Published online Apr 6, 2019. doi: 10.12998/wjcc.v7.i7.809
Peer-review started: January 21, 2019
First decision: February 13, 2019
Revised: March 8, 2019
Accepted: March 16, 2019
Article in press: March 16, 2019
Published online: April 6, 2019
Since it has been recognized that sarcoidosis (SA) is not an exclusive disorder of the lungs but can also affect other organs such as the liver and spleen, efforts have been made to define specific imaging criteria for the diagnosis of the single organ involvement, and the concept has been reinforced that the exclusion of alternative causes is important to achieve the correct diagnosis. Ultrasound (US) is a useful tool to evaluate patients with suspected abdominal SA, such as of the liver, spleen, kidney, pancreas and other organs, showing findings such as organomegaly, focal lesions and lymphadenopathy. While the diagnosis of abdominal SA is more predictable in the case of involvement of other organs (e.g., lungs), the problem is more complex in the case of isolated abdominal SA. The recent use of contrast-enhanced ultrasound and endoscopic ultrasound elastography has provided additional information about the enhancement patterns and tissue rigidity in abdominal SA. Here we critically review the role of US in abdominal SA, reporting typical findings and limitations of current evidence and by discussing future perspectives of study.
Core tip: Ultrasound (US) is useful to evaluate patients with suspected abdominal sarcoidosis (SA), showing some findings such as organomegaly, hypoechoic lesions and adenopathy. While the diagnosis of abdominal SA is more predictable in the case of involvement of other organs (e.g., lungs) the problem is more complex in the case of isolated abdominal SA. The recent use of contrast-enhanced ultrasound and endoscopic ultrasound elastography has provided additional information about the enhancement patterns and tissue rigidity in abdominal SA. Our objective was to critically review the role of US in abdominal SA, reporting characteristic findings and limitations of current evidence and discussing future perspectives of study.
- Citation: Tana C, Schiavone C, Ticinesi A, Ricci F, Giamberardino MA, Cipollone F, Silingardi M, Meschi T, Dietrich CF. Ultrasound imaging of abdominal sarcoidosis: State of the art. World J Clin Cases 2019; 7(7): 809-818
- URL: https://www.wjgnet.com/2307-8960/full/v7/i7/809.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v7.i7.809
Since the first evidence in 1899 of sarcoid lesions provided by the Norwegian dermatologist Caesar Boeck[1,2], it has been recognized that sarcoidosis (SA) is not an exclusive disorder of a single organ, but can affect virtually every tissue, manifesting with clinically silent or symptomatic granulomatous lesions[3,4]. In this context the term “great chameleon” was created, indicating a non-specific disease with an undefined etiology that mimicks various disorders in the clinical picture or imaging[5-8].
In the last few years, efforts have been made to define imaging criteria for the diagnosis of specific organ involvement, and the concept has been reinforced that the exclusion of alternative causes is important to achieve the correct diagnosis; a thorough clinical and laboratory assessment is therefore suggested before establishing any imaging approach [9-11]. Conventional US can reveal some useful imaging findings, and the recent advent of novel US techniques, such as contrast-enhanced ultrasound (CEUS) and endoscopic ultrasound (EUS) elastography, has provided more information about the abdominal involvement in this uncommon disease.
Among all granulomatous disorders, SA most often affects the lungs and intra-thoracic lymph nodes by manifesting with restrictive lung disease[12]. However, SA can also involve abdominal organs, such as the liver and spleen, in a non-negligible percentage that varies according to the presence or absence of overt symptoms[13]. In particular, the symptomatic involvement of liver and spleen is estimated to be around 10%-25 % and 5%-10% of cases, respectively but asymptomatic cases can be higher[14]. A computed tomography (CT) analysis conducted by Warshauer et al[15] in 32 patients with abdominal SA documented a high prevalence of organomegaly and adenopathy (76%). Virtually, there is no organ that is spared from SA[16], and the high potential of ubiquitous body involvement raises the problem of the great diagnostic challenge in patients with suspected abdominal SA, whic may occur in a percentage higher than that documented clinically. The issue becomes even more complex because patients with traditional pulmonary SA do not usually undergo extensive imaging evaluation unless they present significant abnormalities at liver tests[17].
Although the evidence of hepatosplenic SA at imaging can be limited if there is only a microscopic involvement, ultrasound (US) can be useful to detect some macroscopic findings. Low cost, wide availability and no radiation exposure are some of the main advantages of this method, and routine inclusion of this examination in patients with suspected abdominal SA could be useful to screen the involvement of the liver and spleen[17]. However, several other disorders can lead to a liver test dysfunction, such as nonalcoholic liver disease and nonalcoholic steatohepatitis[18], and the two disorders can coexist in such a way as to make the imaging distinction even more complicated. Nevertheless, a first imaging evaluation with US should be included in the routine assessment of patients with suspected abdominal SA, in particular if there is clinical evidence of hepatic dysfunction or liver test abnormalities[17].
Second imaging approaches include contrast-enhanced CT, magnetic resonance imaging (MRI) and 18F-FDG PET/CT, which have shown a good diagnostic reliability in the assessment of abdominal SA[19]
US can reveal several non-specific findings in patients with SA of the liver, such as hepatomegaly or bright liver appearance, and can document a significant inho-mogeneity of the parenchyma with a coarse nodular pattern, suggesting a patchy granulomatous infiltration of the parenchyma[17,20]. Given their nonspecific aspect, however, these findings can be easily misdiagnosed with some features that are observed in other very common disorders, such as fatty liver disease and hepatic cirrhosis[20].
The presence of prominent, various-sized nodules can be documented by showing multiple and rounded hypoechoic lesions. Previous US studies have demonstrated that these nodules usually have no specific size and form but are characteristically hypovascular on Doppler US. The features of these lesions have already been discussed and illustrated[15,17,21]. A significant number of disorders (both benign and malignant) enter the differential diagnosis with these lesions, including pseu-dotumours and tuberculosis[22], and US-guided biopsy can be useful to reveal the presence of non-caseating granulomas, which represent a specific hallmark of the disease and are useful for the definitive diagnosis if the clinical picture or imaging is non- specific or inconclusive[23].
The involvement of the spleen is similar to that of the liver, manifesting with non-specific organ enlargement or hypovascular nodules of various size. In contrast, the lesions can show different degrees of echogenecity (hypo, iso or hyper), possibly according to the different degree of fibrosis of the nodules. However, these lesions can be easily misdiagnosed as nodularities from other disorders; in particular, those that are hypoechoic can be wrongly in-terpreted as lesions from splenic lymphoma, a disorder that is characterized by a hypoechoic appearance of their nodules[24-26]. Here again, the problem becomes even more challenging when the two disorders coexist[27]. Several studies have, in fact, documented a strong correlation between SA and malignancy (the so-called SA-lymphoma syndrome) and the coexistence of sarcoid and lymphomatous tissue can result in great difficulty of differential diagnosis[28]. Sometimes a definite diagnosis is reached only through histological examination, a procedure that would inevitably lead to invasive splenectomy.
Another US finding of abdominal SA is the presence of enlarged lymph nodes, which has been described in up to 76% of the cases as inhomogeneous hypoechoic lesions sized generally 1-2 cm and located in periportal, paracaval, paraaortic and celiac sites. They can be interpreted erroneously as the sign of chronic virus hepatitis C[29], primary biliary cirrhosis[30] and primary sclerosing cholangitis[31]; a correct interpretation is therefore mandatory to avoid wrong therapeutic approaches[32]. Unlike these disorders, lymph nodes enlargement in SA can reach higher sizes (up to 6 cm) and show tumor-like patterns, with no clear evidence of hilar lymph nodes[17,33].
The pancreas is rarely involved in SA (1%-3% of cases), and focal sarcoid lesions have been reported in less than 50 cases in the literature[34].
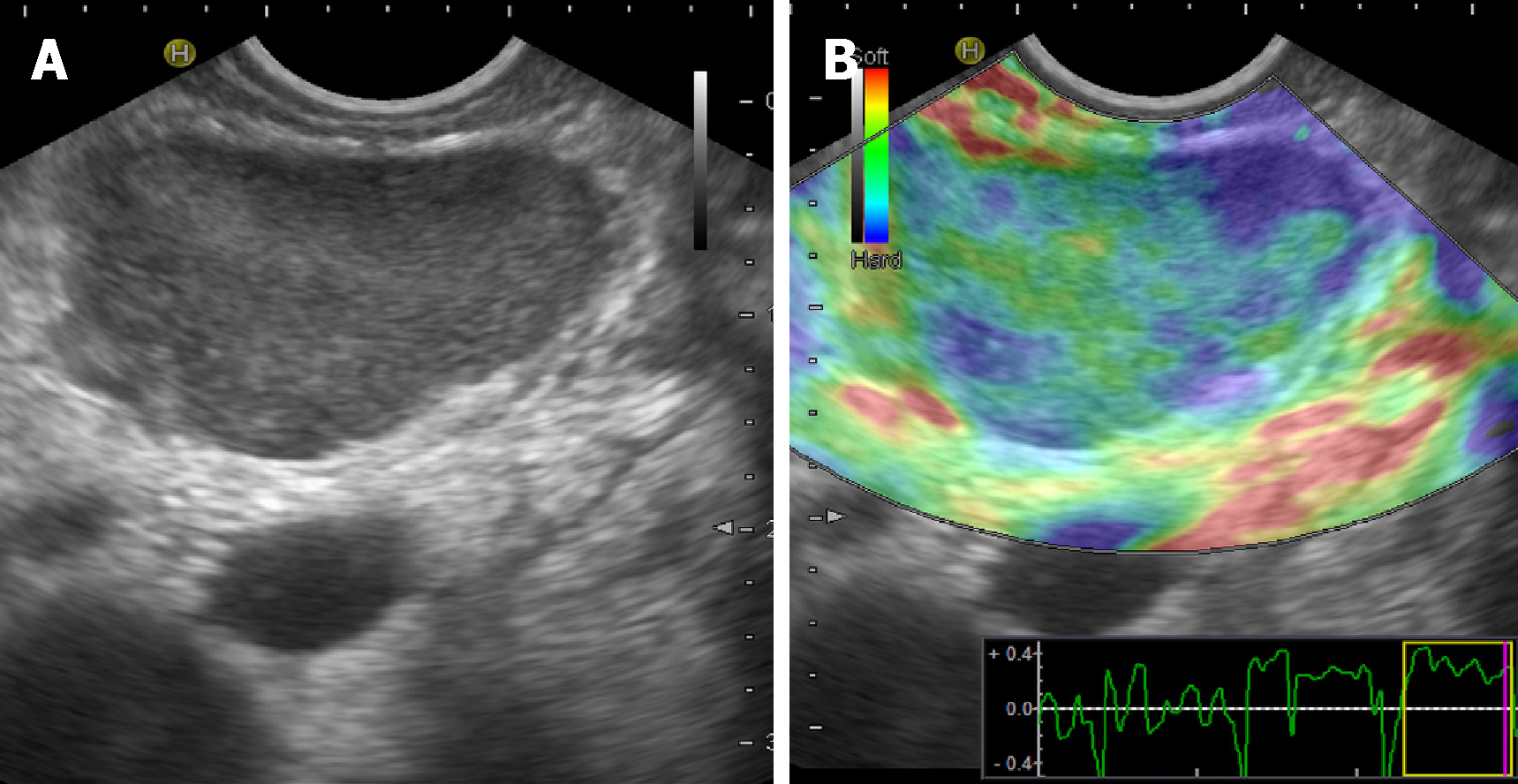
Main imaging findings are the presence of hypoechoic and hypovascular focal masses within the parenchyma, sometimes associated with focal or global common bile and pancreatic ducts dilatation[35]. Size can vary significantly and large hypoechoic masses can be found exceptionally (Figure 1A).
These findings can mimic the imaging features observed in tuberculosis of the pancreas[36]. Further imaging techniques (CEUS and EUS elastography) can be useful to reveal some useful patterns (Figure 1B, see below).
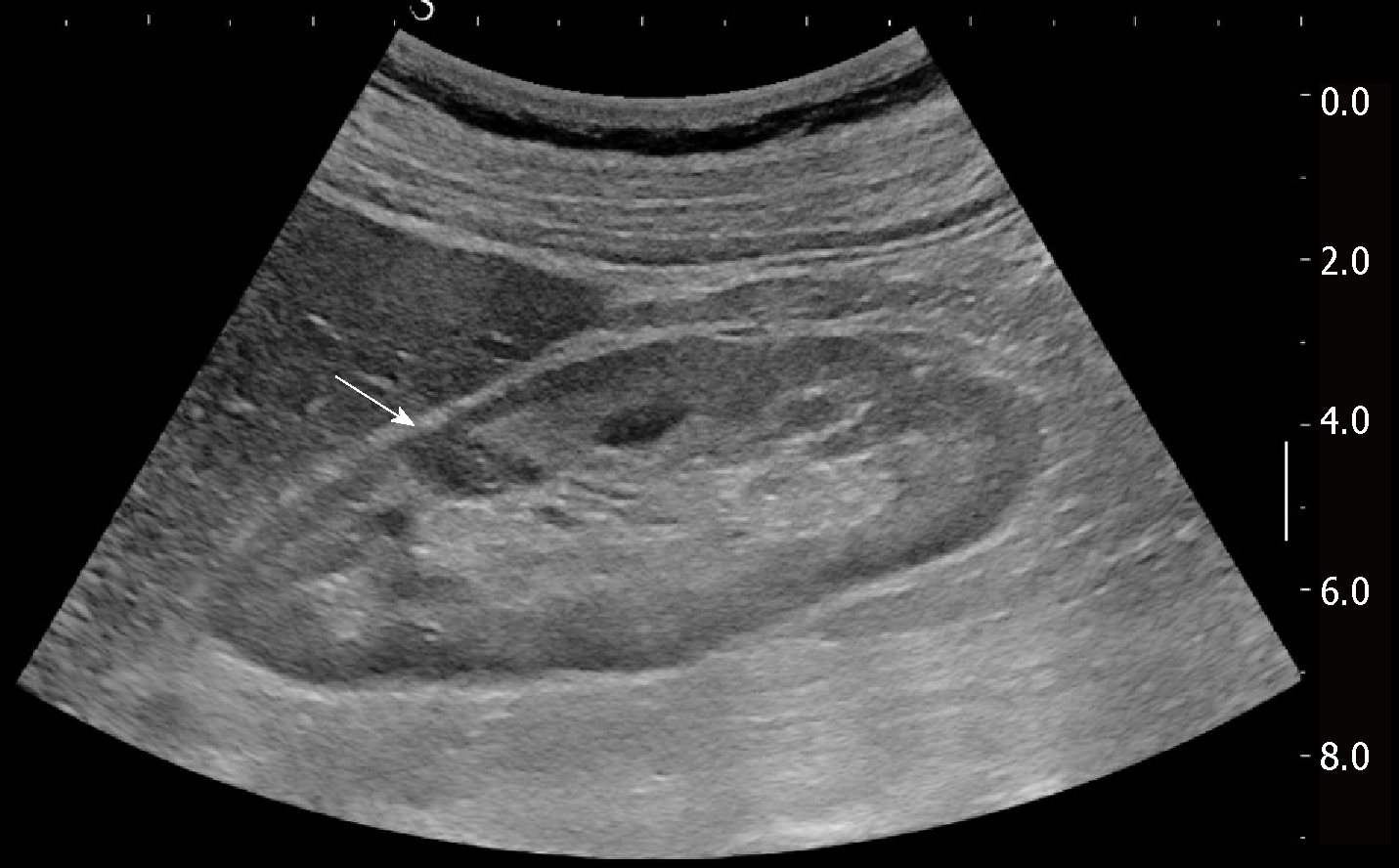
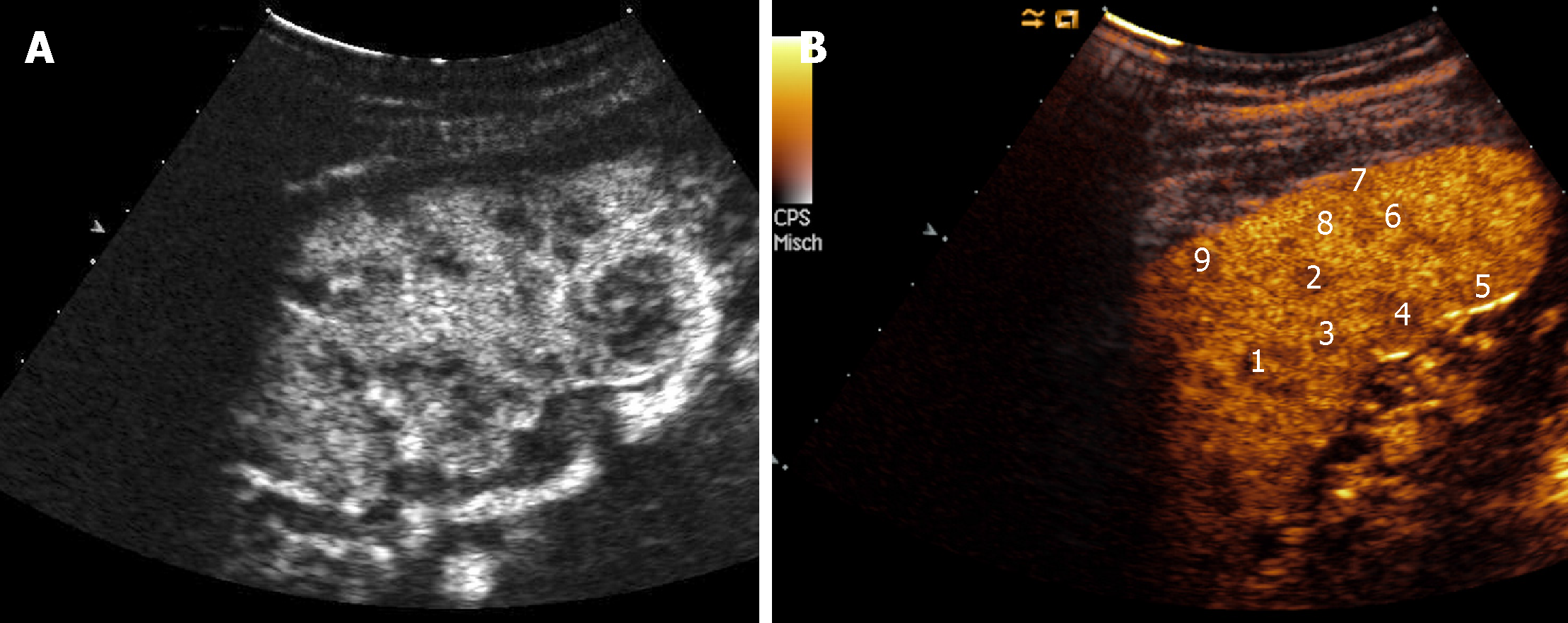
Renal involvement from SA is estimated to be around 25%-30% among all cases, and manifests most commonly with granulomatous interstitial nephritis and disturbances in calcium homeostasis with occurrence of nephrolithiasis and nephrocalcinosis[37]. The direct involvement of kidney by granulomatous tissue and the presence of solid masses, however, are extremely rare. Pseudotumours raise significant problems of differential diagnosis with malignant nodules such as renal cell carcinoma and lymphoma, because they can manifest with similar imaging findings. Such nodules can present as single or multiple hypoechoic masses, hypovascular on Doppler US[38]. They can grow within renal parenchyma (Figure 2) or be characterized by a focal and exophytic growth[39,40].
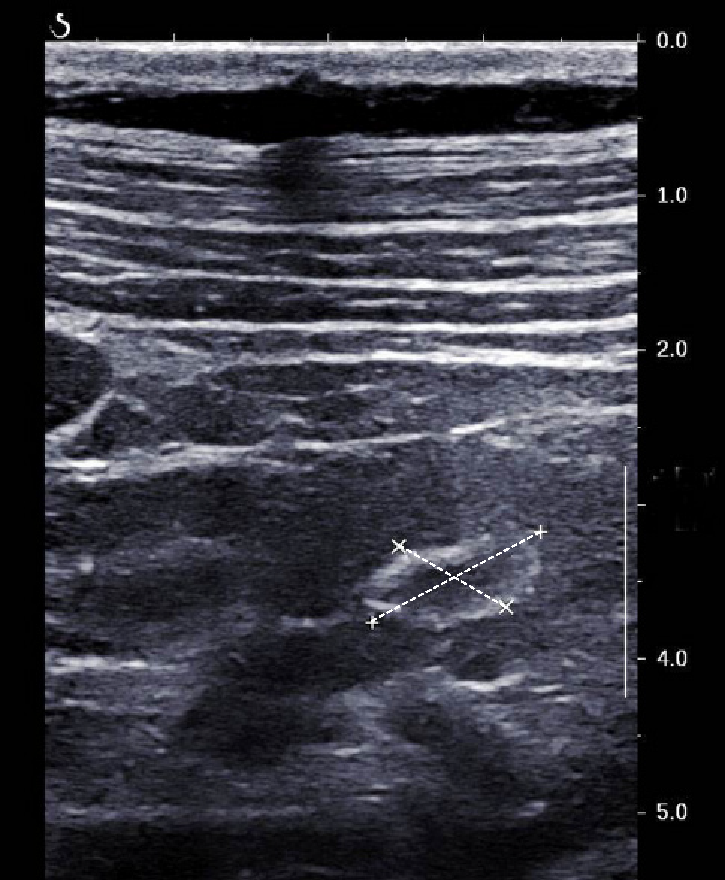
For the first time, we found a case of ring-like echogenic pattern determined by a sarcoid lesion of the parenchyma (Figure 3). Also this pattern can be misdiagnosed with that observed in focal malignant lesions.
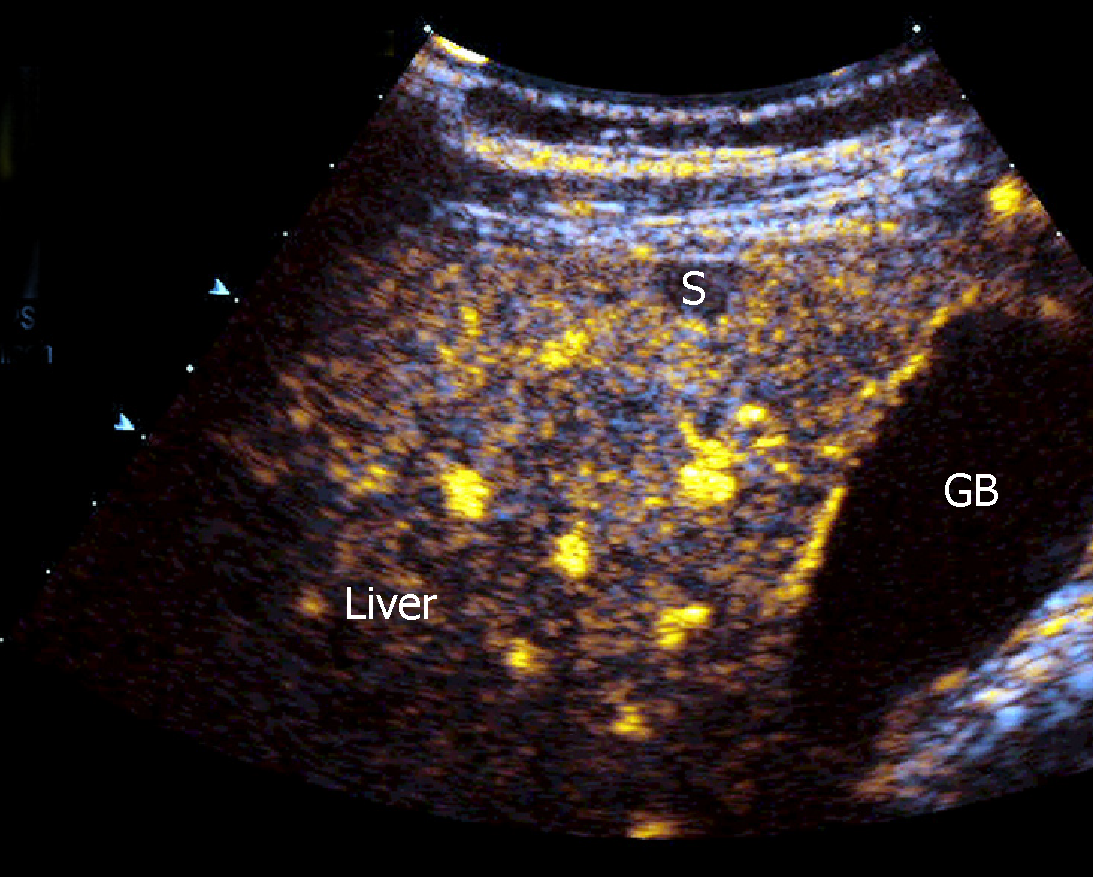
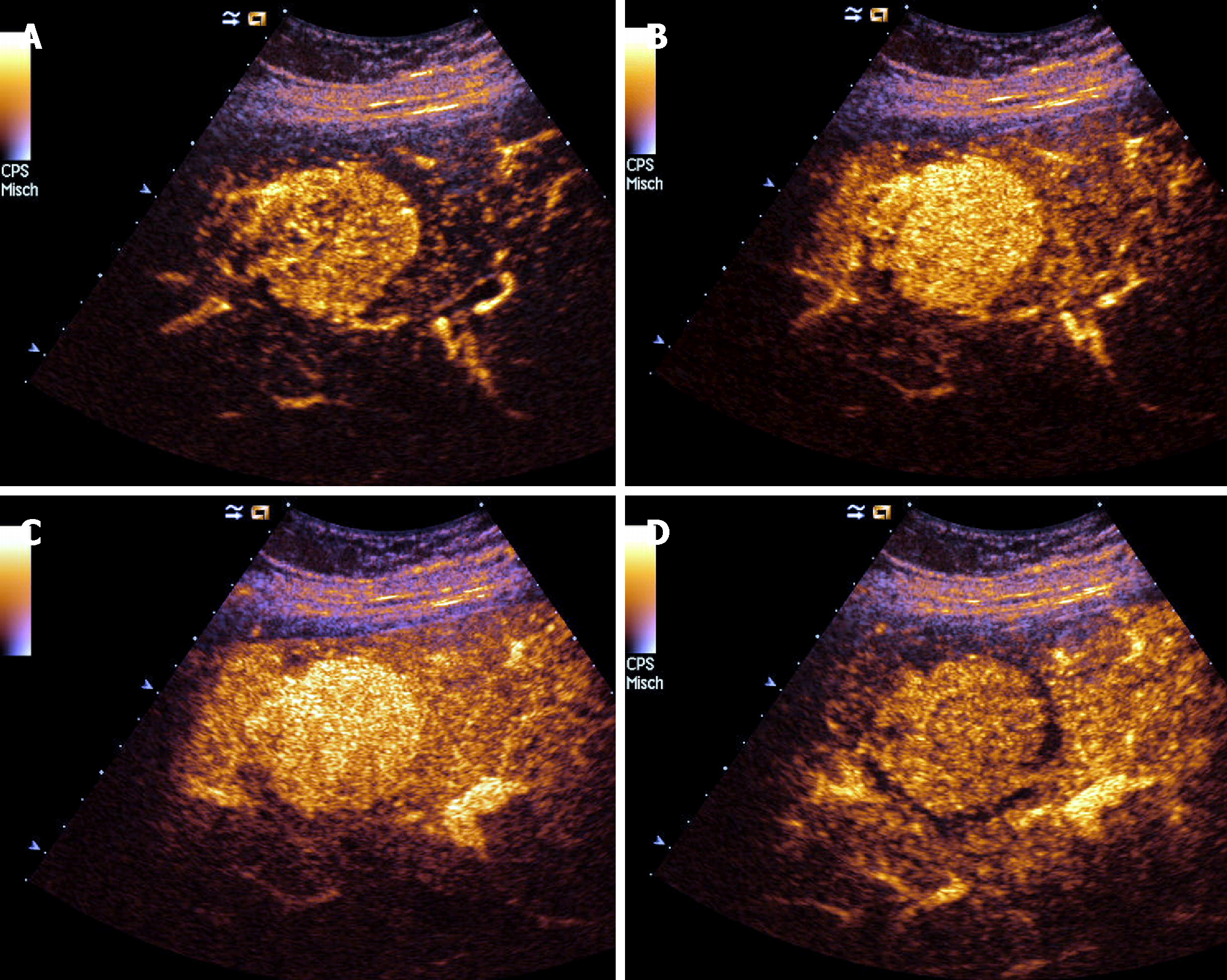
CEUS has demonstrated a high accuracy to detect and characterize the nature of a large amount of liver and splenic nodules[41,42]. Despite this, there are few studies aimed at assessing CEUS patterns in granulomatous infiltration such as from SA, and most evidence derives from large but heterogeneous studies including nodules of different histology[43]. The use of CEUS in liver SA can reveal a variable nodular enhancement and progressive hypoenhancement in the arterial and portal-venous late phases, respectively (Figure 4)[17,44]. Sometimes the nodules can be hyperenhancing for more than 2 min (Figure 5).
For nodular lesions of the spleen, CEUS can show a progressive hypoenhancement pattern in both the arterial and parenchymal phases, with a higher contrast diffusion toward parenchyma in this phase (Figure 6)[45].
Other enhancement patterns have also been documented (e.g., rim-like, homogeneous, dotted) and, in particular cases with peripheral or rim-like enhancement in the arterial phase followed by a rapid washout in the parenchymal phase, can mimick the patterns observed for neoplastic lesions, raising great problems of differential diagnosis with solid cancers and hematologic disorders[46,47].
Stang et al[48] have previously reported two cases of splenic SA with diffuse and homogeneous arterial enhancement followed by a progressive hypoenhancement in the parenchymal phase. In this context, the exclusive use of CEUS to confirm the presence of isolated splenic SA can fail, making histopathological examination necessary[49,50].
Lymph nodes enlargement can also be detected by CEUS, by showing a progressive homogeneous enhancement. This pattern can be useful to confirm the benign nature of the lymph node involvement[51].
In contrast, focal lesions of the pancreas show a rapid and transitory enhancement followed by progressive hypoenhancement; a histological examination is thus important to confirm the benign nature of the lesion and to exclude the presence of malignant tissue[35]. There is low evidence instead about the CEUS patterns of kidney SA[40].
In this complex scenario, a useful diagnostic algorithm can involve the integration of clinical, laboratory and imaging findings if the abdominal involvement from SA is associated with other clinical manifestations (e.g., lung disease). The additional diagnostic support of CEUS could be useful to confirm the presence of the lesions. In the case of suspected isolated hepatosplenic or pancreatic SA, however, the additional use of biopsy is strongly advisable to achieve the correct diagnosis, because many common (and also harmful) conditions can be easily misdiagnosed with SA.
There is a great need for studies aimed at evaluating patients with isolated abdominal SA, because, given the pleomorphic nature of SA, it is not difficult to hypothesize other CEUS patterns.
Although the evidence derives from single case reports or small case series, the greater diffusion of EUS elastography has provided additional information about the tissue rigidity of sarcoid lesions in abdominal SA.
Liver lesions can show a diffuse blue hard pattern within and around the single masses that correlates well with the granulomatous tissue at biopsy[52].
Lymph node enlargement, instead, shows a mixed, predominantly green pattern at EUS elastography, with a 3-point score (range 1-5 soft vs hard/solid, respectively)[53].
Similar patterns of rigidity are observed for pancreatic lesions, and both soft and hard tissues are documented on EUS elastography (Figure 1B). These findings suggest the co-existence of mixed tissue components, which are peculiar to disorders such as SA that show both fibrotic tissue and non-caseating granulomas[53]. Table 1 shows the main US, CEUS and EUS findings of abdominal SA.
| US | CEUS | EUS elastography | |
| Liver | (1) Hepatomegaly; (2) Coarse nodular pattern; (3) Hypoechoic and hypovascular nodules | Variable nodular enhancement and progressive hypoenhancement in the arterial and portal-venous late phases, respectively | Blue hard pattern within and around the single masses that correlate well with the granulomatous tissue at biopsy |
| Spleen | (1) Organ enlargement; (2) Hypo, iso or hyperechoic, hypovascular nodules | (1) Progressive hypoenhancement in both the arterial and parenchymal phases, with a higher contrast diffusion toward parenchyma in this phase; (2) Other patterns: rim-like, homogeneous, dotted | N/A |
| Lymph nodes | (1) Lymphadenopathy; (2) Hypoechoic 1-2 cm sized nodules | Progressive homogeneous enhancement, suggesting a benign pattern | Mixed, predominantly green pattern |
| Pancreas | (1) Focal hypoechoic and hypovascular masses; (2) Pancreatic duct dilatation | Rapid and transitory enhancement followed by progressive hypoenhancement, mimicking a malignant pattern | Both soft and hard tissues, suggesting the co-existence of mixed tissue components |
| Kidneys | (1) Pseudotumors as hypoechoic and hypovascular masses; (2) Ring-like echogenic pattern | Hypoenhancing lesions | N/A |
US is an inexpensive and rapidly available method that can be performed at patient’s bedside. The use of ultrasound contrast agents (UCAs) can improve the detection rate of suspected focal lesions, with a lower risk of hypersensitivity reactions than that associated with X-ray contrast agents, and similar to that encountered with MRI contrast agents[49]. The safer profile of UCAs allows repeating the evaluation of the nodules also more than one time for session, giving more opportunities to study the characteristics of the lesions. However, conventional sonographic methods are operator-dependent and have generally lower specificity and sensitivity than other imaging methods such as CT. This is a great limit of the B-mode US evaluation in cases of suspected abdominal SA, because some lesions can be missed at a first imaging evaluation.
The involvement of abdominal organs by SA is a possible occurrence that should be taken into consideration in patients with a diagnosis of SA. An increase in US examinations as the screening test in asymptomatic patients could be useful to detect asymptomatic nodules, particularly if there are blood test abnormalities indicating for instance liver dysfunction. The challenge remains in the case of isolated hepatosplenic SA, which is often found by chance and is associated with a significant risk of misdiagnosis with other conditions if an appropriate diagnostic approach is not performed.
Due to the limited number of cases of abdominal SA that have been investigated with CEUS and elastography, definite conclusions and recommendations are not possible so far. The wider diffusion of these techniques and the increasing evidence about the diagnostic accuracy for nodular lesions have led to a great need also to assess patients with rare conditions such as SA. In the future, we expect more tailored studies in these patients, also to evaluate the diagnostic accuracy of CEUS and elastography in order to give a presumptive characterization of the nature of nodules, which could be useful to guide (or sometimes avoid) invasive approaches, such as histological examination.
Manuscript source: Invited manuscript
Specialty type: Medicine, Research and Experimental
Country of origin: Italy
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Sun SY, Sipahi AM S-Editor: Dou Y L-Editor: A E-Editor: Wu YXJ
| 1. | Boeck C. Multiple benign sarcoid of the skin. J Cutan Genitourin Dis. 1899;17:543-550. [Cited in This Article: ] |
| 2. | Tchernev G, Cardoso JC, Chokoeva AA, Verma SB, Tana C, Ananiev J, Gulubova M, Philipov S, Kanazawa N, Nenoff P, Lotti T, Wollina U. The "mystery" of cutaneous sarcoidosis: facts and controversies. Int J Immunopathol Pharmacol. 2014;27:321-330. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 3. | Martin WJ 2nd, Iannuzzi MC, Gail DB, Peavy HH. Future directions in sarcoidosis research: summary of an NHLBI working group. Am J Respir Crit Care Med. 2004;170:567-571. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 47] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 4. | Mañá J, Rubio-Rivas M, Villalba N, Marcoval J, Iriarte A, Molina-Molina M, Llatjos R, García O, Martínez-Yélamos S, Vicens-Zygmunt V, Gámez C, Pujol R, Corbella X. Multidisciplinary approach and long-term follow-up in a series of 640 consecutive patients with sarcoidosis: Cohort study of a 40-year clinical experience at a tertiary referral center in Barcelona, Spain. Medicine (Baltimore). 2017;96:e7595. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 67] [Cited by in F6Publishing: 62] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 5. | Chen ES, Moller DR. Etiologies of Sarcoidosis. Clin Rev Allergy Immunol. 2015;49:6-18. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 90] [Cited by in F6Publishing: 97] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 6. | Valeyre D, Prasse A, Nunes H, Uzunhan Y, Brillet PY, Müller-Quernheim J. Sarcoidosis. Lancet. 2014;383:1155-1167. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 725] [Cited by in F6Publishing: 701] [Article Influence: 70.1] [Reference Citation Analysis (0)] |
| 7. | Chokoeva AA, Tchernev G, Tana C, Ananiev J, Wollina U. Sarcoid-like pattern in a patient with tuberculosis. J Biol Regul Homeost Agents. 2014;28:783-788. [PubMed] [Cited in This Article: ] |
| 8. | Tana C, Tchernev G, Chokoeva AA, Wollina U, Lotti T, Fioranelli M, Roccia MG, Maximov GK, Silingardi M. Pulmonary and abdominal sarcoidosis, the great imitators on imaging? J Biol Regul Homeost Agents. 2016;30:45-48. [PubMed] [Cited in This Article: ] |
| 9. | Morgenthau AS, Iannuzzi MC. Recent advances in sarcoidosis. Chest. 2011;139:174-182. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 91] [Cited by in F6Publishing: 95] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 10. | Chen ES, Moller DR. Sarcoidosis--scientific progress and clinical challenges. Nat Rev Rheumatol. 2011;7:457-467. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 116] [Cited by in F6Publishing: 120] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 11. | Chokoeva AA, Tchernev G, Tana M, Tana C. Exclusion criteria for sarcoidosis: A novel approach for an ancient disease? Eur J Intern Med. 2014;25:e120. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 12. | Hirche TO, Hirche H, Cui XW, Wagner TO, Dietrich CF. Ultrasound evaluation of mediastinal lymphadenopathy in patients with sarcoidosis. Med Ultrason. 2014;16:194-200. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 8] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 13. | Palmucci S, Torrisi SE, Caltabiano DC, Puglisi S, Lentini V, Grassedonio E, Vindigni V, Reggio E, Giuliano R, Micali G, Caltabiano R, Andreula C, Foti PV, Ettorre GC, Walsh SL, Vancheri C. Clinical and radiological features of extra-pulmonary sarcoidosis: a pictorial essay. Insights Imaging. 2016;7:571-587. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 29] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 14. | Folz SJ, Johnson CD, Swensen SJ. Abdominal manifestations of sarcoidosis in CT studies. J Comput Assist Tomogr. 1995;19:573-579. [PubMed] [Cited in This Article: ] |
| 15. | Warshauer DM, Molina PL, Hamman SM, Koehler RE, Paulson EK, Bechtold RE, Perlmutter ML, Hiken JN, Francis IR, Cooper CJ. Nodular sarcoidosis of the liver and spleen: analysis of 32 cases. Radiology. 1995;195:757-762. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 97] [Cited by in F6Publishing: 99] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 16. | Tana C, Wegener S, Borys E, Pambuccian S, Tchernev G, Tana M, Giamberardino MA, Silingardi M. Challenges in the diagnosis and treatment of neurosarcoidosis. Ann Med. 2015;47:576-591. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 39] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 17. | Tana C, Dietrich CF, Schiavone C. Hepatosplenic sarcoidosis: contrast-enhanced ultrasound findings and implications for clinical practice. Biomed Res Int. 2014;2014:926203. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 24] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 18. | Harder H, Büchler MW, Fröhlich B, Ströbel P, Bergmann F, Neff W, Singer MV. Extrapulmonary sarcoidosis of liver and pancreas: a case report and review of literature. World J Gastroenterol. 2007;13:2504-2509. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 39] [Cited by in F6Publishing: 31] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 19. | Tana C. FDG-PET Imaging in Sarcoidosis. Curr Med Imaging Rev. 2019;15:2-3. [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 20. | Sartori S, Galeotti R, Calia N, Gualandi M, Nielsen I, Trevisani L, Ceccotti P, Abbasciano V. Sonographically guided biopsy and sonographic monitoring in the diagnosis and follow-up of 2 cases of sarcoidosis with hepatic nodules and inconclusive thoracic findings. J Ultrasound Med. 2002;21:1035-1039. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 21. | Tana C, Silingardi M, Dietrich CF. New trends in ultrasound of hepatosplenic sarcoidosis. Z Gastroenterol. 2015;53:283-284. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 22. | Schuessler G, Fellbaum C, Fauth F, Jacobi V, Schmidt-Matthiesen A, Ignee A, Dietrich CF. [The infammatory pseudotumour -- an unusual liver tumour]. Ultraschall Med. 2006;27:273-279. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 23. | Thanos L, Zormpala A, Brountzos E, Nikita A, Kelekis D. Nodular hepatic and splenic sarcoidosis in a patient with normal chest radiograph. Eur J Radiol. 2002;41:10-11. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 22] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 24. | Saboo SS, Krajewski KM, O'Regan KN, Giardino A, Brown JR, Ramaiya N, Jagannathan JP. Spleen in haematological malignancies: spectrum of imaging findings. Br J Radiol. 2012;85:81-92. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 87] [Cited by in F6Publishing: 83] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 25. | Wan YL, Cheung YC, Lui KW, Tseng JH, Lee TY. Ultrasonographic findings and differentiation of benign and malignant focal splenic lesions. Postgrad Med J. 2000;76:488-493. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 31] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 26. | Ignee A, Cui X, Hirche T, Demolo C, Barreiros AP, Schuessler G, Dietrich CF. Percutaneous biopsies of splenic lesions--a clinical and contrast enhanced ultrasound based algorithm. Clin Hemorheol Microcirc. 2014;58:529-541. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 27. | Oskuei A, Hicks L, Ghaffar H, Hoffstein V. Sarcoidosis-lymphoma syndrome: a diagnostic dilemma. BMJ Case Rep. 2017;2017. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 28. | Tana C, Giamberardino MA, Di Gioacchino M, Mezzetti A, Schiavone C. Immunopathogenesis of sarcoidosis and risk of malignancy: a lost truth? Int J Immunopathol Pharmacol. 2013;26:305-313. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 28] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 29. | Dietrich CF, Lee JH, Herrmann G, Teuber G, Roth WK, Caspary WF, Zeuzem S. Enlargement of perihepatic lymph nodes in relation to liver histology and viremia in patients with chronic hepatitis C. Hepatology. 1997;26:467-472. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 68] [Cited by in F6Publishing: 76] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 30. | Dietrich CF, Leuschner MS, Zeuzem S, Herrmann G, Sarrazin C, Caspary WF, Leuschner UF. Peri-hepatic lymphadenopathy in primary biliary cirrhosis reflects progression of the disease. Eur J Gastroenterol Hepatol. 1999;11:747-753. [PubMed] [Cited in This Article: ] |
| 31. | Hirche TO, Russler J, Braden B, Schuessler G, Zeuzem S, Wehrmann T, Seifert H, Dietrich CF. Sonographic detection of perihepatic lymphadenopathy is an indicator for primary sclerosing cholangitis in patients with inflammatory bowel disease. Int J Colorectal Dis. 2004;19:586-594. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 30] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 32. | Cui XW, Jenssen C, Saftoiu A, Ignee A, Dietrich CF. New ultrasound techniques for lymph node evaluation. World J Gastroenterol. 2013;19:4850-4860. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 80] [Cited by in F6Publishing: 86] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 33. | Lyttkens K, Prytz H, Forsberg L, Hederström E, Hägerstrand I. Ultrasound, hepatic lymph nodes and primary biliary cirrhosis. J Hepatol. 1992;15:136-139. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 17] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 34. | Bihun T, Diaz Y, Wenig S. Granulomatous Pancreas: A Case Report of Pancreatic Sarcoid. Case Rep Gastrointest Med. 2017;2017:1620392. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 35. | Azemoto N, Kumagi T, Koizumi M, Kuroda T, Yamanishi H, Ohno Y, Imamura Y, Takeshita E, Soga Y, Ikeda Y, Onji M, Hiasa Y. Diagnostic Challenge in Pancreatic Sarcoidosis Using Endoscopic Ultrasonography. Intern Med. 2018;57:231-235. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 36. | Dong Y, Jürgensen C, Puri R, D'Onofrio M, Hocke M, Wang WP, Atkinson N, Sharma M, Dietrich CF. Ultrasound imaging features of isolated pancreatic tuberculosis. Endosc Ultrasound. 2018;7:119-127. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 37. | Bergner R, Löffler C. Renal sarcoidosis: approach to diagnosis and management. Curr Opin Pulm Med. 2018;24:513-520. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 38. | Gezer NS, Başara I, Altay C, Harman M, Rocher L, Karabulut N, Seçil M. Abdominal sarcoidosis: cross-sectional imaging findings. Diagn Interv Radiol. 2015;21:111-117. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 44] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 39. | Heldmann M, Behm W, Reddy MP, Bozeman C, Welman G, Abreo F, Minagar A. Pseudotumoral renal sarcoid: MRI, PET, and MDCT appearance with pathologic correlation. AJR Am J Roentgenol. 2005;185:697-699. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 21] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 40. | Lockhart ME, Smith JK, Kenney PJ, Urban DA. Pseudotumorous renal involvement of sarcoidosis. J Urol. 2001;165:895. [PubMed] [Cited in This Article: ] |
| 41. | Dietrich CF, Tana C, Caraiani C, Dong Y. Contrast enhanced ultrasound (CEUS) imaging of solid benign focal liver lesions. Expert Rev Gastroenterol Hepatol. 2018;12:479-489. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 42. | Berzigotti A, Ferraioli G, Bota S, Gilja OH, Dietrich CF. Novel ultrasound-based methods to assess liver disease: The game has just begun. Dig Liver Dis. 2018;50:107-112. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 42] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 43. | Sidhu PS, Cantisani V, Dietrich CF, Gilja OH, Saftoiu A, Bartels E, Bertolotto M, Calliada F, Clevert DA, Cosgrove D, Deganello A, D'Onofrio M, Drudi FM, Freeman S, Harvey C, Jenssen C, Jung EM, Klauser AS, Lassau N, Meloni MF, Leen E, Nicolau C, Nolsoe C, Piscaglia F, Prada F, Prosch H, Radzina M, Savelli L, Weskott HP, Wijkstra H. The EFSUMB Guidelines and Recommendations for the Clinical Practice of Contrast-Enhanced Ultrasound (CEUS) in Non-Hepatic Applications: Update 2017 (Long Version). Ultraschall Med. 2018;39:e2-e44. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 398] [Cited by in F6Publishing: 477] [Article Influence: 79.5] [Reference Citation Analysis (1)] |
| 44. | Stryckers M, Voet D, Vogelaers D, Afschrift M, Verstraete K, Van Belle S. Contrast-enhanced ultrasonography in hepatosplenic sarcoidosis. Acta Clin Belg. 2011;66:429-431. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 4] [Reference Citation Analysis (0)] |
| 45. | Yu X, Yu J, Liang P, Liu F. Real-time contrast-enhanced ultrasound in diagnosing of focal spleen lesions. Eur J Radiol. 2012;81:430-436. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 32] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 46. | Tana C, Iannetti G, Mezzetti A, Schiavone C. Splenic sarcoidosis remains a diagnostic challenge. J Clin Ultrasound. 2014;42:156. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 47. | von Herbay A, Barreiros AP, Ignee A, Westendorff J, Gregor M, Galle PR, Dietrich C. Contrast-enhanced ultrasonography with SonoVue: differentiation between benign and malignant lesions of the spleen. J Ultrasound Med. 2009;28:421-434. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 29] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 48. | Stang A, Keles H, Hentschke S, von Seydewitz CU, Dahlke J, Malzfeldt E, Braumann D. Differentiation of benign from malignant focal splenic lesions using sulfur hexafluoride-filled microbubble contrast-enhanced pulse-inversion sonography. AJR Am J Roentgenol. 2009;193:709-721. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 40] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 49. | Chiorean L, Tana C, Braden B, Caraiani C, Sparchez Z, Cui XW, Baum U, Dietrich CF. Advantages and Limitations of Focal Liver Lesion Assessment with Ultrasound Contrast Agents: Comments on the European Federation of Societies for Ultrasound in Medicine and Biology (EFSUMB) Guidelines. Med Princ Pract. 2016;25:399-407. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 29] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 50. | Tana C, Iannetti G, D'Alessandro P, Tana M, Mezzetti A, Schiavone C. Pitfalls of contrast-enhanced ultrasound (CEUS) in the diagnosis of splenic sarcoidosis. J Ultrasound. 2013;16:75-80. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 51. | Chiorean L, Cui XW, Klein SA, Budjan J, Sparchez Z, Radzina M, Jenssen C, Dong Y, Dietrich CF. Clinical value of imaging for lymph nodes evaluation with particular emphasis on ultrasonography. Z Gastroenterol. 2016;54:774-790. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 52. | Rustemovic N, Hrstic I, Opacic M, Ostojic R, Jakic-Razumovic J, Kvarantan M, Pulanic R, Vucelic B. EUS elastography in the diagnosis of focal liver lesions. Gastrointest Endosc. 2007;66:823-4; discussion 824. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 25] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 53. | Rahimi E, Younes M, Zhang S, Thosani N. Endoscopic ultrasound elastography to diagnose sarcoidosis. Endosc Ultrasound. 2016;5:212-214. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |