Published online Jul 6, 2019. doi: 10.12998/wjcc.v7.i13.1599
Peer-review started: January 29, 2019
First decision: March 14, 2019
Revised: April 10, 2019
Accepted: May 1, 2019
Article in press: May 1, 2019
Published online: July 6, 2019
There is a close relationship between cirrhosis and hepatocellular carcinoma (HCC). Transjugular intrahepatic portosystemic shunt (TIPS) has good clinical effect in treating the complication of portal hypertension. However, because of the risk of postoperative liver failure, severe complications, and low survival rate for HCC, TIPS is contraindicated in patients with portal hypertension and liver cancer. We studied a large cohort of patients with cirrhosis and HCC who underwent TIPS for recurrent variceal bleeding and/or ascites.
To assess the safety, efficacy, and survival rate in patients with HCC who underwent TIPS.
Group A comprised 217 patients with HCC and portal hypertension who underwent the TIPS procedure between 1999 and 2014. After TIPS deployment, these patients received palliative treatment for HCC. Group B comprised a cohort of 136 HCC patients with portal hypertension who did not undergo TIPS placement. Group B received palliative treatment for HCC plus medical therapy for portal hypertension. The clinical outcomes and survival rate were assessed.
In Group A, the primary technical success rate was 97.69% for TIPS placement, and no severe procedure-related complications of TIPS placement were reported. The control of variceal bleeding (VB) within 1 mo did not differ significantly between the groups (P = 0.261). Absorption of refractory ascites within 1 mo, recurrence of VB, and recurrence of refractory ascites differed significantly between the groups (P = 0.017, 0.023, and 0.009, respectively). By comparison, the rate of hepatic encephalopathy in Group B was lower than that in Group A (P = 0.036). The 1-, 2-, 3-, 4-, and 5-year survival rates were significantly different between Groups A and B (χ2 = 12.227, P = 0.018; χ2 = 12.457, P = 0.014; χ2 = 26.490, P = 0.013; χ2 = 21.956, P = 0.009, and χ2 = 24.596, P = 0.006, respectively). The mean survival time was 43.7 mo in Group A and 31.8 mo in Group B. Median survival time was 50.0 mo in Group A and 33.0 mo in Group B. Mean and median survival differed significantly between the two groups (P = 0.000, χ2 = 35.605, log-rank test). The mortality rate from VB in Group A was low than that in Group B (P = 0.006), but the rates of hepatic tumor, hepatic failure, and multiorgan failure did not differ significantly between the two groups (P = 0.173, 0.246 and 0.257, respectively).
TIPS combined with palliative treatment is safe and effective for portal hypertension in patients with HCC.
Core tip: We studied a large cohort of patients with cirrhosis and hepatocellular carcinoma (HCC) who underwent transjugular intrahepatic portosystemic shunt (TIPS) for recurrent variceal bleeding and/or ascites. They were compared with patients with cirrhosis and HCC who did not undergo TIPS placement. We conclude that TIPS combined with palliative treatment is safe and effective for portal hypertension in patients with HCC.
- Citation: Luo SH, Chu JG, Huang H, Yao KC. Safety and efficacy of transjugular intrahepatic portosystemic shunt combined with palliative treatment in patients with hepatocellular carcinoma. World J Clin Cases 2019; 7(13): 1599-1610
- URL: https://www.wjgnet.com/2307-8960/full/v7/i13/1599.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v7.i13.1599
Liver cirrhosis and hepatic cancer can occur after further evolution of liver damage[1]. Primary hepatocellular carcinoma (HCC) usually occurs due to cirrhosis of the liver, although the main cause of cirrhosis is not the same worldwide. There is a close relationship between cirrhosis and HCC; liver cancer is often complicated by cirrhosis, while cirrhosis often leads to the occurrence of liver cancer[2].
The transjugular intrahepatic portosystemic shunt (TIPS) reduces portal pressure and relieves the clinical symptoms associated with various medical conditions[3]. TIPS has found a wide range of applications, including treatment of portal hypertension due to cirrhosis, variceal bleeding (VB), refractory ascites (RA), hepatic hydrothorax, hepatorenal syndrome, Budd–Chiari syndrome, hepatopulmonary syndrome, and portal thrombosis and as a bridge to liver transplantation[4].
Because of the risk of postoperative liver failure, severe complications and low survival rate of HCC patients, TIPS is contraindicated in patients with portal hypertension and liver cancer[5,6]. We conducted a retrospective analysis of 217 cases of portal hypertension and liver cancer that were treated in our hospital between January 1999 and January 2014 with TIPS plus palliative treatment and radiofre-quency ablation (RFA) and 136 cases of liver cancer patients treated with palliative treatment and RFA to compare the safety and efficacy and survival rate.
Between January 1999 and January 2014, 353 patients with portal hypertension and HCC were treated in our center. The Institutional Review Board approved the study protocol. We reviewed the patients’ medical records and medical images to gather information regarding the underlying etiology, clinical presentation, and severity of cirrhosis and patients’ age and sex. Patients admitted to our center with HCC pres-ented with ascites, VB, or both. No differences were seen in terms of age, sex, underlying etiology, clinical presentation, Child–Pugh score, model for end-stage liver disease (MELD) score, and severity of cirrhosis (Table 1).
| Characteristic | Group A | Group B | P value |
| Gender, M/F | 115/97 | 76/60 | 0.526 |
| Age, mean ± SD, yr | 46.32 ± 12.43 | 44.79 ± 13.62 | 0.539 |
| Child–Pugh A/B/C | 54/129/34 | 33/83/20 | 0.462 |
| MELD score, mean ± SD | 10.21 ± 5.25 | 11.37 ± 4.17 | 0.645 |
| BCLC staging A/B/C/D | 18/107/53/34 | 12/67/34/23 | 0.518 |
| Viral hepatitis | 125 | 88 | 0.803 |
| Chronic ethanol consumption | 66 | 31 | 0.461 |
| Cryptogenic hepatitis | 26 | 17 | 0.724 |
| VB | 170 | 114 | 0.163 |
| RA | 47 | 22 | 0.217 |
| Both VB and RA | 69 | 35 | 0.167 |
| Laboratory tests | |||
| AFP, ng/mL | 468.53 ± 34.27 | 513.64 ± 25.19 | 0.625 |
| Alanine transaminase, U/L | 58.24 ± 14.32 | 61.14 ± 12.06 | 0.723 |
| Aspartate transaminase, U/L | 63.42 ± 16.21 | 59.34 ± 14.16 | 0.439 |
| Alkaline phosphatase, U/L | 196.23 ± 64.38 | 183.34 ± 84.64 | 0.376 |
| Total bilirubin, μmol/L | 29.13 ± 4.35 | 31.06 ± 5.24 | 0.634 |
| Albumin, g/L | 28.41 ± 4.37 | 27.13 ± 5.43 | 0.361 |
| Prothrombin time, s | 17.21 ± 5.34 | 19.42 ± 6.43 | 0.428 |
| Platelet count, × 109/L | 73.18 ± 21.43 | 67.46 ± 18.54 | 0.621 |
| Clinical presentation | |||
| Abdominal distention | 127 | 78 | 0.153 |
| Abdominal pain | 146 | 86 | 0.167 |
| Weakness | 153 | 89 | 0.184 |
| Poor appetite | 167 | 92 | 0.076 |
| Jaundice | 23 | 11 | 0.129 |
| Splenomegaly | 117 | 73 | 0.289 |
| Lower limbs edema | 25 | 14 | 0.141 |
This was a retrospective study that compared the clinical efficacy of the combination of TIPS, transarterial chemoembolization (TACE), RFA, and palliative treatment in liver cirrhosis complicated with HCC. All the patients were diagnosed with liver cirrhosis and portal hypertension by medical history, ultrasound, computed tomogra-phy (CT), magnetic resonance imaging, and gastroscopy. HCC was confirmed by imaging, tumor marker, or pathological examination. The patients were randomly divided into two groups. Group A comprised 217 patients with portal hypertension and HCC who were treated with TIPS plus palliative treatment and RFA. Group B comprised a cohort of 136 patients with HCC and portal hypertension who did not undergo TIPS placement and received palliative treatment and RFA.
The indications for TIPS included HCC with portal hypertension-related complica-tions such as recurrent VB after variceal sclerotherapy, RA, or both, which required TIPS placement. The exclusion criteria were: Portal vein thrombosis, history of hepatic encephalopathy (HE), severe right-sided heart failure, polycystic liver disease, dilated biliary ducts, age > 75 years, bilirubin > 5 mg/dL, creatinine > 3 mg/dL, Child–Pugh score > 11, MELD score > 18, sepsis, spontaneous bacterial peritonitis, and patients who had undergone liver transplantation. This study was to compare the clinical efficacy of the two groups. Patients who received oral sorafenib were also excluded.
Response to TIPS treatment was defined as follows: absence of clinically detectable ascites, with or without diuretic therapy, or ascites requiring no further paracentesis, and/or no further VB episodes after TIPS implantation. The development of a large amount of ascites as well as VB after TIPS implantation was defined as non-response. HCC treatment response was assessed using contrast-enhanced CT or magnetic resonance imaging and the modified Response Evaluation Criteria in Solid Tumors criteria were applied.
The anesthetic procedure used standard local anesthesia. TIPS was performed through a transjugular approach, as described previously[7]. The entire length of the intrahepatic tract was covered by the stent graft (BARD, Fluency, Voisins le Bretonneux, France; Viatorr, W.L. Gore & Associates, Flagstaff, AZ, United States). Hepatic venous pressure gradient and portal vein pressure were measured during the procedure, and the shunts were dilated to their full nominal diameter to reach a target portosystemic gradient (PSG) of < 12 mmHg and prominent gastroesophageal collateral vessels observed during TIPS were embolized with coils (Cook Inc., Bloomington, IL, United States). Subsequent direct portography was performed to evaluate whether the portal venous system was completely patent. After the TIPS procedure, intravenous heparin (4000 U/d; Chase Sun Pharma Co. Ltd, Tianjing, China) was given for 3 d and then oral warfarin (2.5 mg/d; Orion Pharma Co. Ltd, Orionintie, Finland) was prescribed to achieve an international normalized ratio of up. TACE/TAE was performed through the femoral artery as described previously[8], and RFA was guided by CT or ultrasound[9].
All patients underwent follow-up examination. A baseline duplex sonogram was obtained the day after TIPS creation, and subsequent shunt velocities were compared to this baseline result during follow-up. After TIPS, the patients were placed on a routine follow-up protocol that was identical for each group.
Follow-up visits and imaging (multiphase CT or MRI) took place 1 mo following the procedure, and every 3 mo thereafter. Treatment was repeated if follow-up imaging demonstrated persistent enhancement of tumors. Patients were seen as outpatients 1 mo after the procedure and then every mo or as needed for HCC cases. Each consul-tation included a clinical examination, blood chemistry, enhanced-CT or MRI examination, and assessment of HE. Ultrasound was performed at 1 wk and 4 wk after TIPS and then at 3 mo and 6 mo, and at 6-mo intervals thereafter, or in case of recurrent bleeding or ascites.
Shunt dysfunction requiring revision during TIPS venography or significant recurrent symptoms occurred. TIPS angiography was performed in patients with recurrent symptoms of suspected shunt dysfunction. TIPS revision was performed when a hemodynamically significant shunt stenosis (> 50%) was present with recurrent VB, recurrent or gradually worsening ascites, and the PSG was at least 15 mmHg unless grade III/IV encephalopathy (West Haven Criteria) was present. Patients lost to follow-up were censored at the time of the last known imaging of the shunt (duplex ultrasound or shunt venography).
Results are expressed as mean ± standard deviation. A logistic regression analysis was performed for the variables. The differences between the groups were compared using one-way analysis of variance followed by least significant difference t tests. Categorical variables were expressed as frequencies and compared using χ2 tests. Differences were considered significant at P < 0.05. The statistical analyses were performed with SPSS version 21.0 (SPSS, Armonk, NY, United States).
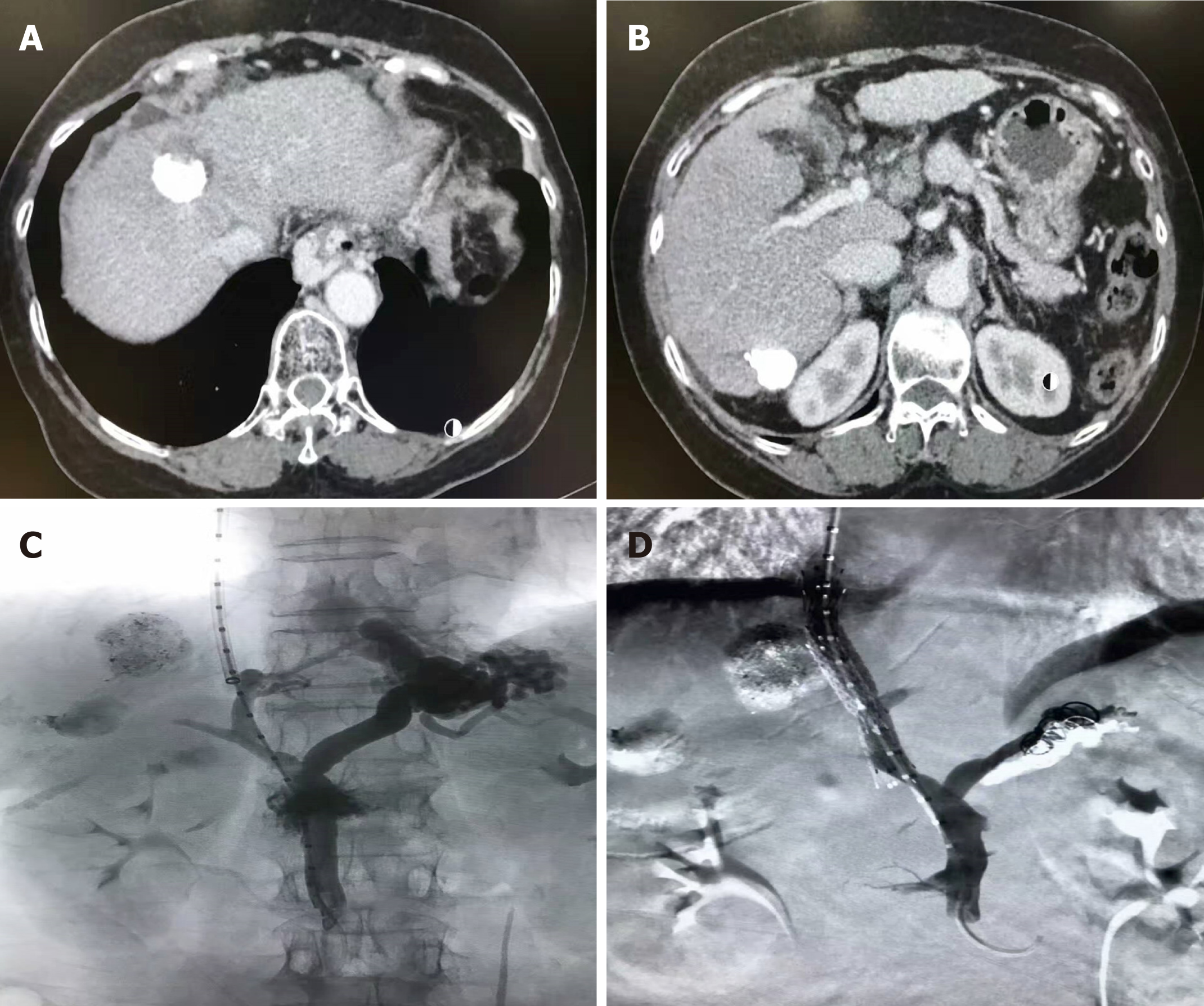
Group A comprised 217 patients; 170 underwent TIPS for treatment of VB, 47 for RA, and 69 for both. Two hundred and twelve cases had successful TIPS placement (Figure 1). The primary technical success rate was 97.69% (212/217), and the five cases that did not undergo successful TIPS placement were excluded. After TIPS placement, the mean PSG decreased from 23.37 ± 6.51 mmHg to 9.43 ± 3.14 mmHg (P = 0.016). There was no bleeding due to rupture or puncture of the tumor during TIPS. One week after the procedure, no acute stent stenosis or occlusion occurred. There were 114 cases of VB, 22 RA, and 35 with both in Group B.
In Group A, 212 cases underwent TACE for a total of 483 times. Among the 212 cases, 133 underwent TACE first followed by TIPS after liver function recovered from TACE injury; 79 cases underwent TIPS first and then after 1 wk recovery, they underwent TACE. RFA was performed 364 times. The 136 cases in Group B underwent TACE 269 times and RFA 175 times (Table 2). There were minor differences in the number of TACE (P = 0.043) and RFA (P = 0.037) performed between the two groups.
| Method | Group A n = 212 | Group B n = 136 | P value |
| TACE, No. of times | 483 | 269 | 0.043 |
| RFA, No. of times | 364 | 175 | 0.037 |
The timing of TIPS placement was different in Group A. Among the 133 cases undergoing TIPS placement followed by TACE; 81 cases were treated after 2 wk when liver function recovered to the preoperative level, 46 cases were treated 4 wk after the liver function recovered to the preoperative level, and six cases were treated when hepatic failure occurred. In the latter cases, four recovered after the treatment, and two died.
In Group A, 79 cases underwent TIPS placement before TACE. Among them, 17 cases were treated 2 wk after liver function recovered to the preoperative level, 49 cases were treated after 4 wk when liver function recovered to the preoperative level, and 13 cases were treated when hepatic failure occurred. In the latter cases, seven recovered after the treatment, and six died. By comparison, liver function recovery in the cases of TIPS placement followed by TACE was more satisfactory than in the cases of TIPS placement before TACE, and there was a significant difference (P < 0.05) (Table 3).
| Timing of TIPS | TACE first n = 133 | TIPS first n = 79 | P value |
| 2 wk | 81 | 17 | 0.008 |
| 4 wk | 46 | 49 | 0.014 |
| Hepatic failure | 6 | 13 | 0.012 |
In the course of follow-up, in Group A, VB was controlled in 153/168 (91.07%) cases within 30 d (2 cases were excluded due to TIPS failure). There were 28 cases of postoperative bleeding, with a rebleeding rate of 16.67% (28/168). This was because patients with TIPS stent dysfunction had PSG > 12 mmHg, after shunt revision, without recurrence of bleeding. Without TIPS treatment in Group B, VB was controlled in 98/114 (85.96%) cases within 30 d. There were 56 (49.12%) cases of rebleeding. These 114 cases were treated by endoscopy for a total of 391 times. In the two groups, there was no difference in the rate of control of VB (P = 0.261), but there was a significant difference in the rate of rebleeding (P = 0.023).
In Group A, after TIPS treatment, ascites was significantly reduced in 14 cases, and symptoms disappeared in 25 cases with complete remission (three cases were excluded due to TIPS failure) within 30 d (39/44, 86.63%). Thirteen of 44 (29.54%) patients had recurrent ascites due to stenosis or occlusion of TIPS stent, which resulted in PSG > 12mmHg. After revision of the stent, ascites symptoms disappe-ared. In six cases, shunt dysfunction was due to tumor invasion. CT and hepatic arteriography confirmed that the symptoms disappeared after balloon dilation and stent implantation. In Group B, 22 cases were treated with diuretic therapy and paracentesis, and in nine (40.90%) cases the amount of ascites was reduced within 30 d. Five cases were converted to middle volume, and 14 cases had no obvious remission (19/22, 86.36%). There was a significant difference between the two groups in the rate of absorption of ascites (P = 0.017) and recurrence of ascites (P = 0.009).
There were 32 patients with 37 times (17.45%, 37/212) occurrences of HE in Group A: 28 with grade I/II and nine with grade III/IV HE. After medical treatment to relieve symptoms, none of the patients died due to HE. There were eight patients with 12 times (8.82%, 12/136) occurrences of HE in Group B: Seven with grade I/II and five with grade III/IV HE. Ten cases of HE were relieved in symptoms after treatment, and two patients died from HE. By comparison, the rate of HE in Group B was significantly lower than in Group A (P = 0.036) (Table 4).
| Symptom | Group A | Group B | P value |
| Control of VB within 1 mo | 153 (153/168, 91.07%) | 98 (98/114, 85.96%) | 0.261 |
| Absorption of RA within 1 mo | 39 (39/44, 88.63%) | 9 (9/22, 40.90%) | 0.017 |
| Recurrence of VB | 28 (28/168, 16.67%) | 56 (56/114, 49.12%) | 0.023 |
| Recurrence of RA | 13 (13/44, 29.54%) | 19 (19/22, 86.36%) | 0.009 |
| HE | 37 (37/212, 17.45%) | 12 (12/136, 8.82%) | 0.036 |
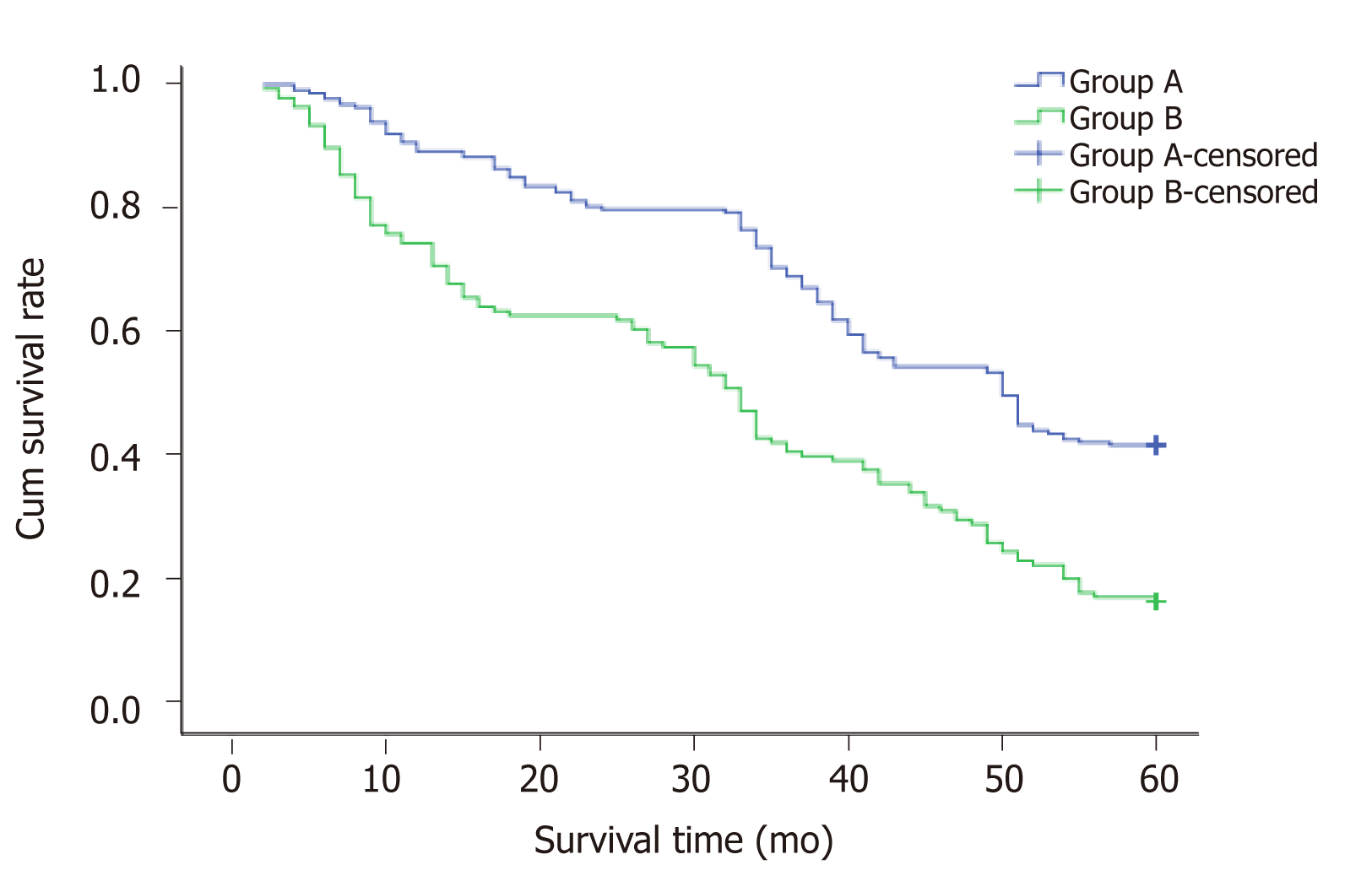
During follow-up at 1 year, 188 patients (188/212, 88.67%) survived in Group A and 101 (101/136, 74.26%) in Group B. At 2 years, 169 patients (169/212, 79.71%) survived in Group A and 85 (85/136, 62.50%) in Group B. At 3 years, 145 patients (145/212, 68.39%) survived in Group A and 55 (55/136, 40.44%) in Group B. At 4 years, 115 patients (115/212, 54.24%) survived in Group A and 39 (39/136, 28.67%) in Group B. The endpoint of this study was at 5 years, when 88 patients (88/212, 41.51%) survived in Group A and 22 (22/136, 16.18%) in Group B. The 1-, 2-, 3-, 4-, and 5-year survival rates differed significantly between Groups A and B (P = 0.018, 0.014, 0.013, 0.009, 0.006, respectively) (Table 5). The mean survival time was 43.7 mo in Group A and 31.8 mo in Group B. Median survival time was 50.0 mo in Group A and 33.0 mo in Group B, and there was a significant difference between the two groups (P = 0.000, χ2 = 35.605, log-rank test) (Figure 2).
| Time | Group | Survival | Survival rate, % | χ2 | P value | |
| Yes | No | |||||
| 1 yr | A | 188 | 24 | 88.67 | 12.227 | 0.018 |
| B | 101 | 35 | 74.26 | |||
| 2 yr | A | 169 | 43 | 79.71 | 12.457 | 0.014 |
| B | 85 | 51 | 62.50 | |||
| 3 yr | A | 145 | 67 | 68.39 | 26.490 | 0.013 |
| B | 55 | 81 | 40.44 | |||
| 4 yr | A | 115 | 97 | 54.24 | 21.956 | 0.009 |
| B | 39 | 97 | 28.67 | |||
| 5 yr | A | 88 | 124 | 41.51 | 24.596 | 0.006 |
| B | 22 | 114 | 16.18 | |||
At the end of follow-up in this study, in Group A, five patients achieved CR, 37 patients achieved PR, 62 patients achieved SD, 108 patients achieved PD, and the disease control rate (CR+PR+SD/total number of cases) was approximately 49.05%. In Group B, three patients achieved CR, 28 patients achieved PR, 41patients achieved SD, 64 patients achieved PD, and the disease control rate was approximately 52.94%. There was no difference between the two groups (P = 0.249) (Table 6).
| HCC treatment response | Group A n = 212 | Group B n = 136 | P value |
| CR | 5 | 3 | |
| PR | 37 | 28 | |
| SD | 62 | 41 | |
| PD | 108 | 64 | |
| Disease control rate | 49.05% | 52.94% | 0.249 |
In Group A, seven patients died from VB, 56 from hepatic tumor, 25 from hepatic failure, 24 from multi-organ failure, and 12 from other causes. In Group B, 42 patients died from VB, 29 from hepatic tumor, 23 from hepatic failure, 17 from multi-organ failure, and three from other causes. The mortality rate for VB in Group A was significantly lower than in Group B (P = 0.006), but the rates of hepatic tumor, hepatic failure, and multi-organ failure did not differ significantly between the two groups (P = 0.173, 0.246, 0.257, respectively) (Table 7).
| Classification of death | Group A | Group B | P value |
| VB | 7 (7/212, 3.30%) | 42 (42/136, 30.88%) | 0.006 |
| Hepatic tumor | 56 (56/212, 26.41%) | 29 (29/136, 21.32%) | 0.173 |
| Hepatic failure | 25 (25/212, 11.79%) | 23 (23/136, 16.91%) | 0.246 |
| Multiorgan failure | 24 (24/212, 11.32%) | 17 (17/136, 12.50%) | 0.257 |
| Others | 12 | 3 | / |
Portal hypertension and HCC are common late complications of liver cirrhosis and sometimes occur simultaneously[10]. There are various treatments for HCC, depending on the nature of the tumor, and TAE/TACE and RFA have become important approaches in recent years, along with RFA for small HCC[11]. The treatment of portal hypertension included administration of oral medicine, such as non-selective beta blockers, and surgical shunts, but TIPS is used more widely due to its safety and effectiveness in patients complicated with portal hypertension. However, according to the current guidelines for the treatment of portal hypertension, TIPS is contrain-dicated in patients with liver cancer.
One of the factors limiting the application of TIPS placement in patients with portal hypertension and liver cancer is its feasibility and safety. Liu et al[12] reported 58 HCC patients with portal vein tumor thrombosis with TIPS treatment; 8.6% of the tumors ruptured and required emergency treatment. Although it has been reported that large liver tumors are prone to rupture[13], there is no evidence that the risk of rupture is associated with tumor size in the TIPS process. Qiu et al[14] reported the largest group of 209 cases, but did not find severe complications, such as abdominal bleeding and tumor rupture. Importantly, we believe that advanced surgical techniques and meticulous preoperative preparation may reduce the occurrence of serious adverse events; however, further prospective studies are needed to confirm this.
In this study, 97.69% of the TIPS procedures were completed successfully, with no serious procedure-related complications, such as tumor rupture or bleeding, either from tumor puncture or directly from venous puncture in the tumor tissue. These results suggest that, as long as there is strict selection of suitable cases combined with the experience of skilled TIPS placement, severe complications will continue to be rare.
TACE takes the advantage of the hepatic dual blood supply resulting in emboli-zation of the tumor-feeding hepatic arteries, while portal venous flow to normal hepatocytes is preserved[15]. TACE in TIPS patients may be associated with increased hepatotoxicity[16]. This important change in the blood supply may aggravate hepatic necrosis[17], and when arteries that supply HCC are embolized, many factors can compensate for liver failure[18]. In a patient with TIPS, the hepatic portal venous perfusion is altered as portal venous flow is decompressed to the systemic circulation[19]. When TIPS is created, the liver loses most of its blood supply, and if TACE is performed in the short term, local liver necrosis increases the possibility of liver failure. In Group A, 79 cases underwent TIPS first then TACE; hepatic function recovery was difficult, and 13 cases developed hepatic failure. As for safety, we suggest that TACE should precede TIPS.
Another limiting factor in the application of TIPS in patients with portal hypertension and hepatic tumor is that although it has greatly improved the survival rate of patients with HCC, it has resulted in complications in portal hypertension cases. Whether it can improve the symptoms and the overall survival rate of the patients remains unknown[20]. After TIPS treatment, in the present study, the recurrence of VB and ascites was controlled, and the response to treatment was significantly greater compared with group B. In the present study, the 1-, 2-, 3-, 4-, and 5-year survival rates were significantly different between Groups A and B. The mean survival time was 43.7 mo in Group A and 31.8 mo in Group B. Median survival time was 50.0 mo in Group A and 33.0 mo in Group B. There was a significant difference between the two groups. The Response Evaluation Criteria in Solid Tumors criteria were applied to evaluate the HCC response, and we found that the disease control rate was no different between the two groups. However, the mortality rate for VB in Group A was lower than in Group B, and the rates of hepatic tumor, hepatic failure, and multiorgan failure did not differ significantly between the two groups. It showed that there was no significant difference between the two groups in the response to interventional therapy for HCC, and TIPS significantly improved the symptoms of portal hypertension and gave patients more opportunities and time for interventional treatment of liver cancer.
This showed that cirrhosis is a major threat in patients with primary liver cancer complicated with portal hypertension. In cases of emergency during long-term cancer treatment, stopping bleeding to extend life should be prioritized when there is a conflict between treatments. Therefore, in this study, TIPS treatment was conducted first, followed by treatment of liver cancer, in order not to lose the opportunity for implementing TIPS.
Lung metastasis was reported[21] after 5 and 10 mo in nine patients with liver cancer who received TIPS treatment[21], but Bettinger et al[22] reported no metastasis in 26 patients. A hypothetical risk is the development of pulmonary metastasis from the portal vein system[23], although this possibility is low. In the present study, only 50 patients had shunt dysfunction due to tumor invasion, and therefore, we did not correlate this transfer with TIPS deployment.
Timely detection and early treatment through close postoperative observation and regular follow-up are recommended to prevent serious adverse events, such as TIPS shunt dysfunction and HE, which are inherent in TIPS placement[24]. In the present study, control of VB within 1 mo did not differ significantly between the groups. However, absorption of RA within 1 mo, recurrence of VB, and recurrence of RA were significantly different between the groups. By comparison, the rate of HE in Group B was lower than in Group A. We showed that TIPS placement in Group A and palliative treatment in Group B both controlled VB, but for other complications of portal hypertension with HCC, TIPS demonstrated good clinical outcomes.
Although TIPS showed several advantages in this study, there were still some limitations to this study (e.g., it was a single-center study with a small sample size). Overall, this study demonstrates that TIPS treatment can better control the symptoms of portal hypertension in patients with both portal hypertension and liver cancer, while further treatment of liver cancer can improve survival rates. For these reasons, TIPS is recommended for further evaluation in multicenter studies with a larger sample size.
In conclusion, TIPS combined with palliative treatment seems to be effective and safe for portal hypertension in patients with HCC. We recommend that TIPS can be used for patients with portal hypertension and liver cancer.
The authors thank all the patients who were involved in this study and colleagues of the Department of Radiology of Air Force Medical Center of PLA for their contribu-tions to the data collection.
There is a close relationship between cirrhosis and hepatocellular carcinoma (HCC). Tra-nsjugular intrahepatic portosystemic shunt (TIPS) has a good clinical effect in treating the complication of portal hypertension. Because of the risk of postoperative liver failure, severe complications, and low survival rate for HCC, TIPS is contraindicated in patients with portal hypertension and liver cancer. We studied a large cohort of patients with cirrhosis and HCC who underwent TIPS for recurrent variceal bleeding and/or ascites. They were compared with patients with cirrhosis and HCC who did not undergo TIPS placement. We conclude that TIPS combined with palliative treatment is safe and effective for portal hypertension in patients with HCC.
Liver cancer is often accompanied by cirrhosis, which often leads to the occurrence of liver cancer. TIPS can reduce portal pressure and relieve the clinical symptoms associated with various medical conditions. Because of the risk of postoperative liver failure, severe complications, and low survival rate for HCC, TIPS is contraindicated in patients with portal hypertension and liver cancer. We conducted a retrospective analysis of portal hypertension and liver cancer that were treated in our hospital with TIPS plus palliative treatment and radiofrequency ablation (RFA) and liver cancer patients treated with palliative treatment and RFA in order to compare the safety, efficacy, and survival rate between the two groups. In the future, randomized controlled trials are needed to verify our results.
The main objective of our study was to confirm our hypothesis that TIPS combined with palliative treatment and RFA for patients with HCC and portal hypertension is safe and effective, and it increases the survival rate of the patients.
We conducted a retrospective study to compare the clinical efficacy of the combination of TIPS, transarterial TACE, RFA, and palliative treatment in liver cirrhosis complicated with HCC. The patients were divided into two groups. Group A comprised 217 patients with portal hypertension and HCC who were treated with TIPS plus palliative treatment and RFA. Group B comprised a cohort of 136 patients with HCC and portal hypertension who did not undergo TIPS placement and received palliative treatment and RFA. A logistic regression analysis was performed for the variables. The differences between the groups were compared using one-way analysis of variance followed by least significant difference t tests. Categorical variables were expressed as frequencies and compared using χ2 tests. Differences were considered significant at P < 0.05. The statistical analyses were performed with SPSS version 21.0 (SPSS, Armonk, NY, United States).
This study showed that TIPS combined with palliative treatment and RFA for patients with HCC and portal hypertension is safe. Mean survival and median survival were longer than in the group without TIPS treatment, which verified our hypothesis.
TIPS combined with palliative treatment and RFA for patients with HCC and portal hypertension is safe and effective, and it prolongs the survival of the patients. We suggest that for patients with HCC and portal hypertension, TIPS procedure is not contraindicated in such circumstances of variceal bleeding and/or ascites. Patients with HCC and portal hypertension can be treated with TIPS procedure in future clinical practice.
Based on the findings in this study that TIPS combined with palliative treatment and RFA for patients with HCC and portal hypertension is safe and effective and that it prolongs the survival of patients, patients with HCC and portal hypertension can be treated with TIPS. In future research, randomized controlled trials are needed to verify our results.
Manuscript source: Unsolicited Manuscript
Specialty type: Gastroenterology and Hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Garbuzenko DV, Namisaki T S-Editor: Ma YJ L-Editor: Filipodia E-Editor: Wang J
| 1. | Dezső K, Rókusz A, Bugyik E, Szücs A, Szuák A, Dorogi B, Kiss M, Nemeskéri Á, Nagy P, Paku S. Human liver regeneration in advanced cirrhosis is organized by the portal tree. J Hepatol. 2017;66:778-786. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 2. | Ahmed Mohammed HA, Yang JD, Giama NH, Choi J, Ali HM, Mara KC, Harmsen WS, Wiesner RH, Leise MD, Therneau TM, Roberts LR. Factors Influencing Surveillance for Hepatocellular Carcinoma in Patients with Liver Cirrhosis. Liver Cancer. 2017;6:126-136. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 3. | Riggio O, Ridola L, Angeloni S, Cerini F, Pasquale C, Attili AF, Fanelli F, Merli M, Salvatori FM. Clinical efficacy of transjugular intrahepatic portosystemic shunt created with covered stents with different diameters: results of a randomized controlled trial. J Hepatol. 2010;53:267-272. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 95] [Cited by in F6Publishing: 100] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 4. | Ascha M, Abuqayyas S, Hanouneh I, Alkukhun L, Sands M, Dweik RA, Tonelli AR. Predictors of mortality after transjugular portosystemic shunt. World J Hepatol. 2016;8:520-529. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 5. | European Association For The Study Of The Liver. ; European Organisation For Research And Treatment Of Cancer. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2012;56:908-943. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4059] [Cited by in F6Publishing: 4345] [Article Influence: 362.1] [Reference Citation Analysis (2)] |
| 6. | Bruix J, Sherman M; American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020-1022. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5972] [Cited by in F6Publishing: 6341] [Article Influence: 487.8] [Reference Citation Analysis (1)] |
| 7. | Bai M, He CY, Qi XS, Yin ZX, Wang JH, Guo WG, Niu J, Xia JL, Zhang ZL, Larson AC, Wu KC, Fan DM, Han GH. Shunting branch of portal vein and stent position predict survival after transjugular intrahepatic portosystemic shunt. World J Gastroenterol. 2014;20:774-785. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 31] [Cited by in F6Publishing: 29] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 8. | Gbolahan OB, Schacht MA, Beckley EW, LaRoche TP, O'Neil BH, Pyko M. Locoregional and systemic therapy for hepatocellular carcinoma. J Gastrointest Oncol. 2017;8:215-228. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 49] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 9. | Kang TW, Rhim H. Recent Advances in Tumor Ablation for Hepatocellular Carcinoma. Liver Cancer. 2015;4:176-187. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 62] [Cited by in F6Publishing: 70] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 10. | Nakayama H, Takayama T. Management before hepatectomy for hepatocellular carcinoma with cirrhosis. World J Hepatol. 2015;7:2292-2302. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 11. | Díaz-González Á, Reig M, Bruix J. Treatment of Hepatocellular Carcinoma. Dig Dis. 2016;34:597-602. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 47] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 12. | Liu L, Zhao Y, Qi X, Cai G, He C, Guo W, Yin Z, Chen H, Chen X, Fan D, Han G. Transjugular intrahepatic portosystemic shunt for symptomatic portal hypertension in hepatocellular carcinoma with portal vein tumor thrombosis. Hepatol Res. 2014;44:621-630. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 30] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 13. | Chan AC, Dai JW, Chok KS, Cheung TT, Lo CM. Prognostic influence of spontaneous tumor rupture on hepatocellular carcinoma after interval hepatectomy. Surgery. 2016;159:409-417. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 42] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 14. | Qiu B, Zhao MF, Yue ZD, Zhao HW, Wang L, Fan ZH, He FL, Dai S, Yao JN, Liu FQ. Combined transjugular intrahepatic portosystemic shunt and other interventions for hepatocellular carcinoma with portal hypertension. World J Gastroenterol. 2015;21:12439-12447. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 18] [Cited by in F6Publishing: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 15. | Matsui O, Miyayama S, Sanada J, Kobayashi S, Khoda W, Minami T, Kozaka K, Gabata T. Interventional oncology: new options for interstitial treatments and intravascular approaches: superselective TACE using iodized oil for HCC: rationale, technique and outcome. J Hepatobiliary Pancreat Sci. 2010;17:407-409. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 35] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 16. | Miura JT, Rilling WS, White SB, Hieb RA, Tutton SM, Patel PJ, Gamblin TC, Hohenwalter EJ. Safety and efficacy of transarterial chemoembolization in patients with transjugular intrahepatic portosystemic shunts. HPB (Oxford). 2015;17:707-712. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 17. | Kang JW, Kim JH, Ko GY, Gwon DI, Yoon HK, Sung KB. Transarterial chemoembolization for hepatocellular carcinoma after transjugular intrahepatic portosystemic shunt. Acta Radiol. 2012;53:545-550. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 19] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 18. | Wang Z, Zhang H, Zhao H, Wang X, Tsauo J, Luo X, Li X. Repeated transcatheter arterial chemoembolization is safe for hepatocellular carcinoma in cirrhotic patients with transjugular intrahepatic portosystemic shunt. Diagn Interv Radiol. 2014;20:487-491. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 19. | Bannas P, Roldán-Alzate A, Johnson KM, Woods MA, Ozkan O, Motosugi U, Wieben O, Reeder SB, Kramer H. Longitudinal Monitoring of Hepatic Blood Flow before and after TIPS by Using 4D-Flow MR Imaging. Radiology. 2016;281:574-582. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 34] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 20. | Wallace MJ, Madoff DC. Transjugular intrahepatic portosystemic shunts in patients with hepatic malignancy. Semin Intervent Radiol. 2005;22:309-315. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 21. | Wallace M, Swaim M. Transjugular intrahepatic portosystemic shunts through hepatic neoplasms. J Vasc Interv Radiol. 2003;14:501-507. [PubMed] [Cited in This Article: ] |
| 22. | Bettinger D, Knüppel E, Euringer W, Spangenberg HC, Rössle M, Thimme R, Schultheiß M. Efficacy and safety of transjugular intrahepatic portosystemic shunt (TIPSS) in 40 patients with hepatocellular carcinoma. Aliment Pharmacol Ther. 2015;41:126-136. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 26] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 23. | Sumiyoshi T, Shima Y, Nishiuchi R, Sasaki K, Kouzuki A, Noda Y, Hata Y, Uka K. Needle tract implantation of hepatoblastoma after percutaneous needle biopsy: report of a case. Surg Today. 2014;44:1138-1141. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 24. | Luo X, Wang Z, Tsauo J, Zhou B, Zhang H, Li X. Advanced Cirrhosis Combined with Portal Vein Thrombosis: A Randomized Trial of TIPS versus Endoscopic Band Ligation Plus Propranolol for the Prevention of Recurrent Esophageal Variceal Bleeding. Radiology. 2015;276:286-293. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 73] [Cited by in F6Publishing: 65] [Article Influence: 7.2] [Reference Citation Analysis (0)] |