Abstract
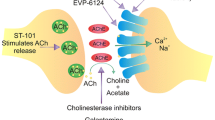
M1 muscarinic receptors (M1 mAChRs) play a role in an apparent linkage of three major hallmarks of Alzheimer’s disease (AD): β-amyloid (Aβ) peptide; tau hyperphosphorylation and paired helical filaments (PHFs); and loss of cholinergic function conducive to cognitive impairments. We evaluated the M1 muscarinic agonists AF102B (Cevimeline, EVOXAC™: prescribed for Sjøgren’s syndrome), AF150(S), and AF267B on some of these hallmarks of AD. Activation of M1 mAChRs with these agonists leads, inter alia, to enhanced secretion of amyloid precursor protein (α-APP), (via α-secretase activation), to decreased Aβ (via γ-secretase inhibition), and to inhibition of Aβ- and/or oxidative stress-induced cell death. In several animal models mimicking different aspects of AD, these drugs restored cognitive impairments, and in select cases induced a decrease in brain Aβ elevation, with a high safety margin, following po administration. Notably, in mice with small hippocampi, unlike rivastigmine and nicotine, AF150(S) and AF267B restored cognitive impairments also on escape latency in a Morris water maze paradigm, in reversal learning. Studies from other labs showed that AF102B and talsaclidine (another M1 agonist) decreased cerbrospinal fluid (CSF) Aβ in AD patients following chronic treatment, being the first reported drugs with such a profile. The clinical significance of these studies remains to be elucidated, yet based on in vivo (rabbits) and in vitro studies (cell cultures), our M1 agonists can decrease brain Aβ, owing to a novel and dual complementary effect (e.g., inhibition of γ-secretase and activation of α-secretase). Remarkably, although M1 agonists can decrease CSF Aβ in AD patients, an increased AD-type pathology in Parkinson’s disease was recently been associated with chronic antimuscarinic treatment. In another aspect, these agonists decreased tau hyperphosphorylation in vitro and in vivo. Notably, nicotinic agonists or cholinesterase inhibitors increased tau hyperphosphorylation. In summary, the M1 agonists tested are effective on cognition and behavior and show unique disease-modifying properties owing to beneficial effects on major hallmarks of AD. This may place such drugs in the first line of modern AD therapies (e.g., β- or γ-secretase inhibitors, vaccines against Aβ, statins, and inhibitors of tau hyperphosphorylation).
Similar content being viewed by others
References
Beach T. G., Potter P. E., Sue L. I., Fisher A., Scott S., Layne K. J., et al. (2002) Cerebral Abeta deposition induced by cortical deafferentiation is reduced by cholinergic therapy. Soc. Neurosci. Abstr. 722, 9.
Beach T. G., Walker D. G., Potter P. E., and Fisher A. (2001) Reduction of cerebrospinal fluid amyloid beta after systemic administration of M1 muscarinic agonists. Brain Res. 905, 220–223.
Buxbaum J. D., Oishi M., Chen H. I., Pinkas-Kramarski R., Jaffe E. A., Gandy S. E., and Greengard P. (1992) Cholinergic agonists and interleukin 1 regulate processing and secretion of the Alzheimer beta-A4 amyloid protein precursor. Proc. Natl. Acad. Sci. USA 89, 10,075–10,078.
Eckols K., Bymaster F. P., Mitch C. H., Shannon H. E., Ward J. S., and DeLapp N. W. (1995) The muscarinic M1 agonist xanomeline increases soluble amyloid precursor protein release from CHOM1 cells. Life Sci. 57, 1183–1190.
Fisher A. (1997) Muscarinic agonists for the treatment of Alzheimer’s disease: progress and perspectives. Exp. Opin. Invest. Drugs 6, 1395–1414.
Fisher A. (1999) Muscarinic receptor agonists in Alzheimer’s disease. More than just symptomatic treatment. CNS Drugs 12, 197–214.
Fisher A. (2000) Therapeutic strategies in Alzheimer’s Disease: M1 muscarinic agonists. Jpn. J. Pharmacol. 84, 101–112.
Forlenza O. V., Spink J. M., Dayanandan R., Anderton B. H., Olensen O. F., and Lovestone S. (2000) Muscarinic agonists reduce τ phosphorylation in non-neuronal cells via GSK-3β inhibition and in neurons. J. Neural Transm. 107, 1201–1212.
Genis I., Fisher A., and Michaelson D. M. (1999) Site-specific dephosphorylation of tau in apolipoprotein E-deficient and control mice by M1 muscarinic agonist treatment. J. Neurochem. 12, 206–213.
Haring R., Fisher A., Marciano D., Pittel Z., Kloog Y., Zuckerman A., et al. (1998) Mitogen-activated protein kinase-dependent and protein kinase C-dependent pathways link the M1 muscarinic receptor to amyloid precursor protein secretion. J. Neurochem. 71, 2094–2103.
Haring R., Gurwitz D., Barg J., Pinkas-Kramarski R., Heldman E., Pittel Z., et al. (1994) Amyloid precursor protein secretion via muscarinic receptors: Reduced desensitization using the M1-selective agonist AF102B. Biochem. Biophys. Res. Comm. 203, 652–658.
Haring R., Gurwitz D., Barg J., Pinkas-Kramarsky R., Heldman E., Pittel Z., et al. (1995) NGF promotes amyloid protein secretion via muscarinic receptor activation. Biochem. Biophys. Res. Comm. 213, 15–23.
Haring R., Pittel Z., Eizenberg O., and Fisher A. (2000) M1 muscarinic agonists protect PC12M1 cells from growth factor deprivation and beta-amyloid-induced apoptosis. World Conference on Alzheimer’s Disease, Washington, D.C., July 9–13.
Hartmann T., Runz H., Grimm H., Grziwa B., Bergmann C., Simons M., et al. (2002) γ-Secretase inhibition via cholesterol depletion involves presenilin. Soc. Neurosci. Abstr. 122, 6.
Hellstrom-Lindahl E., Moore H., and Nordberg A. (2000) Increased levels of τ protein in SH-SY5Y cells after treatment with cholinesterase inhibitors and nicotinic agonists. J. Neurochem. 74, 777–784.
Hock C., Maddalena A., Heuser I., Naber D., Oertel W., von Der Krame H., et al. (2000) Treatment with the selective muscarinic agonist talsclidine decreases cerebrospinal fluid levels of total amyloid beta-peptide in patients with Alzheimer’s disease. Ann. N.Y. Acad. Sci. 920, 285–291.
Hung A. Y., Haass C., Nitsch R., Qiu W. Q., Citron M., Wurtman R. J., et al. (1993) Activation of protein kinase C inhibits cellular production of the amyloid beta-protein. J. Biol. Chem. 268, 22,959–22,962.
Kurumatani T., Fastbom J., Bonkale W. L., Bogdanovic N., Winblad B., Ohm T. G., and Cowburn R. F. (1998) Loss of inositol 1,4,5-trisphosphate receptor sites and decreased PKC levels correlate with staging of Alzheimer’s disease neurofibrillary pathology. Brain Res. 796, 209–221.
Leloup C., Michaelson D. M., Fisher A., Hartmann T., Beyreuther K., and Stein R. (2000) M1 muscarinic receptors blocks caspase activation by phosphatidylinositide 3-kinase and MAPK/ERK-independent pathways. Cell Death Differ. 7, 825–833.
Lucas J., Hernandez F., Gomez-Ramos P., Moran M. A., Hen R., and Avila J. (2001) Decreased nuclear β-catenin, τ hyperphosphorylation and neurodegeneration in GSK-3β conditional transgenic mice. EMBO J. 20, 27–39.
Mattson M. P. (1997) Central role of oxyradicals in the mechanism of amyloid beta-peptide cytotoxicity. Alzheimer’s Dis. Rev. 2, 1–14.
Muller D. M., Mendla K., Farber S. A., and Nitsch R. M. (1997) Muscarinic M1 receptor agonists increase the secretion of the amyloid precursor protein ectodomain. Life Sci. 60, 985–991.
Nitsch R. M., Deng M., Tennis M., Schoenfield D., and Growdon J. H. (2000) The selective muscarinic M1 agonist AF102B decreases levels of total A (beta) in cerebrospinal fluid of patients with Alzheimer’s disease. Ann. Neurol. 48, 913–918.
Nitsch R. N., Slack B. E., Wurtman R. J., and Growdon J. H. (1992) Release of Alzheimer amyloid precursor derivatives stimulated by activation of muscarinic acetylcholine receptors. Science 58, 304–307.
Perry E. K., Burn D. J., Kilford L., Lees A. J., and Perry R. H. (2002) Increased Alzheimer pathology in Parrkinson’s disease is associated with chronic antimuscarinic drug treatment. Seventh International Meeting on Parkinson Disease and Movement Disorders, Nov., Miami FL.
Pittel Z., Heldman E., Barg J., Haring R., and Fisher A. (1996) Muscarinic control of amyloid precursor protein secretion in rat cerebral cortex and cerebellum. Brain Res. 742, 299–304.
Sadot E., Gurwitz D., Barg J., Behar L., Ginzburg I., and Fisher A. (1996) Activation of m1-muscarinic acetylcholine receptor regulates tau phosphorylation in transfected PC12 cells. J. Neurochem. 66, 877–880.
Schwarz R. D., Callahan M. J., Davis R. E., Jaen J. C., and Tecle H. (1997) Development of M1 subtype selective muscarinic agonists for Alzheimer’s disease: Translation of in vitro selectivity into in vivo efficacy. Drug Dev. Res. 40, 133–143.
Simons M., Schwarzler F., Lutjohann D., von Bergmann K., Beyreuther K., Dichgans J., et al. (2002) Treatment with simavastatin in normocholesterolemic patients with Alzheimer’s disease: a 26-week randomized, placebo-controlled, double-blind trial. Ann. Neurol. 52, 346–350.
Vincent G. P. and Sepinwall J. (1992) AF102B, a novel M1 agonist, enhanced spatial learning in C57BL/10 mice with a long duration of action. Brain Res. 597, 264–268.
Wolf B. A., Wertkin A. M., Jolly Y. C., Yasuda R. O., Wolfe B. B., Konrad R. J., et al. (1995) Muscarinic regulation of Alzheimer’s disease amyloid precursor protein secretion and amyloid beta-protein production in human neuronal NT2N cells. J. Biol. Chem. 270, 4916–4922.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fisher, A., Pittel, Z., Haring, R. et al. M1 muscarinic agonists can modulate some of the hallmarks in Alzheimer’s disease. J Mol Neurosci 20, 349–356 (2003). https://doi.org/10.1385/JMN:20:3:349
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1385/JMN:20:3:349