Abstract
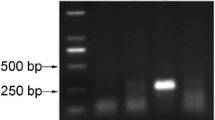
We report the simple and rapid method for detection of tomato yellow leaf curl Thailand virus (TYLCTHV) based on the direct capture of virus particles to the surface of a polymerase chain reaction (PCR) tube. This method allowed PCR without the time-consuming procedures of DNA extraction from infected plant tissue. A small amount of tomato tissue (∼10 mg) was ground in extraction buffer to release viruses from plant tissues. The constituents of the plant extract that might inhibit PCR activity were discarded by washing the tube with PBST buffer before adding the PCR mixture to the tube. This method was used for detection of TYLCTHV with plant sap solution diluted up to 1:20,000 and was more sensitive than an enzyme-linked immunosorbent assay (ELISA) method. In addition, this method can be used for detection of TYLCTHV in viruliferous whiteflies. The PCR tubes with captured TYLCTHV could be used for PCR, after storage at 4°C for 4 wk. The method presented here was used for detection of begomoviruses in cucurbit and pepper. In addition, this method was effectively used to detect papaya ringspot virus in papaya and zucchini yellow mosaic virus in cucumber by reverse transcriptase (RT)-PCR.
Similar content being viewed by others
References
Gafni, Y. (2003) Tomato yellow leaf curl virus, the intracellular dynamics of a plant DNA virus. Mol. Plant Pathol. 4, 9–15.
Moriones, E. and Navas-Castillo, J. (2000) Tomato yellow leaf curl virus, and emerging virus complex causing epidemics worldwide. Virus Res. 71, 123–134.
Clark, M. F. and Adam, A. N. (1997) Characteristics of the microplate method of enzyme-linked immunosorbent assay (ELISA) for the detection of plant viruses. J. Gen. Virol. 34, 475–483.
Lommel, S. A., McCain, A. H., and Morris, T. J. (1982) Evaluation of indirect enzyme-linked immunosorbent assay for detection of plant viruses. Phytopath. 72, 1018–1022.
Gilbertson, R. L., Rojas, M. R., Russell, D. R., and Maxwell, D. P. (1991) Use of asymmetric polymerase chain reaction and DNA sequencing to determine genetic variability of bean golden mosaic geminivirus in the Dominican Republic. J. Gen. Virol. 72, 2843–2848.
Polston, J. E., Dodds, J. A., and Perring, T. M. (1989) Nucleic acid probes for detection and strain discrimination of cucurbit geminiviruses. Phytopath. 79, 1123–1127.
Navot, N., Zeidan, M., Pichersky, F., Zamir, D., and Czosnek, H. (1992) Use of the polymerase chain reaction to amplify tomato leaf curl virus DNA from infected plant and viruliferous whiteflies. Phytopath. 82, 1199–1202.
Rojas, M. R., Gilbertson, R. L., Russell, D. R., and Maxwell, D. P. (1993) Use of degenerate primers in the polymerase chain reaction to detect whitefly-transmitted geminivirus. Plant Dis. 77, 340–347.
Wyatt, S. D. and Brown, J. K. (1996) Detection of subgroup III geminivirus isolate in leaf extracts by degenerate primers and polymerase chain reaction. Phytopath. 86, 1288–1293.
Henson, J. and French, R. (1993) Polymerase chain reaction and plant disease diagnosis. Ann. Rev. Phytopath. 31, 81–109.
Nolasco, G., de Blas, C., Torres, V., and Ponz, F. (1993) A method combining immunocapture and PCR amplification in a microtiter plate for the detection of plant viruses and subviral pathogens. J. Virol. Methods 45, 201–218.
Wetzel, T., Candresse, T., Macquaire, G., Raelonandro, M., and Dunez, J. (1992) A highly sensitive immunocapture polymerase chain reaction method for plum pox potyvirus detection. J. Virol. Methods 39, 17–37.
Sano, T., Smith, C. L., and Cantor, C. R. (1992) Immuno-PCR: very sensitive antigen detection by means of specific antibody-DNA complexes. Science 258, 120–122.
Dellaporta, S. L., Wood, J., and Hicks, H. B. (1983) A plant DNA minipreparation: Version II. Plant Mol. Biol. Rep. 14, 19–21.
Thomson, D. and Dietzen, R. G. (1995) Detection of DNA and RNA plant viruses by PCR and RT-PCR using a rapid virus release protocol without tissue homogenization. J. Virol. Methods 54, 85–95.
Fukata, S., Kato, S., Yoshida, K., et al. (2003) Detection of tomato yellow leaf curl virus by loop-mediated isothermal amplification reaction. J. Virol. Methods 112, 35–40.
Pico, B., Diez, M. J., and Nuez, F. (1999) Improved diagnosis techniques for tomato yellow leaf curl virus in tomato breeding programs. Plant Dis. 83, 1006–1012.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ieamkhang, S., Riangwong, L. & Chatchawankanphanich, O. Detection of tomato yellow leaf curl thailand virus by PCR without DNA extraction. Mol Biotechnol 31, 233–238 (2005). https://doi.org/10.1385/MB:31:3:233
Issue Date:
DOI: https://doi.org/10.1385/MB:31:3:233