is also an important risk factor for psoriasis, given that the relationship between obesity and psoriasis is mutually interdependent[29]. Recent evidence has suggested an addition role of vitamin D in the pathogenesis of several inflammatory skin diseases including psoriasis[31]. An association between low levels of vitamin D and psoriasis has been described[31]. Vitamin D also participates in keratinocyte proliferation and maturation. Nevertheless, the potential value of vitamin D supplementation for psoriasis is still under debate[31]. Dietary antioxidants including omega 3 polyunsaturated fatty acids derived from fish oil, vitamin B12, vitamin D and selenium have the capacity to decrease oxidative stress and consequently lower reactive oxygen species production, and this could be relevant in the pathogenesis of psoriasis. Based on these findings, the Mediterranean diet has been proposed to slow disease progression[32,33]. All of these endogenous factors contribute to the composition of the so-called “exposome”, a measure of the whole complex of endogenous, ingested and not ingested, substances that interact with the body that are capable of perturbing, modifying and modulating vital functions[34]. Exposures capable of modulating psoriasis include tobacco, which increases severity, flare frequency, and even the incidence of the psoriasis[35], alcohol[36] and pollutants[37], even if the role of both alcohol and pollutants in psoriasis is still debatable. Interestingly, the use of marijuana was recently related to secukinumab resistance in a cohort of erythrodermic patients, focusing the attention on addiction screening during intake medical history of psoriatic patients[38]. Anti-psoriatic drugs are also capable of modulating taste, and consequently, the patient’s diet, as recently described for both methotrexate[39] and apremilast[40].
Since the melatoninergic system was discovered in the epidermis, circadian rhythm has been regarded as a possible modulator of inflammation[41], healing[42], aging[43], neuroendocrine[44], and neoplastic conditions[45]. The cyclic nature of sunlight which influences the suprachiasmatic nucleus (central clock) also influences other peripheral tissue, including skin (peripheral clock), in different ways that are slowly beginning to be understood by examining the mechanism(s) of their dysfunction[46,47]. The shift in circadian rhythm may be occasional and transitory, as in the case of jet-lag during intercontinental flights[48], or in the case of the Ramadan fasting months for Muslims[49,50], or more chronic, as in night shift workers[51,52]; however this effect is particularly evident in psoriatic patients. Interestingly, night-shift workers exhibited not only an increase in severity of psoriatic flares, but also an increased incidence of psoriasis, suggesting that shifting the circadian rhythm (i.e., sleep and diet) may be a risk factor for psoriasis[52]. Sunlight, in the form of narrow band UVB (NB-UVB), may also be curative for psoriatic skin, allowing a reprograming of the circadian clock and inhibition of autoimmune phenomena[53], however skin may also marginally adapt to NB-UVB, an outcome termed photoadaptation[54]. Thus, sunlight is considered an integral part of the exposome.
The possible influence of nutrients on gene expression and clinical progression or remission of psoriasis is not fully explored and may represent the main challenge in approaching complementary therapy for psoriasis. It is reported that many dietetic factors may exert beneficial effects, while others can aggravate inflammatory and immune networks, thus leading to psoriasis comorbidities[55]. Previous studies have reported the positive effects of low-energy diets, vegetarian diets, formula diet weight loss programs, gluten-free or very low-calorie carbohydrate-free diet. It is believed that certain vitamins (e.g., A, E and C), and oligo-elements (e.g., iron, copper, manganese, zinc, and selenium) are anti-oxidants, leading to a reduction in oxidative stress and decreased production of reactive oxygen species[55]. In fact, the disruption of cell redox signaling and involvement of oxidative stress in the pathogenesis of psoriasis was previously suggested, indicating that the potential therapeutic use of dietary antioxidants in psoriasis may represent a novel complementary strategy[56]. Although limited data exist regarding the role of specific diet regimens in psoriasis, the main goal for clinicians is to reduce cardiac risk factors and obesity-related comorbidities.
Interestingly, psoriatic patients displayed a higher risk of cancer compared to the general population; furthermore, this increase is not fully explained by anti-psoriatic immunosuppressive therapies, so several real-life or ecological studies have suggested an intimate relation with diet[57]. This theory is supported by the evidence that different foods modify microRNA expression in psoriatic patients[28]. The influence of a restrictive caloric diet was documented to be beneficial in relapsing plaque psoriasis, and may also decrease the risk of cancer[58-60]. Some lipid components, such as omega-3 polyunsaturated fatty acids may protect cells against UV-induced DNA damage by increasing the expression of tumor-suppressor protein p53, thus promoting cell cycle arrest and preventing melanoma development[61-63]. Solid cancers in psoriasis have been reported, especially those linked to alcohol and smoking. A higher risk of non-melanoma skin cancers, especially squamous cell carcinoma has been shown, possibly as a result of previous exposure to 8-methoxypsoralen-ultraviolet-A (PUVA), cyclosporin, tumor necrosis factor-inhibitors and/or methotrexate[64-66]. Consideration of malignancy risk associated with individual treatments and personal nutritional phenotype may help clinicians to make optimal therapeutic decisions for individual patients.
The "omics" era has provided researchers with new powerful techniques (e.g., metagenomics) and has elucidated a myriad of dysregulated immune responses that are heavily associated with the gut microbiota. Previous research suggested that ingested nutrients heavily affect the body’s microbial composition and community. This has led to experimental approaches in mouse models of psoriasis. In recent years, nutrition and microbial influence has been heavily implicated in psoriasis onset and disease severity. The contribution of nutrients in mouse models has provided valuable insight into human disease regulation. For instance, 12-O-tetradecanoylphorbol-13-acetate (TPA), a known inflammatory signal transducer, can induce psoriasis-like skin lesions in mice, while lesions and proinflammatory cytokine expression were significantly reduced in TPA-induced psoriasis by tangerine-derived nutrient flavonoids: Nobiletin (Nob) and 5-hydroxy-6,7,8,3′,4′-pentamethoxyflavone (5-HPMF)[67]. In addition, obesity, a result of poor nutrition, has been shown to exacerbate the severity of psoriasiform dermatitis in imiquimod-induced rodent models[68]. Collectively, these results support the need to elucidate nutritional impact on psoriasis severity. Research has suggested that monounsaturated fatty acid-rich diets, such as the Mediterranean Diet have anti-inflammatory effects and slow the progression of psoriasis in patients[33]. Poor nutrition has been associated with dysregulated metabolic functions and even more so, has been shown to be critical in skin disease metabolic homeostasis compared to healthy individuals. Previous research has shown evidence for biochemical skin barrier restoration through topical administration of solenopsin, a compound of fire ant venom chemically similar to ceramides, and its derivates by reducing inflammatory markers and improving acanthosis in KC-Tie2 mice, an established rodent-model of psoriasis[69].
Several lines of evidence confirmed the relationship between skin microorganisms and psoriatic lesions, such as Group A β-hemolytic streptococcal infections linked to guttate psoriasis[70]. Other microorganisms including Staphylococcus aureus, Malassezia and Candida albicans also appear to be involved in psoriasis pathogenesis[71]. The deep inter-relationship between the mycobiome and microbiome seem to act as a disease-modifier in psoriatic patients. Although it is well known that the gut and skin microbiome deeply interact, sparse information is available on the gut and skin mycobiome. Using high-throughput 16S rRNA gene sequencing, Alekseyenko et al[72], found that psoriatic plaques had an abundance of the following bacteria: Corynebacterium, Propionibacterium, Staphylococcus, and Streptococcus. Baker et al[73], found that peptidoglycan, a cell wall component of Gram-positive bacteria including Streptococci and Staphylococci, acts as a T cell activator in psoriasis. The authors observed that dermal papillae and cellular infiltrates of guttate and chronic plaque skin lesions had higher numbers of peptidoglycan-containing cells compared to non-lesional psoriatic skin. Psoriatic dermal Streptococcal- and Staphylococcal-specific CD4+ T cell lines proliferated and produced IFN-alfa in response to the respective peptidoglycan structures. Overall, these results suggest that peptidoglycans may be responsible for T cell activation in psoriasis. Moreover, some studies have linked gut microbiota and psoriasis.
Up to 10% of patients with inflammatory bowel disease (IBD) are diagnosed with psoriasis[74]. Patients with psoriasis have a 3-fold higher risk of developing Crohn’s disease as compared to the general population; and Crohn’s disease patients have a 7-fold higher risk of developing psoriasis[75]. Recently, Scher et al[76], using pyrosequencing, found that patients with psoriatic arthritis and patients with skin psoriasis had a decreased bacterial diversity and a reduced relative abundance of some bacterial taxa such as Akkermansia, Ruminococcus, and Pseudobutyrivibrio, as compared to healthy controls. Among the risk factors for psoriatic diseases summarized in Table 1, overall, the alteration of gut microbiota may translate into physiological consequences including poor regulation of intestinal immune responses that may then affect distant organ systems[77-85]. Given the gut microbiome’s influence on the Gut-Skin axis, probiotic supplementation may have a promising role in the management of psoriatic patients. On this point, Gueniche et al[77], in a randomized double-blind placebo-controlled clinical study, showed that oral supplementation with the probiotic strain Lactobacillus paracasei decreased skin sensitivity and increased the rate of barrier function recovery.
| Ref. | Year | Studied population | Psoriasis risk factors | Type of psoriasis |
| Barrea et al[29] | 2016 | Human | Obesity | Not specified |
| Kanemaru et al[68] | 2015 | Animal - Mice | Obesity | Psoriasiform dermatitis |
| Dauden et al[30] | 2018 | Human | Metabolic disorders | Not specified |
| Barrea et al[31] | 2017 | Human | Reduction vitamin D | Not specified |
| Lin et al[56] | 2016 | Human | Oxidative stress | Not specified |
| Yang et al[67] | 2018 | Animal - Mice | 12-O-tetradecanoylphorbol-13-acetate administration | Psoriasis-like skin lesions |
| Ottman et al[70] | 2012 | Human | Beta-hemolytic streptococcal infections | Guttate psoriasis |
| Zeng et al[71] | 2017 | Human | Staphylococcus aureus, Malassezia and Candida albicans infections | Not specified |
| Alekseyenko et al[72] | 2013 | Human | Corynebacterium, Propionibacterium, Staphylococcus, and Streptococcus infections | Psoriatic plaques |
| Baker et al[73] | 2006 | Human | Higher numbers of peptidoglycan-containing cells | Guttate and chronic plaques |
| Oliveira Mde et al[75] | 2015 | Human | Inflammatory bowel disease (i.e., Crohn’s disease) | Not specified |
| Scher et al[76] | 2015 | Human | Significant reduction in Akkermansia, Ruminococcus, and Pseudobutyrivibrio in gut microbiota | Psoriasic arthritis |
| Tan et al[85] | 2018 | Human | Reduction of Coprococcus species and Akkermansia muciniphila in gut microbiota | Psoriatic arthritis |
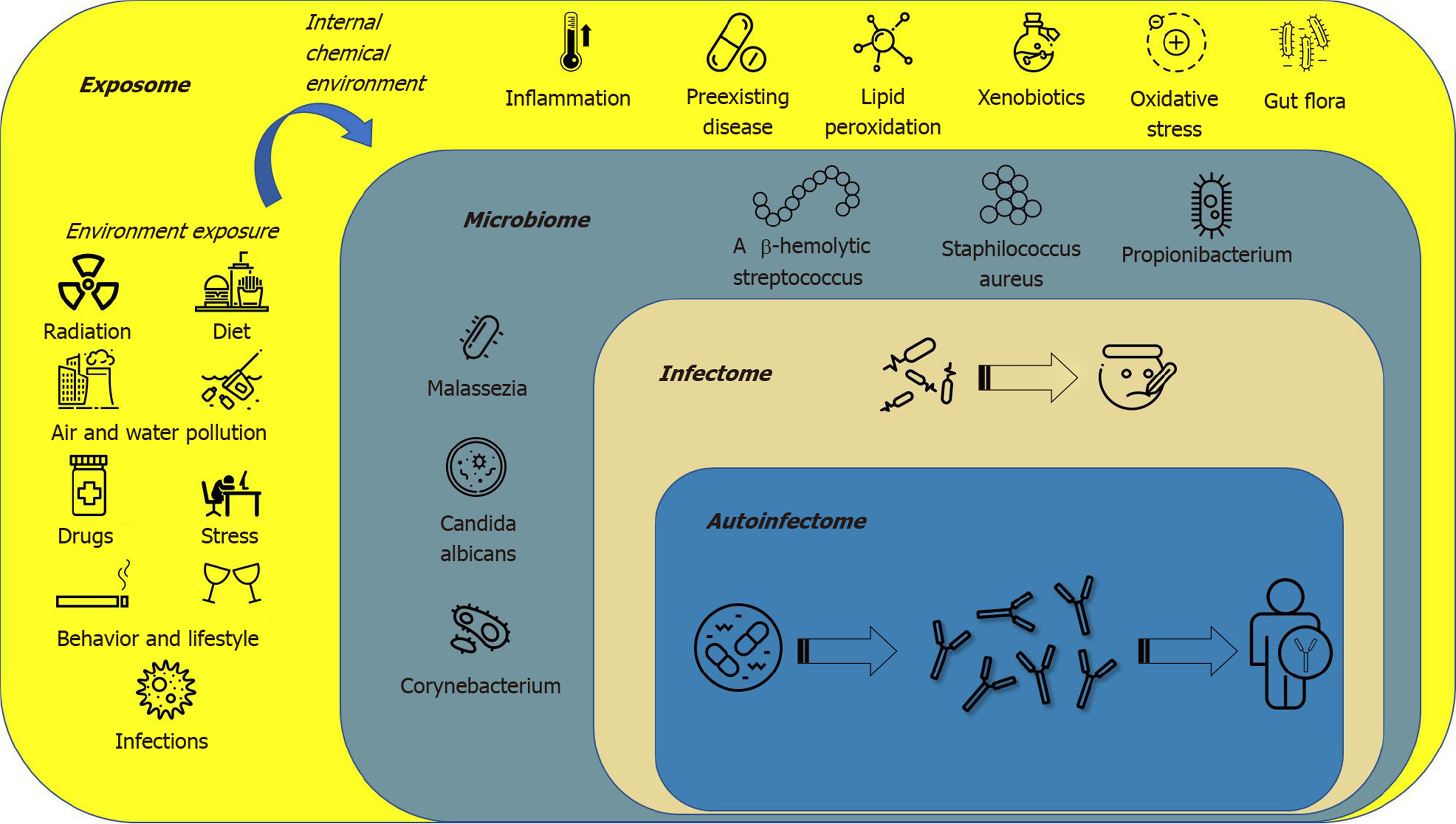
The term “exposome” defines all environmental factors, including infectious and non-infectious agents, to which a human is exposed over a lifetime[80]. The “microbiota” is a term used to describe the 10-100 trillion symbiotic microbes harbored by each human; the “microbiome” consists of the genes that these microbes harbor[81]; the “infectome” is a part of the microbiome, referring to the collection of human exposure to infectious agents; the “autoinfectome” describes a part of the microbiome that includes the infectious agents linked to the presence of autoimmune diseases[82]. Figure 1 summarizes the main interactions between the exposome, microbiome, infectome and autoinfectome. Recently a systematic review, which included 933 psoriatic arthritis patients and 1611 controls, aimed to evaluate the link between infections (viral and bacterial infections) and the risk of psoriatic arthritis and reported a controversial result that exhibited a trend but failed to achieve significance[83]. However, differences exist between infection, colonization and dysbiosis, as suggested by several studies highlighting a different mycobiome and microbiome in psoriasis, psoriatic arthritis and control subjects[84]. In fact, a dysregulation in the ratio of Firmicutes/Bacteroidetes was highlighted in the gut microbiome of psoriatic patients; furthermore, Actinobacteria was reduced in the gut of psoriatic patients. Gut dysbiosis was also found to be related to skin dysbiosis as decreased beta-diversity in psoriatic skin microbiome is related to an increased risk of developing psoriatic arthritis, and skin flora are now regarded as possible sensitive and specific biomarkers to predict comorbidities in psoriatic patients[84]. The skin microbiota in psoriasis patients seems to be less diverse when compared to healthy persons with a decrease in Coprococcus species[76], and more recently Akkermansia muciniphila[85]. The characteristic proinflammatory mediators of psoriatic skin lesions have been reported to be the innate antimicrobial peptides and proteins (AMPs). AMPs are a diverse group of small molecules (12–100 amino acid residues) that constitute the primary effector system of innate immunity against microbes[86]. Although medical history certainly plays a crucial role in psoriasis management, it has some limitations such as recall bias. In particular, it has already been demonstrated that not all infections or even dysbiosis that are not clinically evident are still capable of triggering an immune/autoimmune response. Currently, there is limited, evolving information suggesting some benefit from fecal microbiota transplantation, although durability of response is a concern and currently under investigation[87,88].
Drug therapies are an important consideration for alteration of the cutaneous and gut microbiome[78,89] as well as the mycobiome; however, few studies have explored this topic, and are summarized in Table 2.
| Ref. | Year | Study type | Studied population | Proposed treatment | Type of psoriasis |
| Barrea et al[32], Phan et al[33] | 2015, 2018 | Cohort | Human | Dietary antioxidants (omega 3 polyunsatured fatty acids derived from fish oil, vitamin B12, vitamin D and selenium) | Not specified |
| Subbiah et al[55] | 2010 | Review | - | Low-energy diets, vegetarian diets, formula diet weight loss programs, gluten-free or very low-calorie carbohydrate-free diet | Not specified |
| Subbiah et al[55], Lin et al[85] | 2010, 2016 | Review | - | Dietary antioxidants: Vitamins (A, E and C), and oligo-elements (iron, copper, manganese, zinc, and selenium) | Not specified |
| Murzaku et al[61], Upala et al[62], Morken et al[63] | 2014, 2017, 2011 | Review, systematic review, pilot study | Human | Omega-3 polyunsaturated fatty acids | Not specified |
| Yang et al[67] | 2018 | RCT | Animal – mice | Nobiletin (Nob) and 5-hydroxy-6,7,8,3′,4′-pentamethoxyflavone (5-HPMF) | Not specified |
| Arbiser et al[69] | 2017 | RCT | Animal - mice | Topical administration of solenopsin analogs | Not specified |
| Gueniche et al[77] | 2014 | RCT | Human | Oral supplementation with the probiotic Lactobacillus paracasei | Not specified |
| Eppinga et al[78] | 2014 | Review | - | Th17, TNF-α and IL-22 inhibitors | Psoriatic arthritis |
| Castelino et al[79] | 2014 | Review | - | Transplant of fecal microbiome | Psoriatic arthritis |
To date IL-17/IL-17RA signaling has been demonstrated to be a key component in regulating Candida in the gut microbiome[79]. Furthermore, psoriatic arthritis and IBD have genetic and environmental similarities, highlighting that microbiome dysbiosis may affect autoimmune diseases[78]. T-cell activation is an important mechanism of psoriasis, and dysbiosis has been associated with the differentiation of T-cells into effector T cells with fewer regulatory T-cells resulting in changes in the levels of cytokines. In particular, Th17 inhibitors produced the best response compared to patients treated with tumor necrosis factor-α and IL-23 inhibitors[78]. It is interesting to note that this Th-17 mediated response may not translate to the skin, as the skin microbiome could prevent the development of psoriatic plaques in these individuals[3,79]. It is possible that transplanting fecal microbiota could improve or resolve the dysbiosis present in psoriatic arthritis[79]. Fecal microbiota transplants have been used with success in IBD. In fact, Kragsnaes et al[90] are currently exploring fecal microbiota transplantation (FMT) in patients with psoriatic arthritis currently on methotrexate to examine their treatment response. Evaluating evidence of the efficacy of FMT is likely due to be complicated by various factors including antibiotic use, prior psoriasis therapy, subtype of psoriasis and comorbidities which similarly affect the gut or skin microbiome[91]. Future studies are needed to identify potential modulators of the gut and skin microbiome, and identify which medications would be optimal for a patient’s individual microbiome signature.
The interaction of Microbiome-Gut-Nutrients in psoriasis is beginning to be understood with the advent of improved omics technologies and their possible integration with each other in order to more precisely separate psoriasis patient endotypes. The transition from immune-targeted therapy to precision-based therapy will be based on the mix between biological signature, the endotype, and potential specific interaction within the exposome.
Manuscript source: Unsolicited Manuscript
Specialty type: Medicine, research and experimental
Country of origin: Italy
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Rhoads J, Serban ED S-Editor: Ma YJ L-Editor: Webster JR E-Editor: Liu JH
| 1. | Greb JE, Goldminz AM, Elder JT, Lebwohl MG, Gladman DD, Wu JJ, Mehta NN, Finlay AY, Gottlieb AB. Psoriasis. Nat Rev Dis Primers. 2016;2:16082. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 426] [Cited by in F6Publishing: 500] [Article Influence: 62.5] [Reference Citation Analysis (0)] |
| 2. | Damiani G, Cazzaniga S, Conic RR, Naldi L; Psocare Registry Network. Pruritus Characteristics in a Large Italian Cohort of Psoriatic Patients. J Eur Acad Dermatol Venereol. 2019;33:1316-1324. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 26] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 3. | Lande R, Botti E, Jandus C, Dojcinovic D, Fanelli G, Conrad C, Chamilos G, Feldmeyer L, Marinari B, Chon S, Vence L, Riccieri V, Guillaume P, Navarini AA, Romero P, Costanzo A, Piccolella E, Gilliet M, Frasca L. The antimicrobial peptide LL37 is a T-cell autoantigen in psoriasis. Nat Commun. 2014;5:5621. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 324] [Cited by in F6Publishing: 367] [Article Influence: 36.7] [Reference Citation Analysis (0)] |
| 4. | Arakawa A, Siewert K, Stöhr J, Besgen P, Kim SM, Rühl G, Nickel J, Vollmer S, Thomas P, Krebs S, Pinkert S, Spannagl M, Held K, Kammerbauer C, Besch R, Dornmair K, Prinz JC. Melanocyte antigen triggers autoimmunity in human psoriasis. J Exp Med. 2015;212:2203-2212. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 209] [Cited by in F6Publishing: 237] [Article Influence: 26.3] [Reference Citation Analysis (0)] |
| 5. | Liang Y, Sarkar MK, Tsoi LC, Gudjonsson JE. Psoriasis: a mixed autoimmune and autoinflammatory disease. Curr Opin Immunol. 2017;49:1-8. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 111] [Cited by in F6Publishing: 130] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 6. | Al-Mutairi N, Al-Farag S, Al-Mutairi A, Al-Shiltawy M. Comorbidities associated with psoriasis: an experience from the Middle East. J Dermatol. 2010;37:146-155. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 101] [Cited by in F6Publishing: 108] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 7. | Santus P, Rizzi M, Radovanovic D, Airoldi A, Cristiano A, Conic R, Petrou S, Pigatto PDM, Bragazzi N, Colombo D, Goldust M, Damiani G. Psoriasis and Respiratory Comorbidities: The Added Value of Fraction of Exhaled Nitric Oxide as a New Method to Detect, Evaluate, and Monitor Psoriatic Systemic Involvement and Therapeutic Efficacy. Biomed Res Int. 2018;2018:3140682. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 46] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 8. | Fiore M, Leone S, Maraolo AE, Berti E, Damiani G. Liver Illness and Psoriatic Patients. Biomed Res Int. 2018;2018:3140983. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 42] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 9. | Della Valle V, Maggioni M, Carrera C, Cattaneo A, Marzano AV, Damiani G. A mysterious abdominal pain during active psoriasis. Intern Emerg Med. 2018;13:889-892. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 10. | Damiani G, Radaeli A, Olivini A, Calvara-Pinton P, Malerba M. Increased airway inflammation in patients with psoriasis. Br J Dermatol. 2016;175:797-799. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 28] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 11. | Malerba M, Damiani G, Radaeli A, Ragnoli B, Olivini A, Calzavara-Pinton PG. Narrowband ultraviolet B phototherapy in psoriasis reduces proinflammatory cytokine levels and improves vitiligo and neutrophilic asthma. Br J Dermatol. 2015;173:1544-1545. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 31] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 12. | Watad A, Bragazzi NL, McGonagle D, Damiani G, Comaneshter D, Cohen A, Amital H. Systemic Sclerosis is Linked to Psoriasis and May Impact on Patients' Survival: A Large Cohort Study. J Clin Med. 2019;8. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 13. | Villanova F, Di Meglio P, Nestle FO. Biomarkers in psoriasis and psoriatic arthritis. Ann Rheum Dis. 2013;72 Suppl 2:ii104-ii110. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 90] [Cited by in F6Publishing: 96] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 14. | Asa'ad F, Fiore M, Alfieri A, Pigatto PDM, Franchi C, Berti E, Maiorana C, Damiani G. Saliva as a Future Field in Psoriasis Research. Biomed Res Int. 2018;2018:7290913. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 15. | Diani M, Perego S, Sansoni V, Bertino L, Gomarasca M, Faraldi M, Pigatto PDM, Damiani G, Banfi G, Altomare G, Lombardi G. Differences in Osteoimmunological Biomarkers Predictive of Psoriatic Arthritis among a Large Italian Cohort of Psoriatic Patients. Int J Mol Sci. 2019;20. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 16. | Menter MA, Griffiths CE. Psoriasis: the future. Dermatol Clin. 2015;33:161-166. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 13] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 17. | Cowen EW, Goldbach-Mansky R. DIRA, DITRA, and new insights into pathways of skin inflammation: what's in a name? Arch Dermatol. 2012;148:381-384. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 48] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 18. | Capon F. The Genetic Basis of Psoriasis. Int J Mol Sci. 2017;18. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 103] [Cited by in F6Publishing: 110] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 19. | Zhao M, Lu Q. The Aberrant Epigenetic Modifications in the Pathogenesis of Psoriasis. J Investig Dermatol Symp Proc. 2018;19:S81-S82. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 20. | Ovejero-Benito MC, Reolid A, Sánchez-Jiménez P, Saiz-Rodríguez M, Muñoz-Aceituno E, Llamas-Velasco M, Martín-Vilchez S, Cabaleiro T, Román M, Ochoa D, Daudén E, Abad-Santos F. Histone modifications associated with biological drug response in moderate-to-severe psoriasis. Exp Dermatol. 2018;27:1361-1371. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 41] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 21. | Kerdel F, Zaiac M. An evolution in switching therapy for psoriasis patients who fail to meet treatment goals. Dermatol Ther. 2015;28:390-403. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 46] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 22. | Damiani G, Conic RRZ, de Vita V, Costanzo A, Regazzini R, Pigatto PDM, Bragazzi NL, Pacifico A, Malagoli P. When IL-17 inhibitors fail: Real-life evidence to switch from secukinumab to adalimumab or ustekinumab. Dermatol Ther. 2019;32:e12793. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 28] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 23. | Damiani G, Cazzaniga S, Naldi L; PsoReal Study Group. Use of fumaric acid derivatives (FADs) in Italian reference centres for psoriasis. G Ital Dermatol Venereol. 2019;. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 3] [Reference Citation Analysis (0)] |
| 24. | Damiani G, Conic RRZ, Pigatto PDM, Bragazzi NL, Pacifico A, Malagoli P; Young Dermatologists Italian Network. From randomized clinical trials to real life data. An Italian clinical experience with ixekizumab and its management. Dermatol Ther. 2019;32:e12886. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 25. | Yan D, Issa N, Afifi L, Jeon C, Chang HW, Liao W. The Role of the Skin and Gut Microbiome in Psoriatic Disease. Curr Dermatol Rep. 2017;6:94-103. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 65] [Cited by in F6Publishing: 51] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 26. | Conic RR, Damiani G, Schrom KP, Ramser AE, Zheng C, Xu R, McCormick TS, Cooper KD. Psoriasis and Psoriatic Arthritis Cardiovascular Disease Endotypes Identified by Red Blood Cell Distribution Width and Mean Platelet Volume. J Clin Med. 2020;9. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 46] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 27. | Seth D, Ehlert AN, Golden JB, Damiani G, McCormick TS, Cameron MJ, Cooper KD. Interaction of Resistin and Systolic Blood Pressure in Psoriasis Severity. J Invest Dermatol. 2019;. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 28. | Kocic H, Damiani G, Stamenkovic B, Tirant M, Jovic A, Tiodorovic D, Peris K. Dietary compounds as potential modulators of microRNA expression in psoriasis. Ther Adv Chronic Dis. 2019;10:2040622319864805. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 42] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 29. | Barrea L, Nappi F, Di Somma C, Savanelli MC, Falco A, Balato A, Balato N, Savastano S. Environmental Risk Factors in Psoriasis: The Point of View of the Nutritionist. Int J Environ Res Public Health. 2016;13. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 63] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 30. | Dauden E, Blasco AJ, Bonanad C, Botella R, Carrascosa JM, González-Parra E, Jodar E, Joven B, Lázaro P, Olveira A, Quintero J, Rivera R. Position statement for the management of comorbidities in psoriasis. J Eur Acad Dermatol Venereol. 2018;32:2058-2073. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 49] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 31. | Barrea L, Savanelli MC, Di Somma C, Napolitano M, Megna M, Colao A, Savastano S. Vitamin D and its role in psoriasis: An overview of the dermatologist and nutritionist. Rev Endocr Metab Disord. 2017;18:195-205. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 149] [Cited by in F6Publishing: 135] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 32. | Barrea L, Balato N, Di Somma C, Macchia PE, Napolitano M, Savanelli MC, Esposito K, Colao A, Savastano S. Nutrition and psoriasis: is there any association between the severity of the disease and adherence to the Mediterranean diet? J Transl Med. 2015;13:18. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 79] [Cited by in F6Publishing: 89] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 33. | Phan C, Touvier M, Kesse-Guyot E, Adjibade M, Hercberg S, Wolkenstein P, Chosidow O, Ezzedine K, Sbidian E. Association Between Mediterranean Anti-inflammatory Dietary Profile and Severity of Psoriasis: Results From the NutriNet-Santé Cohort. JAMA Dermatol. 2018;154:1017-1024. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 59] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 34. | Vermeulen R, Schymanski EL, Barabási AL, Miller GW. The exposome and health: Where chemistry meets biology. Science. 2020;367:392-396. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 288] [Cited by in F6Publishing: 387] [Article Influence: 96.8] [Reference Citation Analysis (0)] |
| 35. | Naldi L. Psoriasis and smoking: links and risks. Psoriasis (Auckl). 2016;6:65-71. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 24] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 36. | Svanström C, Lonne-Rahm SB, Nordlind K. Psoriasis and alcohol. Psoriasis (Auckl). 2019;9:75-79. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 37. | Puri P, Nandar SK, Kathuria S, Ramesh V. Effects of air pollution on the skin: A review. Indian J Dermatol Venereol Leprol. 2017;83:415-423. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 109] [Cited by in F6Publishing: 123] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 38. | Damiani G, Pacifico A, Russo F, Pigatto PDM, Bragazzi NL, Bonifati C, Morrone A, Watad A, Adawi M. Use of Secukinumab in a Cohort of Erythrodermic Psoriatic Patients: A Pilot Study. J Clin Med. 2019;8. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 39. | Duhra P, Foulds IS. Methotrexate-induced impairment of taste acuity. Clin Exp Dermatol. 1988;13:126-127. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 16] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 40. | Damiani G, Bragazzi NL, Grossi E, Petrou S, Radovanovic D, Rizzi M, Atzeni F, Sarzi-Puttini P, Santus P, Pigatto PD, Franchi C. Severe bitter taste associated with apremilast. Dermatol Ther. 2019;32:e12876. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 41. | Lyons AB, Moy L, Moy R, Tung R. Circadian Rhythm and the Skin: A Review of the Literature. J Clin Aesthet Dermatol. 2019;12:42-45. [PubMed] [Cited in This Article: ] |
| 42. | Vorotelyak EA, Malchenko LA, Rogovaya OS, Lazarev DS, Butorina NN, Brodsky VY. Melatonin Stimulates Epithelium Migration in Wound Models In Vitro and In Vivo. Bull Exp Biol Med. 2019;168:242-246. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 43. | Nanzadsuren T, Myatav T, Dorjkhuu A, Byamba K. Association between serum melatonin and skin aging in an urban population of Mongolia. J Cosmet Dermatol. 2019;. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 44. | Bocheva G, Slominski RM, Slominski AT. Neuroendocrine Aspects of Skin Aging. Int J Mol Sci. 2019;20. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 49] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 45. | Pourhanifeh MH, Mahdavinia M, Reiter RJ, Asemi Z. Potential use of melatonin in skin cancer treatment: A review of current biological evidence. J Cell Physiol. 2019;234:12142-12148. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 30] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 46. | Plikus MV, Van Spyk EN, Pham K, Geyfman M, Kumar V, Takahashi JS, Andersen B. The circadian clock in skin: implications for adult stem cells, tissue regeneration, cancer, aging, and immunity. J Biol Rhythms. 2015;30:163-182. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 105] [Cited by in F6Publishing: 113] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 47. | Bragazzi NL, Sellami M, Salem I, Conic R, Kimak M, Pigatto PDM, Damiani G. Fasting and Its Impact on Skin Anatomy, Physiology, and Physiopathology: A Comprehensive Review of the Literature. Nutrients. 2019;11. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 28] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 48. | Damiani G, Bragazzi NL, Garbarino S, Chattu VK, Shapiro CM, Pacifico A, Malagoli P, Pigatto PDM, Conic RRZ, Tiodorovic D, Watad A, Adawi M. Psoriatic and psoriatic arthritis patients with and without jet-lag: does it matter for disease severity scores? Insights and implications from a pilot, prospective study. Chronobiol Int. 2019;36:1733-1740. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 49. | Adawi M, Damiani G, Bragazzi NL, Bridgewood C, Pacifico A, Conic RRZ, Morrone A, Malagoli P, Pigatto PDM, Amital H, McGonagle D, Watad A. The Impact of Intermittent Fasting (Ramadan Fasting) on Psoriatic Arthritis Disease Activity, Enthesitis, and Dactylitis: A Multicentre Study. Nutrients. 2019;11. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 39] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 50. | Damiani G, Watad A, Bridgewood C, Pigatto PDM, Pacifico A, Malagoli P, Bragazzi NL, Adawi M. The Impact of Ramadan Fasting on the Reduction of PASI Score, in Moderate-To-Severe Psoriatic Patients: A Real-Life Multicenter Study. Nutrients. 2019;11. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 51] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 51. | Li WQ, Qureshi AA, Schernhammer ES, Han J. Rotating night-shift work and risk of psoriasis in US women. J Invest Dermatol. 2013;133:565-567. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 48] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 52. | Cohen JM, Jackson CL, Li TY, Wu S, Qureshi AA. Sleep disordered breathing and the risk of psoriasis among US women. Arch Dermatol Res. 2015;307:433-438. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 53. | Chen X, Yang M, Cheng Y, Liu GJ, Zhang M. Narrow-band ultraviolet B phototherapy versus broad-band ultraviolet B or psoralen-ultraviolet A photochemotherapy for psoriasis. Cochrane Database Syst Rev. 2013;CD009481. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 54. | Pacifico A, Damiani G, Iacovelli P, Conic RR, Scarabello A, Filoni A, Malagoli P, Bragazzi NL, Pigatto PD, Morrone A. Photoadaptation to UVB TL01 in psoriatic patients. J Eur Acad Dermatol Venereol. 2020;. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 55. | Subbiah MT. Application of nutrigenomics in skin health: nutraceutical or cosmeceutical? J Clin Aesthet Dermatol. 2010;3:44-46. [PubMed] [Cited in This Article: ] |
| 56. | Lin X, Huang T. Oxidative stress in psoriasis and potential therapeutic use of antioxidants. Free Radic Res. 2016;50:585-595. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 78] [Cited by in F6Publishing: 92] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 57. | Geller S, Xu H, Lebwohl M, Nardone B, Lacouture ME, Kheterpal M. Malignancy Risk and Recurrence with Psoriasis and its Treatments: A Concise Update. Am J Clin Dermatol. 2018;19:363-375. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 76] [Cited by in F6Publishing: 57] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 58. | Castaldo G, Galdo G, Rotondi Aufiero F, Cereda E. Very low-calorie ketogenic diet may allow restoring response to systemic therapy in relapsing plaque psoriasis. Obes Res Clin Pract. 2016;10:348-352. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 59. | Fontana L, Klein S, Holloszy JO. Long-term low-protein, low-calorie diet and endurance exercise modulate metabolic factors associated with cancer risk. Am J Clin Nutr. 2006;84:1456-1462. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 70] [Cited by in F6Publishing: 77] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 60. | Nagui N, El Nabarawy E, Mahgoub D, Mashaly HM, Saad NE, El-Deeb DF. Estimation of (IgA) anti-gliadin, anti-endomysium and tissue transglutaminase in the serum of patients with psoriasis. Clin Exp Dermatol. 2011;36:302-304. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 17] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 61. | Murzaku EC, Bronsnick T, Rao BK. Diet in dermatology: Part II. Melanoma, chronic urticaria, and psoriasis. J Am Acad Dermatol. 2014;71:1053.e1-1053.e16. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 33] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 62. | Upala S, Yong WC, Theparee T, Sanguankeo A. Effect of omega-3 fatty acids on disease severity in patients with psoriasis: A systematic review. Int J Rheum Dis. 2017;20:442-450. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 63. | Morken T, Bohov P, Skorve J, Ulvik R, Aukrust P, Berge RK, Livden JK. Anti-inflammatory and hypolipidemic effects of the modified fatty acid tetradecylthioacetic acid in psoriasis--a pilot study. Scand J Clin Lab Invest. 2011;71:269-273. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 14] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 64. | Lee JH, Kim HJ, Han KD, Kim HN, Park YM, Lee JY, Park YG, Lee YB. Cancer risk in 892 089 patients with psoriasis in Korea: A nationwide population-based cohort study. J Dermatol. 2019;46:95-102. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 27] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 65. | Fiorentino D, Ho V, Lebwohl MG, Leite L, Hopkins L, Galindo C, Goyal K, Langholff W, Fakharzadeh S, Srivastava B, Langley RG. Risk of malignancy with systemic psoriasis treatment in the Psoriasis Longitudinal Assessment Registry. J Am Acad Dermatol. 2017;77:845-854.e5. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 93] [Cited by in F6Publishing: 105] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 66. | Pouplard C, Brenaut E, Horreau C, Barnetche T, Misery L, Richard MA, Aractingi S, Aubin F, Cribier B, Joly P, Jullien D, Le Maître M, Ortonne JP, Paul C. Risk of cancer in psoriasis: a systematic review and meta-analysis of epidemiological studies. J Eur Acad Dermatol Venereol. 2013;27 Suppl 3:36-46. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 137] [Cited by in F6Publishing: 145] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 67. | Yang G, Li S, Yang Y, Yuan L, Wang P, Zhao H, Ho CT, Lin CC. Nobiletin and 5-Hydroxy-6,7,8,3',4'-pentamethoxyflavone Ameliorate 12- O-Tetradecanoylphorbol-13-acetate-Induced Psoriasis-Like Mouse Skin Lesions by Regulating the Expression of Ki-67 and Proliferating Cell Nuclear Antigen and the Differentiation of CD4+ T Cells through Mitogen-Activated Protein Kinase Signaling Pathways. J Agric Food Chem. 2018;66:8299-8306. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 68. | Kanemaru K, Matsuyuki A, Nakamura Y, Fukami K. Obesity exacerbates imiquimod-induced psoriasis-like epidermal hyperplasia and interleukin-17 and interleukin-22 production in mice. Exp Dermatol. 2015;24:436-442. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 41] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 69. | Arbiser JL, Nowak R, Michaels K, Skabytska Y, Biedermann T, Lewis MJ, Bonner MY, Rao S, Gilbert LC, Yusuf N, Karlsson I, Fritz Y, Ward NL. Evidence for biochemical barrier restoration: Topical solenopsin analogs improve inflammation and acanthosis in the KC-Tie2 mouse model of psoriasis. Sci Rep. 2017;7:11198. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 70. | Ottman N, Smidt H, de Vos WM, Belzer C. The function of our microbiota: who is out there and what do they do? Front Cell Infect Microbiol. 2012;2:104. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 248] [Cited by in F6Publishing: 260] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 71. | Zeng J, Luo S, Huang Y, Lu Q. Critical role of environmental factors in the pathogenesis of psoriasis. J Dermatol. 2017;44:863-872. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 101] [Cited by in F6Publishing: 111] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 72. | Alekseyenko AV, Perez-Perez GI, De Souza A, Strober B, Gao Z, Bihan M, Li K, Methé BA, Blaser MJ. Community differentiation of the cutaneous microbiota in psoriasis. Microbiome. 2013;1:31. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 271] [Cited by in F6Publishing: 268] [Article Influence: 24.4] [Reference Citation Analysis (0)] |
| 73. | Baker BS, Laman JD, Powles A, van der Fits L, Voerman JS, Melief MJ, Fry L. Peptidoglycan and peptidoglycan-specific Th1 cells in psoriatic skin lesions. J Pathol. 2006;209:174-181. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 52] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 74. | Salem I, Ramser A, Isham N, Ghannoum MA. The Gut Microbiome as a Major Regulator of the Gut-Skin Axis. Front Microbiol. 2018;9:1459. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 293] [Cited by in F6Publishing: 286] [Article Influence: 47.7] [Reference Citation Analysis (0)] |
| 75. | Oliveira Mde F, Rocha Bde O, Duarte GV. Psoriasis: classical and emerging comorbidities. An Bras Dermatol. 2015;90:9-20. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 179] [Cited by in F6Publishing: 197] [Article Influence: 21.9] [Reference Citation Analysis (0)] |
| 76. | Scher JU, Ubeda C, Artacho A, Attur M, Isaac S, Reddy SM, Marmon S, Neimann A, Brusca S, Patel T, Manasson J, Pamer EG, Littman DR, Abramson SB. Decreased bacterial diversity characterizes the altered gut microbiota in patients with psoriatic arthritis, resembling dysbiosis in inflammatory bowel disease. Arthritis Rheumatol. 2015;67:128-139. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 445] [Cited by in F6Publishing: 501] [Article Influence: 55.7] [Reference Citation Analysis (0)] |
| 77. | Gueniche A, Philippe D, Bastien P, Reuteler G, Blum S, Castiel-Higounenc I, Breton L, Benyacoub J. Randomised double-blind placebo-controlled study of the effect of Lactobacillus paracasei NCC 2461 on skin reactivity. Benef Microbes. 2014;5:137-145. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 64] [Cited by in F6Publishing: 64] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 78. | Eppinga H, Konstantinov SR, Peppelenbosch MP, Thio HB. The microbiome and psoriatic arthritis. Curr Rheumatol Rep. 2014;16:407. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 40] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 79. | Castelino M, Eyre S, Upton M, Ho P, Barton A. The bacterial skin microbiome in psoriatic arthritis, an unexplored link in pathogenesis: challenges and opportunities offered by recent technological advances. Rheumatology (Oxford). 2014;53:777-784. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 29] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 80. | Bogdanos DP, Smyk DS, Invernizzi P, Rigopoulou EI, Blank M, Pouria S, Shoenfeld Y. Infectome: a platform to trace infectious triggers of autoimmunity. Autoimmun Rev. 2013;12:726-740. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 70] [Cited by in F6Publishing: 67] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 81. | Turnbaugh PJ, Ley RE, Hamady M, Fraser-Liggett CM, Knight R, Gordon JI. The human microbiome project. Nature. 2007;449:804-810. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3538] [Cited by in F6Publishing: 3360] [Article Influence: 197.6] [Reference Citation Analysis (0)] |
| 82. | Bogdanos DP, Smyk DS, Rigopoulou EI, Sakkas LI, Shoenfeld Y. Infectomics and autoinfectomics: a tool to study infectious-induced autoimmunity. Lupus. 2015;24:364-373. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 23] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 83. | Thrastardottir T, Love TJ. Infections and the risk of psoriatic arthritis among psoriasis patients: a systematic review. Rheumatol Int. 2018;38:1385-1397. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 84. | Benhadou F, Mintoff D, Schnebert B, Thio HB. Psoriasis and Microbiota: A Systematic Review. Diseases. 2018;6. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 64] [Cited by in F6Publishing: 65] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 85. | Tan L, Zhao S, Zhu W, Wu L, Li J, Shen M, Lei L, Chen X, Peng C. The Akkermansia muciniphila is a gut microbiota signature in psoriasis. Exp Dermatol. 2018;27:144-149. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 91] [Cited by in F6Publishing: 96] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 86. | Batycka-Baran A, Maj J, Wolf R, Szepietowski JC. The new insight into the role of antimicrobial proteins-alarmins in the immunopathogenesis of psoriasis. J Immunol Res. 2014;2014:628289. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 41] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 87. | Yermekbayeva B. Rilonacept to improve artery function in patients with atherosclerosis. [accessed 2020 Feb 25]. In: ClinicalTrials.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: http://clinicaltrials.gov/show/NCT03594877 ClinicalTrials.gov Identifier: NCT03594877. [Cited in This Article: ] |
| 88. | ProgenaBiome. Rilonacept to improve artery function in patients with atherosclerosis. [accessed 2020 Feb 25]. In: ClinicalTrials.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: http://clinicaltrials.gov/show/NCT04099979 ClinicalTrials.gov Identifier: NCT04099979. [Cited in This Article: ] |
| 89. | Flint HJ, Scott KP, Louis P, Duncan SH. The role of the gut microbiota in nutrition and health. Nat Rev Gastroenterol Hepatol. 2012;9:577-589. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1207] [Cited by in F6Publishing: 1259] [Article Influence: 104.9] [Reference Citation Analysis (0)] |
| 90. | Kragsnaes MS, Kjeldsen J, Horn HC, Munk HL, Pedersen FM, Holt HM, Pedersen JK, Holm DK, Glerup H, Andersen V, Fredberg U, Kristiansen K, Christensen R, Ellingsen T. Efficacy and safety of faecal microbiota transplantation in patients with psoriatic arthritis: protocol for a 6-month, double-blind, randomised, placebo-controlled trial. BMJ Open. 2018;8:e019231. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 41] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 91. | Langan EA, Griffiths CEM, Solbach W, Knobloch JK, Zillikens D, Thaçi D. The role of the microbiome in psoriasis: moving from disease description to treatment selection? Br J Dermatol. 2018;178:1020-1027. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 43] [Article Influence: 7.2] [Reference Citation Analysis (0)] |