Published online Oct 16, 2015. doi: 10.12998/wjcc.v3.i10.887
Peer-review started: April 8, 2015
First decision: June 4, 2015
Revised: July 12, 2015
Accepted: August 10, 2015
Article in press: August 11, 2015
Published online: October 16, 2015
AIM: To examine the usefulness of a new tapered metallic stent (MS) in patients with unresectable malignant hilar bile duct obstruction.
METHODS: This new tapered MS was placed in 11 patients with Bismuth II or severer unresectable malignant hilar bile duct obstruction, as a prospective study. The subjects were six patients with bile duct carcinoma, three with gallbladder cancer, and two with metastatic bile duct obstruction. Stenosis morphology was Bismuth II: 7, IIIa: 3, and IV: 1. UMIN Clinical Trial Registry (UMIN000004758).
RESULTS: MS placement was 100% (11/11) successful. There were no procedural accidents. The mean patency period was 208.401 d, the median survival period was 142.000 d, and the mean survival period was 193.273 d. Occlusion rate was 36.4% (4/11); the causes of occlusion were ingrowth and overgrowth in 2 patients each, 18.2%, respectively. Patients with occlusion underwent endoscopic treatment one more time and all were treatable.
CONCLUSION: The tapered MS proved useful in patients with unresectable malignant hilar bile duct obstruction because it provided a long patency period, enabled re-treatment by re-intervention, and no procedural accidents occurred.
Core tip: Placement of a tapered metallic stent in patients with unresectable malignant hilar bile duct obstruction proved useful because it allowed a longer patency period without procedural accidents.
- Citation: Sakai Y, Tsuyuguchi T, Nishikawa T, Sugiyama H, Sasaki R, Sakamoto D, Watanabe Y, Nakamura M, Yasui S, Mikata R, Yokosuka O. New tapered metallic stent for unresectable malignant hilar bile duct obstruction. World J Clin Cases 2015; 3(10): 887-893
- URL: https://www.wjgnet.com/2307-8960/full/v3/i10/887.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v3.i10.887
The guidelines for biliary cancer diagnosis recommend drainage as frequent as possible in patients with unresectable malignant hilar bile duct obstruction for improvement of patient’s quality of life or when performing chemotherapy[1]. As an approach route for hilar bile duct occlusion, there are surgical, percutaneous, and transpapillary routes; and the region where drainage can be carried out differs depending on the location of the tumor, thus it is very difficult to establish treatment strategies. An endoscopic approach is recommended as the drainage route because of the low invasiveness and high success rate of internal drainage[1]. Metallic stents (MSs) are considered useful for internal drainage in terms of patency period[1]. Even past randomized controlled trials reported that MSs are associated with a longer patency period and lower occlusion rate than plastic stents (PSs)[2,3]. Under such considerations, it may be necessary to set the strategies to use MSs in patients with unresectable malignant hilar bile duct obstruction. In this study we examine the usefulness of a new tapered MS developed for exclusive use in patients with unresectable malignant hilar bile duct obstruction.
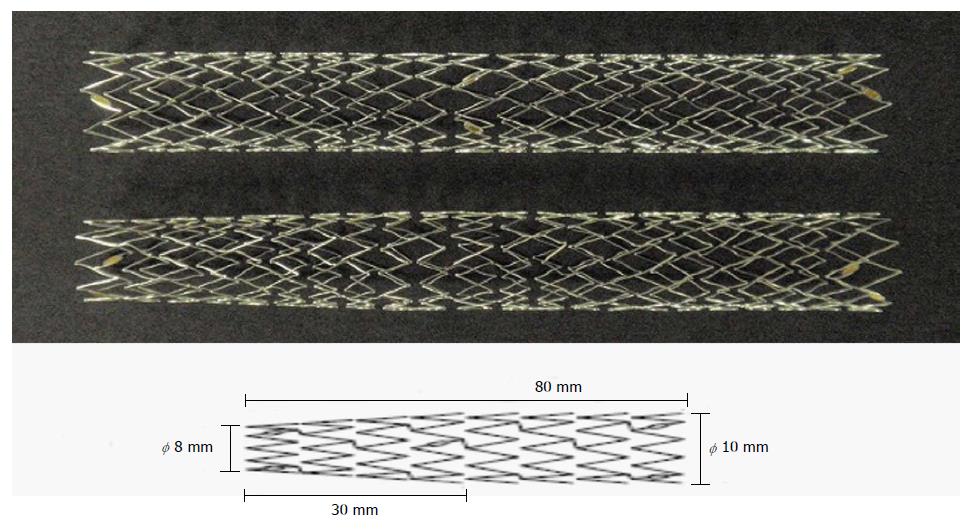
The patients with unresectable malignant hilar bile duct obstruction and showed a remarkable increase of hepatobiliary enzymes, had Bismuth II or higher degree stenosis according to Bismuth classification[4] and had been treated between July 2011 to December 2012 were included in this study (Table 1). There were 11 patients (7 men and 4 women) aged 72.273 ± 10.771 (59-85) years. The diagnosis was established based on a combination of images plus pathological findings. The cause of obstruction was bile duct carcinoma in 6, gallbladder cancer in 3, and metastatic bile duct obstruction in 2 patients. We evaluated the intrahepatic bile duct with a little contrast media. Stenosis morphology was Bismuth II in 7, IIIa in 3, and IV in 1 patient. The stenosis was 22.727 ± 8.545 (10-35) mm long. Remarkable increase of hepatobiliary enzymes was defined as a value double or more the normal value of ALT (IU/L), ALP (IU/L), or T-Bil (mg/dL), or a combination of them. ALT (IU/L) was 114.055 ± 96.915, ALP (IU/L) was 1157.09 ± 420.250, and T-Bil (mg/dL) was 5.427 ± 4.4365 prior to drainage. Inclusion criteria were: (1) Patients with unresectable malignant hilar bile duct obstruction; (2) No criteria on underlying disease, age or sex; and (3) Patients who give gave their informed consent. Exclusion criteria were: (1) Patients in whom the endoscopic approach was difficult; (2) Patients with a bleeding tendency; (3) Patients who had suffered serious procedural accidents; (4) Patients who did not provide their informed consent; and (5) Patients who were determined not to be appropriate by the physician in charge. The MS was placed in all the patients via the endoscopic retrograde cholangiopancreatography (ERCP) route. Magnetic resonance cholangiopancreatography was performed in all of them before drainage. There was no case of cholangitis. Chemotherapy was performed in 6 patients and 5 received the best supportive care. The patients were followed up from MS placement to their death, and if patients were alive by March 2014 they were evaluated. Before ERCP, all patients were given the standard premedication consisting of intravenous administration of midazolam (3 to 10 mg), and the dose depended on age and tolerance. Scopolamine butylbromide or glucagon was used for duodenal relaxation. During ERCP, arterial oxygen saturation was continuously monitored using a pulse oximeter. Patients were kept fasting after the procedure for at least 24 h with drip infusion of 2000 mL and stayed in the hospital for at least 72 h. They received 8-h infusion of a protease inhibitor (nafamostat mesilate, 20 mg/d) and were prescribed antibiotics (SBT/CPZ, 2 g/d) for 2 d. For cannulation, catheters PR-104Q, R110Q-1 and PR233Q were used. Wire-guided cannulation was not performed. A 0.025-inch or 0.035-inch guidewire (Jagwire: Microvasive, Boston Scientific Corp., Natick, MA, Revo Wave: PIOLAX, or VisiGlide: Olympus Corp., Tokyo, Japan) was used. The endoscopes used were JF240, JF260V, TJF260V (Olympus Corp.), backward side-viewing endoscopes. After cholangiography, a guidewire was placed in the bile duct to conduct endoscopic sphincterotomy (EST). Clever-Cut3V (Olympus Corp.) was used as the knife for EST. EST was conducted using a single electrosurgical current generator (PSD-20, Olympus Corp.) at a power of 25 watts. EST was carried out in all the patients. The effect of drainage was determined by placing an endoscopic nasobiliary drainage (ENBD), or a PS in either the right or left bile duct. The effect of drainage was evaluated 7 d after drainage placement, and it was determined effective if the T-Bil was normal or 2/3 or less; then a tapered MS was placed. In patients without effective drainage, ENBD or PS was placed in the bile duct in the side where the drainage is not placed. An ENBD tube of 7 Fr. was used (FLEXIMA: Boston Scientific Corp., Natick, MA, or SD9: SILUX Straight type). Tube stents of 7 Fr., 8.5 Fr. and 10 Fr. were used (FLEXIMA: Boston Scientific Corp., or SD9: SILUX Straight type). And if the drainage was effective, the new tapered MS was placed in the region. As for tapered MSs, the delivery system is a laser-cut MS created for use exclusively in the liver. These MSs are 7 Fr in size, with a full length of 8 cm and a 3-cm tapered tip. The mesh space is 6-8 mm at the center of the stent, and its internal diameter in the papillary side is 10 mm, while in the hepatic side it is 8 mm (PIOLAX: Japan) (Figure 1). In patients for whom two tapered MSs were required, stenting was performed in the partial stent-in-stent manner[5-7]. The axial force and radial force of this MS were evaluated as follows. Axial force is the unbending force of the MS from the curved part. To measure the axial force, a portion of the stent was pushed perpendicularly by a force gauge (model DPX-5TR, Imada, Tokyo) until the angle became 60 degrees, and the force necessary to keep it in place was recorded. The measurement was made in an oven at 37 °C for 3 points distant 20, 40, and 60 mm from the bending point. Radial force is the dilating force of the MS. Radial force was measured using a radial force measurement machine (RX 500, Machine Solutions, Flagstaff, Ariz) in an oven at 37 °C. An MS sample in a fully expanded state was placed in the cylindric space of the machine, and the cylinder was contracted to shrink the MS to its minimum size of 2 mm. Then the force on the cylinder was reserved by an expansion force of the MS until it achieved its fully expanded state of 10 mm in diameter. The placement success rate, patency period, occlusion rate, and success rate of re-intervention of this MS were examined. Procedural accidents during ERCP-related procedures were evaluated according to Cotton’s classification[8]. When the jaundice level was T-Bil 3 mg/dL less, we started chemotherapy. This study was conducted under approval of our ethical committee, and was registered as prospective clinical trial. UMIN Clinical Trial Registry (UMIN000004758).
| Case | Sex | Age | Disease | Stent no. | Stenosis morphology | Stenosis length (mm) | Treatment |
| 1 | Male | 60 | Intrahepatic bile duct carcinoma | 2 | Bismuth II | 25 | BSC |
| 2 | Male | 59 | Colon cancer | 1 | Bismuth IIIa | 33 | Chemotherapy |
| 3 | Female | 85 | Intrahepatic bile duct carcinoma | 1 | Bismuth IIIa | 28 | Chemotherapy |
| 4 | Female | 85 | Bile duct carcinoma | 2 | Bismuth IV | 35 | BSC |
| 5 | Male | 67 | Intrahepatic bile duct carcinoma | 2 | Bismuth II | 18 | Chemotherapy |
| 6 | Female | 61 | Gallbladder cancer | 2 | Bismuth II | 17 | BSC |
| 7 | Male | 65 | Gallbladder cancer | 1 | Bismuth II | 18 | Chemotherapy |
| 8 | Female | 85 | Gallbladder cancer | 2 | Bismuth II | 22 | BSC |
| 9 | Male | 81 | Colon cancer | 1 | Bismuth II | 32 | BSC |
| 10 | Male | 79 | Bile duct carcinoma | 1 | Bismuth IIIa | 10 | Chemotherapy |
| 11 | Male | 68 | Bile duct carcinoma | 1 | Bismuth II | 12 | Chemotherapy |
Fisher’s exact probability test, student’s t-test, and the Mann-Whitney U-test were used for statistical analyses to compare the blood test findings prior to drainage insertion and post MS insertion. A P value < 0.05 was regarded as significant. Cumulative stent patency and survival were estimated using the Kaplan-Meier estimator. Data were analyzed using SPSS software version 17 (SPSS, Chicago, IL).
Initial drainage was successful in 6 (54.5%) of the eleven patients, and the remaining 5 (45.5%) had poor drainage thus drainage in the right and left bile ducts was performed. In the end, drainage was successful in all the patients. Since drainage was effective, MS was placed in all of them. In the six patients who underwent unilateral bile duct drainage one MS was placed, while in the five patients who underwent right and left bile duct drainage, two MS were placed; stenting was successful in all the patients. The mean number of MSs used was 1.545 ± 0.522 (1-2). All the parameters assessed at one week after stenting showed significant improvement compared with those before drainage insertion: ALT 33.00 ± 13.892 (IU/L), ALP 566.91 ± 365.157 (IU/L), and T-Bil 1.373 ± 0.8833 (mg/dL) (Table 2, Table 3 and Table 4). There were no procedural accidents due to stenting. The axial force of this MS was 0.156 ± 0.017 N when evaluated at bending point 20 mm, and radial force was 4.76 ± 0.18 N when evaluated at a dilated diameter of 4 mm. The patency of MS is shown in Table 5. The mean patency period was 208.401 d, the median survival period was 142.000 d (mean 193.273 d). The occlusion rate was 36.4% (4/11), and the occlusion causes were ingrowth in 2 (18.2%) patients and overgrowth in another 2 (18.2%). Patients with occlusion underwent endoscopic treatment one more time and in all of them it was 100% (4/4) successful. In patients who developed overgrowth in the contralateral hepatic side, an MS was placed in the partial stent-in-stent manner. In patients with an MS in each bile duct who developed overgrowth, an MS was additionally placed. In two patients with two MSs in the right and left bile duct who developed ingrowth, two PSs were placed in the right and left bile duct in the stent-in-stent manner. Re-treatment was successful in all the occlusion patients. The accidental occurrence symptom about the ERCP related procedures did not accept it.
| Case | ALT before drainage(IU/L) | ALT after MS insertion(IU/L) | P-value |
| 1 | 63 | 54 | |
| 2 | 165 | 26 | |
| 3 | 182 | 32 | |
| 4 | 263 | 53 | |
| 5 | 75 | 14 | |
| 6 | 326 | 52 | |
| 7 | 69 | 25 | |
| 8 | 37 | 25 | |
| 9 | 115 | 25 | |
| 10 | 39 | 22 | |
| 11 | 212 | 35 | |
| Average | 114.055 ± 96.915 | 33.00 ± 13.892 | P < 0.05 |
| Case | ALP before drainage(IU/L) | ALP after MS insertion(IU/L) | P-value |
| 1 | 1524 | 1288 | |
| 2 | 1113 | 490 | |
| 3 | 1200 | 386 | |
| 4 | 1726 | 775 | |
| 5 | 605 | 354 | |
| 6 | 1289 | 254 | |
| 7 | 1524 | 956 | |
| 8 | 638 | 256 | |
| 9 | 1611 | 956 | |
| 10 | 610 | 238 | |
| 11 | 888 | 283 | |
| Average | 1157.09 ± 420.250 | 566.91 ± 365.157 | P < 0.05 |
| Case | T-Bil before drainage(mg/dL) | T-Bil after MS insertion(mg/dL) | P-value |
| 1 | 13.9 | 3.8 | |
| 2 | 3 | 1 | |
| 3 | 12 | 1 | |
| 4 | 1.4 | 0.7 | |
| 5 | 3.1 | 0.9 | |
| 6 | 10.2 | 2.1 | |
| 7 | 2.3 | 1.3 | |
| 8 | 3 | 1 | |
| 9 | 1.8 | 1.3 | |
| 10 | 3.8 | 1 | |
| 11 | 5.2 | 1 | |
| Average | 5.427 ± 4.4365 | 1.373 ± 0.8833 | P < 0.05 |
| Case | Survival period (d) | Alive or dead | Patency period (d) | Absence or presence of occlusion | Occlusion cause | Re-intervention |
| 1 | 142 | Dead | 126 | + | Ingrowth | PS |
| 2 | 122 | Dead | 86 | + | Ingrowth | PS |
| 3 | 213 | Dead | 213 | - | - | - |
| 4 | 93 | Dead | 93 | - | - | - |
| 5 | 245 | Dead | 245 | - | - | - |
| 6 | 78 | Dead | 78 | - | - | - |
| 7 | 533 | Alive | 130 | + | Overgrowth | MS |
| 8 | 123 | Dead | 123 | - | - | - |
| 9 | 145 | Dead | 75 | + | Overgrowth | MS |
| 10 | 127 | Dead | 127 | - | - | - |
| 11 | 305 | Dead | 305 | - | - | - |
| Mean | 142 | 208.401 | - | - | - | |
| Median | 193.273 | - | - | - | - |
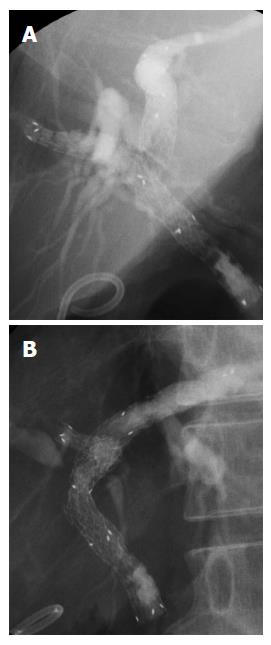
In this study we evaluated a new tapered MS for unresectable malignant hilar bile duct obstruction. The MS used in this study was the laser-cut MS that enables precise stenting because shortening is structurally less[5]. Although evaluation may be partially difficult due to the small sample size, we experienced no procedural accidents during insertion, the patency period was long, and re-intervention was successful in all the patients; thus we consider this is a useful stent. This MS has moderate radial force at low axial force[9]. With regard to procedural accidents, this MS has low axial force, which enables stenting along the bile duct and may prevent kinking. When an MS is placed, usually procedural accidents such as acute pancreatitis or acute cholecystitis do not occur, however, abdominal pain may occur[10]. There may be various causes for this, including stress on the bile duct due to high axial force or strong radial force of the MS, or to a mismatch of the bile duct and MS regarding diameter, especially if the MS is of a diameter larger than that of the hepatic bile duct. The MS used in this study has a low axial force and a moderate radial force as shown in past reports; thus it is useful to treat stenosis and carry out stenting while applying low pressure on the bile duct. Furthermore, the tip is tapered, enabling good positioning of the stent (Figure 2). This may reduce the risk of abdominal pain due to stenting and of procedural complications such as hepatic abscess because the Glisson's sheath is not compressed. In this study no procedural accidents occurred; still if pancreatography is performed frequently during the procedure or it is difficult to catheterize the bile duct, pancreatitis might occur after ERCP[11,12].
As for the patency period, the sample size was small and thus evaluation is difficult. However, the patency period was in the same range as that found in a previous report of similar sample size, and which was considered as satisfactory[13]. The nature of the tumor, effect of chemotherapy, and characteristics of the MS itself may influence the patency period; yet, these should be evaluated in a study involving a large number of patients in the future.
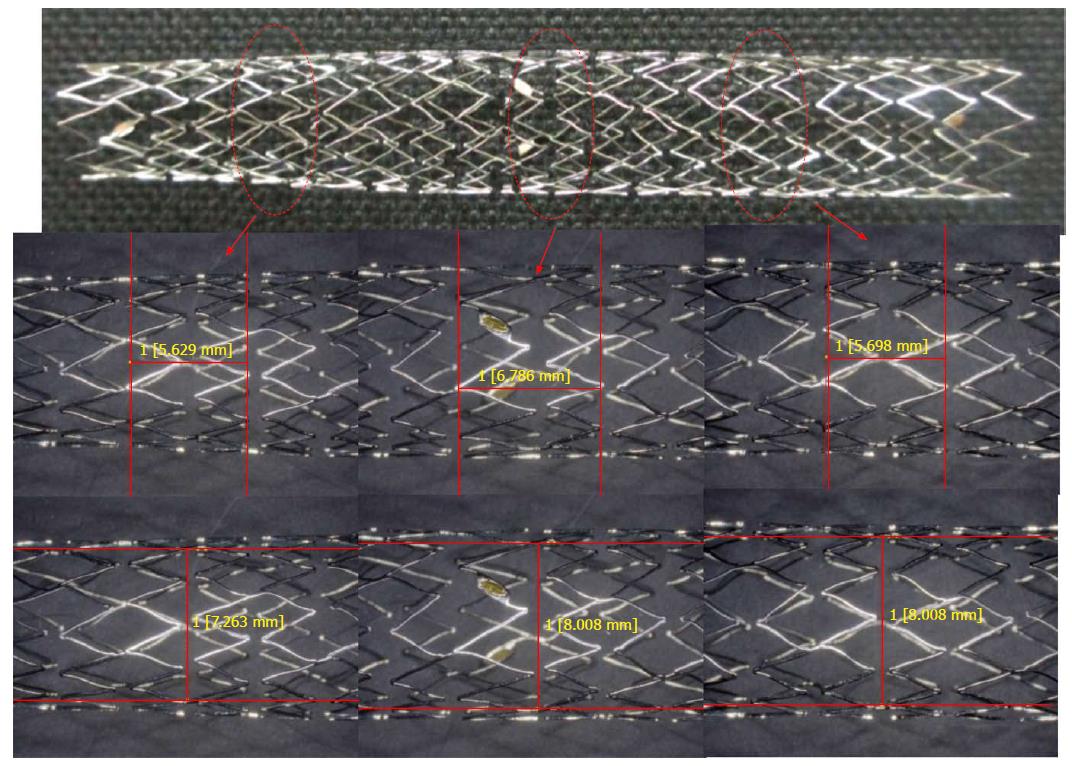
Re-treatment was 100% (4/4) successful. Recent advancement of endoscopes and medical devices has enabled re-treatment in a comparatively easy way. This MS has a large mesh space that facilitates re-intervention. Indeed, Mukai et al[14] reported that in the liver MSs with a large mesh space were an excellent choice because it was easier to re-intervene. Furthermore, other authors have also reported on the usefulness of MSs with a large mesh space that were created for exclusive use in the liver in patients with unresectable malignant hilar bile duct obstruction[13,15]. The MSs used in these reports were of the braided type with a mesh space of 7 mm; that is, a space similar to that of the mesh space of the laser-cut tapered MS used in this study (Figure 3). The laser-cut tapered MS used in this study has a large mesh space, which facilitates manipulation through the mesh and re-intervention. From such results and reports, it is currently considered that MSs with a large mesh space may be an excellent choice for use in the liver. Compared with the braided MS, the laser-cut MS used in this study hardly suffered shortening and enabled precise placement. However, in the future it may be necessary a randomized clinical trial to assess which one is best regarding placement success rate and patency period.
Our results suggested that the new tapered MS was useful for patients with unresectable malignant hilar bile duct obstruction because the patency period was long, re-treatment was possible, and there were no procedural accidents during their insertion.
Even past randomized controlled trials reported that metallic stents (MSs) are associated with a longer patency period and lower occlusion rate than plastic stents. Under such considerations, it may be necessary to set the strategies to use MSs in patients with unresectable malignant hilar bile duct obstruction.
In this study, the authors examine the usefulness of a new tapered MS developed for exclusive use in patients with unresectable malignant hilar bile duct obstruction.
It may be necessary to set the strategies to use MSs in patients with unresectable malignant hilar bile duct obstruction.
A new tapered MS developed for exclusive use in patients with unresectable malignant hilar bile duct obstruction.
The results suggested that the new tapered MS was useful for patients with unresectable malignant hilar bile duct obstruction because the patency period was long, re-treatment was possible, and there were no procedural accidents during their insertion.
This study prospectively estimated the efficacy of an uncovered metal stent with slightly tapered shape in its distal end for the patients with malignant biliary obstruction at the liver hilum.
P- Reviewer: Kanno Y, Singh V S- Editor: Ji FF L- Editor: A E- Editor: Jiao XK
| 1. | Tsuyuguchi T, Takada T, Miyazaki M, Miyakawa S, Tsukada K, Nagino M, Kondo S, Furuse J, Saito H, Suyama M. Stenting and interventional radiology for obstructive jaundice in patients with unresectable biliary tract carcinomas. J Hepatobiliary Pancreat Surg. 2008;15:69-73. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 2. | Wagner HJ, Knyrim K, Vakil N, Klose KJ. Plastic endoprostheses versus metal stents in the palliative treatment of malignant hilar biliary obstruction. A prospective and randomized trial. Endoscopy. 1993;25:213-218. [PubMed] [Cited in This Article: ] |
| 3. | Mukai T, Yasuda I, Nakashima M, Doi S, Iwashita T, Iwata K, Kato T, Tomita E, Moriwaki H. Metallic stents are more efficacious than plastic stents in unresectable malignant hilar biliary strictures: a randomized controlled trial. J Hepatobiliary Pancreat Sci. 2013;20:214-222. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 133] [Cited by in F6Publishing: 142] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 4. | Bismuth H, Nakache R, Diamond T. Management strategies in resection for hilar cholangiocarcinoma. Ann Surg. 1992;215:31-38. [PubMed] [Cited in This Article: ] |
| 5. | Kawamoto H, Tsutsumi K, Harada R, Fujii M, Kato H, Hirao K, Kurihara N, Nakanishi T, Mizuno O, Ishida E. Endoscopic deployment of multiple JOSTENT SelfX is effective and safe in treatment of malignant hilar biliary strictures. Clin Gastroenterol Hepatol. 2008;6:401-408. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 44] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 6. | Kawamoto H, Tsutsumi K, Fujii M, Harada R, Kato H, Hirao K, Kurihara N, Nakanishi T, Mizuno O, Ishida E. Endoscopic 3-branched partial stent-in-stent deployment of metallic stents in high-grade malignant hilar biliary stricture (with videos). Gastrointest Endosc. 2007;66:1030-1037. [PubMed] [Cited in This Article: ] |
| 7. | Kawamoto H, Tsutsumi K, Fujii M, Harada R, Kato H, Hirao K, Kurihara N, Nakanishi T, Mizuno O, Ishida E. Multiple stenting in a patient with a high-grade malignant hilar biliary stricture: endoscopic four-branched partial stent-in-stent deployment of metallic stents. Endoscopy. 2007;39 Suppl 1:E167-E168. [PubMed] [Cited in This Article: ] |
| 8. | Cotton PB, Lehman G, Vennes J, Geenen JE, Russell RC, Meyers WC, Liguory C, Nickl N. Endoscopic sphincterotomy complications and their management: an attempt at consensus. Gastrointest Endosc. 2009;37:383-393. [PubMed] [Cited in This Article: ] |
| 9. | Isayama H, Nakai Y, Toyokawa Y, Togawa O, Gon C, Ito Y, Yashima Y, Yagioka H, Kogure H, Sasaki T. Measurement of radial and axial forces of biliary self-expandable metallic stents. Gastrointest Endosc. 2009;70:37-44. [PubMed] [Cited in This Article: ] |
| 10. | Ho H, Mahajan A, Gosain S, Jain A, Brock A, Rehan ME, Ellen K, Shami VM, Kahaleh M. Management of complications associated with partially covered biliary metal stents. Dig Dis Sci. 2010;55:516-522. [PubMed] [Cited in This Article: ] |
| 11. | Freeman ML, Nelson DB, Sherman S, Haber GB, Herman ME, Dorsher PJ, Moore JP, Fennerty MB, Ryan ME, Shaw MJ. Complications of endoscopic biliary sphincterotomy. N Engl J Med. 1996;335:909-918. [PubMed] [Cited in This Article: ] |
| 12. | Freeman ML, DiSario JA, Nelson DB, Fennerty MB, Lee JG, Bjorkman DJ, Overby CS, Aas J, Ryan ME, Bochna GS. Risk factors for post-ERCP pancreatitis: a prospective, multicenter study. Gastrointest Endosc. 2001;54:425-434. [PubMed] [Cited in This Article: ] |
| 13. | Kogure H, Isayama H, Nakai Y, Tsujino T, Ito Y, Yamamoto K, Mizuno S, Yagioka H, Kawakubo K, Sasaki T. Newly designed large cell Niti-S stent for malignant hilar biliary obstruction: a pilot study. Surg Endosc. 2011;25:463-467. [PubMed] [Cited in This Article: ] |
| 14. | Mukai T, Yasuda I, Isayama H, Nakashima M, Doi S, Iwashita T, Iwata K, Kato T, Tomita E, Moriwaki H. Comparison of axial force and cell width of self-expandable metallic stents: which type of stent is better suited for hilar biliary strictures? J Hepatobiliary Pancreat Sci. 2011;18:646-652. [PubMed] [Cited in This Article: ] |
| 15. | Kogure H, Isayama H, Nakai Y, Tsujino T, Matsubara S, Yashima Y, Ito Y, Hamada T, Takahara N, Miyabayashi K. High single-session success rate of endoscopic bilateral stent-in-stent placement with modified large cell Niti-S stents for malignant hilar biliary obstruction. Dig Endosc. 2014;26:93-99. [PubMed] [DOI] [Cited in This Article: ] |