Published online Jul 6, 2021. doi: 10.12998/wjcc.v9.i19.5287
Peer-review started: February 15, 2021
First decision: March 11, 2021
Revised: March 31, 2021
Accepted: May 6, 2021
Article in press: May 6, 2021
Published online: July 6, 2021
Hemichorea usually results from vascular lesions of the basal ganglia. Most often, the lesion is contralateral to the affected limb but rarely, it may be ipsilateral. The pathophysiology of ipsilateral hemichorea is still poorly understood. We review the literature on hemichorea due to ipsilateral cerebral infarction and explore possible mechanisms for its occurrence.
A 72-year-old woman presented with complaints of involuntary movements of the muscles of the left side of the face and mild weakness of the right limbs. Her symptoms had started suddenly 1 d earlier. After admission to the hospital, the involuntary movements spread to involve the left limbs also. Magnetic resonance imaging revealed a left thalamic infarction. The patient’s hemichorea subsided after treatment with haloperidol (2 mg per time, 3 times/d) for 3 d; the hemiparesis resolved with rehabilitation physiotherapy. She is presently symptom free and on treatment for prevention of secondary stroke. We review the literature on the occurrence of ipsilateral hemichorea following thalamic infarction and discuss the possible pathomechanisms of this unusual presentation.
Ipsilateral hemichorea following a thalamic stroke is rare but it can be explained by structure of the extrapyramidal system. The thalamus is a relay station that exerts a bilateral control of motor function.
Core Tip: Acute hemichorea is usually caused by lacunar infarcts in the contralateral basal ganglia. We present a case of hemichorea due to ipsilateral thalamic infarction. A 72-year-old woman was admitted to our hospital with unilateral hemichorea and contralateral hemiparesis. Magnetic resonance imaging revealed a left thalamic infarction. The hemiparesis improved with rehabilitation physiotherapy, and the hemichorea subsided following treatment with haloperidol. The mechanism of ipsilateral hemichorea is poorly understood, but it could be because some fibers of the extrapyramidal system continue their ipsilateral course, passing through the thalamus, which is a relay station exerting control over motor function of both sides.
- Citation: Li ZS, Fang JJ, Xiang XH, Zhao GH. Hemichorea due to ipsilateral thalamic infarction: A case report. World J Clin Cases 2021; 9(19): 5287-5293
- URL: https://www.wjgnet.com/2307-8960/full/v9/i19/5287.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i19.5287
Acute hemichorea most often results from focal vascular insult (ischemic or hemo
A 72-year-old right-handed Chinese woman was admitted to our hospital 1 d after she suddenly developed mild right hemiparesis and involuntary movements of the muscles on the left side of her face. There was winking and grimacing of the left side of her face.
One day prior to admission, the patient had left-limb weakness abruptly when she was walking, but she was able to carry out actions with her hands and walk normally. She denied headache, nausea, or vomiting. Loss of consciousness was not found during the course. About 10 min later, winking and grimacing of the left side of her face appeared. One day after admission, she developed involuntary irregular movements of her left limbs (Video). The involuntary movements affecting the face and distal parts of the left limbs were non-rhythmic. The involuntary movements were most noticeable when she was at rest. The movements involve the fingers, wrist, forearm and toes, ankle, and leg. The limb movements like twisting. They were aggravated by emotional stress, and disappeared completely when she was asleep.
The patient had a history of hypertension for 10 years, which was well controlled with amlodipine 5 mg/d. She had no history of any medication or infection prior to onset of symptoms. She had no previous history of chorea or other involuntary movements.
The patient denied a past history of drug or alcohol abuse, smoking, or promiscuous sexual behavior. There was no family history of stroke, epilepsy, or other neurologic diseases.
After arriving at our emergency department, the patient's Glasgow Coma Scale score was 15. Neurological examination confirmed involuntary, irregular movements of the left face and limbs consistent with right hemichorea. There was no cranial nerve involvement. Sensation was normal. Muscular tone and deep tendon reflexes were normal, but there was a subtle right limb weakness. The right upper and lower limb muscle strength was level 5. No other positive neurologic signs were found.
Blood cell counts; serum glucose and calcium levels; liver, renal, and thyroid function tests; tumor markers; serology for human immunodeficiency virus and Treponema pallidum; and arterial blood gas analysis were all normal.
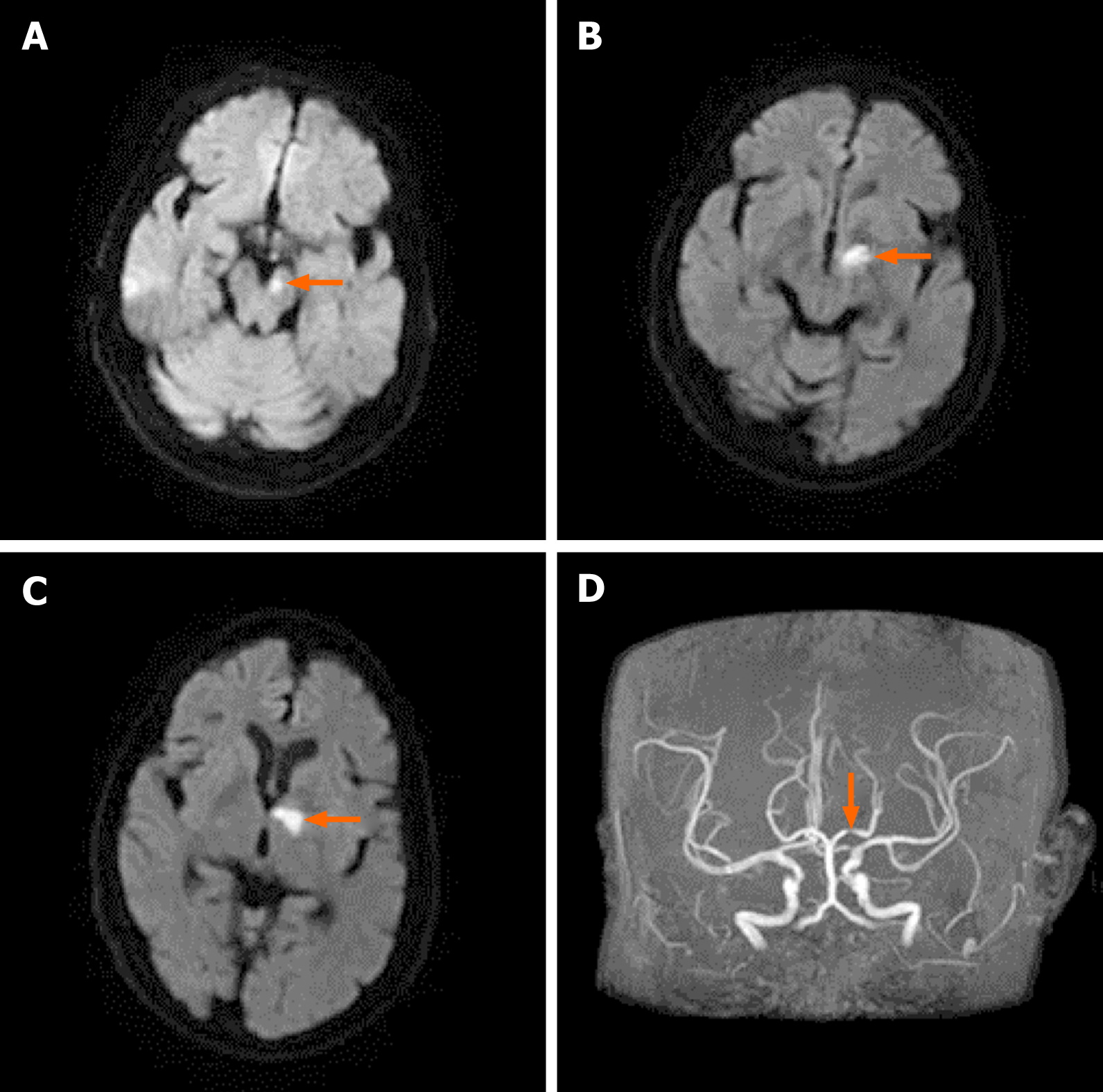
Magnetic resonance imaging (MRI) showed an acute infarction in the left dorsal thalamus and left cerebral peduncle zone (Figure 1A-C), together with signs of chronic ischemic microangiopathy in the periventricular areas. Magnetic resonance angiography showed severe stenosis of the P1 segment of the left posterior cerebral artery (Figure 1D). Carotid duplex sonography showed bilateral nonstenotic carotid atheroma. The 24-h ambulatory electroencephalogram was normal.
Hemichorea due to ipsilateral acute thalamic infarction.
The hemichorea subsided after treatment with haloperidol (2 mg per time, 3 times/d) for 3 d. The hemiparesis gradually improved with rehabilitation physiotherapy for 1 mo. The patient was put on aspirin (100 mg/d) for secondary prevention of ischemic stroke.
At the follow-up 3 mo from the onset of symptoms, the patient was symptom free (without haloperidol medication). The weakness of her right limbs had disappeared.
In this study, we describe the case of a 72-year-old woman with left thalamic and left cerebral peduncle infarction combined with left hemichorea and mild right hemiparesis. Cranial MRI showed an acute infarction in the left dorsal thalamus and left cerebral peduncle zone. The left thalamic infarction of the patient resulted in weakness of the right limb and hemichorea of the left limb. The symptoms of the patient were not thought to have resulted from infarction of the left cerebral peduncle. The lesion of the cerebral peduncle may result in oculomotor nerve palsy and right limb ataxia, but this patient did not have this symptom.
The most common movement disorders caused by basal ganglia infarction are chorea and ballism. Chorea is characterized by irregular, sudden, and brief contractions of the whole body, but most often presents in the distal parts of the body. Ballism is considered a very severe form of chorea which mainly involves the proximal limbs[7]. Unlike dystonia, which is not associated with stroke, approximately 80% of cases of chorea develop immediately after a stroke[8]. There is evidence of the significant association between movement disorders following a stroke and plaques in the basal ganglia and the thalamus (44% and 37%)[9]. It is three times greater to develop a movement disorder after a deep nuclei infarction (such as of the basal ganglia and thalamus) than after a cortical infarction[10]. Krauss et al[11] reported two patients with basal ganglia lesions who developed ipsilateral hyperkinetic movement disorders and contralateral hemiparesis. One study, using cerebral single photon emission computed tomography with technetium-99m ethyl cysteinate dimer, demonstrated that hypoperfusion of the subthalamic nucleus and globus pallidus internus (GPi) is associated with the development of chorea after stroke[12].
The pathophysiology of hemichorea is related to direct and indirect pathways in the basal ganglia. The cortico-striato-pallido-thalamico-cortical loop is the primary circuit of the basal ganglia. The thalamus is disinhibited by lesions in the direct pathway, which promotes movements. Damages in the external GPi and thalamus constitute the indirect pathway, which inhibit movements. Changes in contralateral subthalamic nucleus are responsible for the occurrence of hemichorea. Usually, the subthalamic nucleus could stimulate the GPi and substantia nigra. The outflow of the GPi to the thalamus has an inhibitory effect, while the activity of the thalamus is inhibited by the motor cortex, leading to normal controlled movements. Abnormal choreic movements are caused by damage to the thalamus in hemichorea[3]. Infarction in the thalamus has been shown to change several neurotransmitter systems. Under normal circumstances, γ-aminobutyric acid (GABA) and dopamine are in a state of balance. The declined concentrations of the inhibitory neurotransmitter, GABA, may be related to hypoxic-ischemic necrosis of thalamic neurons. On the contrary, the relative upregulation of the dopamine after hypoxia-ischaemia leads to contralateral limb hyperkinetic movement disorders.
Mechanisms underlying ipsilateral hemiparesis after stroke, including non-decussation of corticospinal tracts or inhibitory impulses of ipsilateral basal ganglia to the motor cortex of the opposite side through the corpus callosum, suggested that potential differences among individuals in neural pathways may be relevant. Diffusion tensor imaging showed no decussation of pyramidal tracts in the caudal medulla in a previous case[13]. However, our patient developed contralateral hemiplegia, ruling out this rare possibility. The evidence that unilateral functional neurosurgery in patients with Parkinson’s disease can bilaterally improve motor function supports the fact that the basal ganglia have bilateral effects on motor function[14].
Contralateral hemiparesis may be a conditioning factor for the occurrence of hemichorea ipsilateral to cerebral lesions. Among previously reported patients with hyperkinesia ipsilateral to cerebral lesions, the majority had contralateral hemiparesis. It is theoretically possible that the patients might have had bilateral chorea if they had not had concomitant hemiparesis. However, it is also possible that the motor deficit contralateral to the thalamic lesion triggers the ipsilateral hyperkinesia. It remains unclear how such activity is mediated. Commissural connections between subthalamic nuclei have been described, but their functional relevance in humans is unclear[15]. Meanwhile, the pedunculopontine nucleus is connected to both subthalamic nuclei[16,17]. The possibility that the thalamus and subthalamic nuclei exert bilateral control of motor function may explain this. Improvement of involuntary movements on both sides in Parkinson’s disease patients receiving unilateral stereotaxic neurosurgery supports this explanation.
Hemichorea ipsilateral to thalamic lesions could also be mediated at a higher subcortical level. Fibers from the subthalamic nucleus pass through the GPi to the pre-supplementary motor area, Brodmann 24 area, and the dorsolateral prefrontal cortex through thalamic relay nuclei. Effective subthalamic nucleus stimulation has been shown to result in increased activation of the contralateral dorsolateral prefrontal cortex[18].
In a previous study, 18F-fluorodeoxyglucose-positron emission tomography performed 1 wk after onset of hemichorea due to ipsilateral subthalamic nucleus infarction revealed hypometabolism in the ipsilateral hemispheric cortex and the caudate nucleus and putamen, probably owing to the nerve conduction block between the subthalamic nucleus and basal ganglia system and the cortex of the ipsilateral side, respectively[19]. The anatomic pathways related to this bilateral control are not known.
Yang et al[20] reported ipsilateral hemichorea and hemiballism in three patients with acute cerebrovascular disease. Cranial computed tomography showed deviation of the midline structures to one side in two patients, with signs of cerebellar herniation in one of the two patients. The authors postulated that brain edema or a space-occupying lesion could produce ipsilateral involuntary movement by compressing and stimulating the relevant structures on the opposite side.
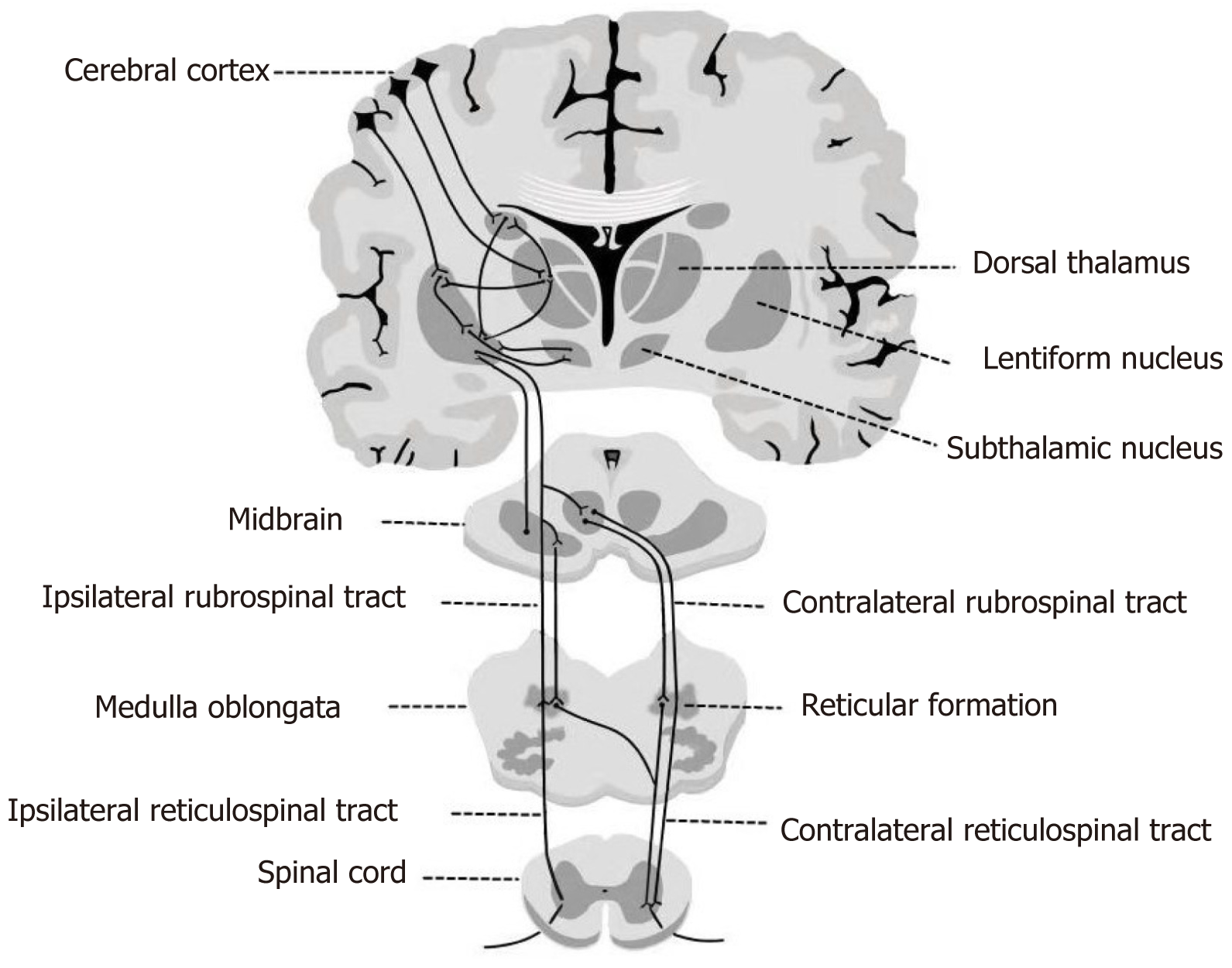
Our patient had no intracranial edema or space-occupying lesion. We believe that the explanation of the ipsilateral hemichorea lies in the structure of the extrapyramidal system. The extrapyramidal system is a group of nuclei and related tracts whose main role is to facilitate and coordinate motor signals. In the pyramidal decussation, 70%-90% of the pyramidal tracts cross to the contralateral side to form the lateral pyramidal tract. The remaining 10%-20% continue descending on the same side, forming the anterior pyramidal tract. Similarly, some descending fibers of the extrapyramidal system continue their ipsilateral course. The extrapyramidal system is parallel to the pyramidal tracts, from the cerebral cortex to the spinal cord. With the bilateral connections of the central tegmental tract decussating in the pons and the thalamus acting as a relay station, the adjustments of postural muscles on both sides can be affected by each cerebral hemisphere[21] (Figure 2). Bilateral Brodmann area 6 directly controls the extrapyramidal effects of both sides through a tract that decussates in the pons[22]. Riddle et al[23] found in an animal model that the frequency and strength of excitatory reticulomotoneuronal connections in hand motoneuron groups are similar with those in forearm or upper arm motoneuron groups. The main subcortical component of the circuits connecting the cerebral cortex and thalamus is located at the basal ganglia. Efferent impulse from the basal ganglia passes through the ventral anterior nucleus of the thalamus to reach the cerebral cortex, helping to mediate the preparatory stage of movements. Basal ganglia nuclei receive afferent nerve impulse from the cerebral cortex and send the transmission, via the thalamus, to the cerebellum and spinal cord. The direct and indirect pathways provide positive and negative feedbacks, respectively, in the circuit between the basal ganglia and thalamus. Previous neuroimaging studies have shown that the most common lesions associated with hemichorea are located in the subthalamic nucleus, putamen, and caudate nucleus[24]. In an animal model, chorea developed due to the inactivation of the subthalamic nucleus and reduced output of the basal ganglia[25].
It is acknowledged that chorea and ballism have a similar pathophysiology[26]. Clarification of the role of extrapyramidal system networks may have important clinical implications.
We report a patient with unilateral hemichorea and contralateral hemiparesis associated with ipsilateral thalamic lesion. We believe that this unusual phenomenon might be mediated by the descending fibers of the ipsilateral extrapyramidal system. Contralateral hemiparesis may be a conditioning factor for the occurrence of hemichorea ipsilateral to cerebral lesions. It is also possible that the motor deficit contralateral to the thalamic lesion triggers the ipsilateral hyperkinesia.
We are thankful to the patient for permission to submit this paper for publication.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Tee YK, Zhang ZX S-Editor: Gao CC L-Editor: Wang TQ P-Editor: Zhang YL
| 1. | Dewey RB Jr, Jankovic J. Hemiballism-hemichorea. Clinical and pharmacologic findings in 21 patients. Arch Neurol. 1989;46:862-867. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 193] [Cited by in F6Publishing: 207] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 2. | Crozier S, Lehéricy S, Verstichel P, Masson C, Masson M. Transient hemiballism/hemichorea due to an ipsilateral subthalamic nucleus infarction. Neurology. 1996;46:267-268. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 21] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 3. | Kannepalli NR, Yadav R, Vazhayil V, Somanna S, Pal PK. Ipsilateral Hemichorea-hemiballism in a Case of Postoperative Stroke. Tremor Other Hyperkinet Mov (N Y). 2016;6:359. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 5] [Reference Citation Analysis (0)] |
| 4. | Moersch FP. Hemiballismus. Arch Neurol Psychiatry. 1939;41:365. [Cited in This Article: ] |
| 5. | Baik JS, Park JH, Kim JY, Minn YK. A case of hemichorea with an ipsilateral thalamic infarction. Mov Disord. 2002;17:S315-S316. [Cited in This Article: ] |
| 6. | Kim JT, Baik JS, Kim JY, Park JH. A Case of Ipsilateral Hemichorea Due to Thalamic Infarction. J Korean Neurol Assoc. 2002;20:540-543. [Cited in This Article: ] |
| 7. | Bhidayasiri R, Truong DD. Chorea and related disorders. Postgrad Med J. 2004;80:527-534. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 58] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 8. | Bansil S, Prakash N, Kaye J, Wrigley S, Manata C, Stevens-Haas C, Kurlan R. Movement disorders after stroke in adults: a review. Tremor Other Hyperkinet Mov (N Y). 2012;2. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 27] [Reference Citation Analysis (0)] |
| 9. | Ghika-Schmid F, Ghika J, Regli F, Bogousslavsky J. Hyperkinetic movement disorders during and after acute stroke: the Lausanne Stroke Registry. J Neurol Sci. 1997;146:109-116. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 162] [Cited by in F6Publishing: 158] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 10. | Siniscalchi A, Gallelli L, Labate A, Malferrari G, Palleria C, Sarro GD. Post-stroke Movement Disorders: Clinical Manifestations and Pharmacological Management. Curr Neuropharmacol. 2012;10:254-262. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 34] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 11. | Krauss JK, Pohle T, Borremans JJ. Hemichorea and hemiballism associated with contralateral hemiparesis and ipsilateral basal ganglia lesions. Mov Disord. 1999;14:497-501. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 2] [Reference Citation Analysis (0)] |
| 12. | Takahashi T, Kanamori H, Shigehara R, Takahashi SN, Tamura M, Takasu T, Murakami M. Pure hemi-chorea resulting from an acute phase of contralateral thalamic lacunar infarction: a case report. Case Rep Neurol. 2012;4:194-201. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 13. | Reddy YM, Parida S, Murthy JMK. Teaching NeuroImages: All Hemiparesis Are Not Contralateral. Neurology. 2021;96:e478-e479. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 14. | Alberts JL, Hass CJ, Vitek JL, Okun MS. Are two leads always better than one: an emerging case for unilateral subthalamic deep brain stimulation in Parkinson's disease. Exp Neurol. 2008;214:1-5. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 47] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 15. | Mettler FA. Anatomy of the basal ganglia. In: Vinken PJ, Bruyn GW. Handbook of Clinical Neurology, vol 6. Amsterdam: North Holland, 1968: 1-55. [Cited in This Article: ] |
| 16. | Lavoie B, Parent A. Pedunculopontine nucleus in the squirrel monkey: projections to the basal ganglia as revealed by anterograde tract-tracing methods. J Comp Neurol. 1994;344:210-231. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 237] [Cited by in F6Publishing: 228] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 17. | Jackson A, Crossman AR. Nucleus tegmenti pedunculopontinus: efferent connections with special reference to the basal ganglia, studied in the rat by anterograde and retrograde transport of horseradish peroxidase. Neuroscience. 1983;10:725-765. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 248] [Cited by in F6Publishing: 254] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 18. | Limousin P, Greene J, Pollak P, Rothwell J, Benabid AL, Frackowiak R. Changes in cerebral activity pattern due to subthalamic nucleus or internal pallidum stimulation in Parkinson's disease. Ann Neurol. 1997;42:283-291. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 326] [Cited by in F6Publishing: 289] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 19. | Renard D, Le Floch A, Castelnovo G, Collombier L, Kotzki PO, Labauge P. Hemiballism due to an ipsilateral subthalamic nucleus lesion. J Neurol. 2011;258:507-509. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 20. | Yang X, He ML, Liu LX, Wu X. Ipsilateral hemichorea and hemiballism in acute cerebrovascular disease. Zhonghua Shenjingke Zazhi. 1996;29:222-224. [Cited in This Article: ] |
| 21. | Guo GW, Wang X. Colour Atlas of Human Anatomy. People's Medical Publishing House, 1986: 302. [Cited in This Article: ] |
| 22. | Denny-Brown D. The fundamental organization of motor behavior. In: Yahr MD, Purpura DP. The Neurophysiological Basis of Normal and Abnormal Motor Activities. New York: Raven Press, 1967: 415-444. [Cited in This Article: ] |
| 23. | Riddle CN, Edgley SA, Baker SN. Direct and indirect connections with upper limb motoneurons from the primate reticulospinal tract. J Neurosci. 2009;29:4993-4999. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 190] [Cited by in F6Publishing: 205] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 24. | Chung SJ, Im JH, Lee MC, Kim JS. Hemichorea after stroke: clinical-radiological correlation. J Neurol. 2004;251:725-729. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 78] [Cited by in F6Publishing: 82] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 25. | Crossman AR, Sambrook MA, Jackson A. Experimental hemichorea/hemiballismus in the monkey. Studies on the intracerebral site of action in a drug-induced dyskinesia. Brain. 1984;107 (Pt 2):579-596. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 153] [Cited by in F6Publishing: 168] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 26. | Albin RL. The pathophysiology of chorea/ballism and Parkinsonism. Parkinsonism Relat Disord. 1995;1:3-11. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 68] [Cited by in F6Publishing: 71] [Article Influence: 5.9] [Reference Citation Analysis (0)] |