-
PDF
- Split View
-
Views
-
Cite
Cite
Tiina Ojala, Jukka Salminen, Juha-Matti Happonen, Jaana Pihkala, Eero Jokinen, Heikki Sairanen, Excellent functional result in children after correction of anomalous origin of left coronary artery from the pulmonary artery – a population-based complete follow-up study, Interactive CardioVascular and Thoracic Surgery, Volume 10, Issue 1, January 2010, Pages 70–75, https://doi.org/10.1510/icvts.2009.209627
Close - Share Icon Share
Abstract
Surgical strategy to construct a two-coronary system for a patient with anomalous origin of left coronary artery from pulmonary artery (ALCAPA) has evolved with time. Limited long-term follow-up data are available on these children. We report population-based follow-up in children operated on for ALCAPA. In total, 29 patients underwent aortic reimplantation of ALCAPA between 1979 and 2006. Twenty (69%) children were repaired with direct aortic implantation, five (17%) with a modified tubular extension technique, and four (14%) patients with an intrapulmonary baffling technique. Early postoperative mortality (<30 days) was 17%. No late mortality (>30 days) was detected. The median length of follow-up was 11 years (range 10 months–27 years). Global left ventricular function by echocardiography (M-mode) was within normal limits (>30%) in all patients one year after operation. Functionally, 80% of patients were classified in NYHA class I, 20% in NYHA II, and 0% in NYHA classes III/IV at the time of the last examination. Excellent results with good long-term outcome can be achieved in infants with ALCAPA using reimplantation techniques. Normalization of cardiac function is expected within the first year in all operative survivors with a patent coronary system.
1. Introduction
Anomalous origin of left coronary artery from pulmonary artery (ALCAPA) is a rare malformation with an incidence of 0.25–0.50% in children with congenital heart defects [1]. It carries a mortality of up to 90% in infancy, if left unoperated [2]. When medically treated the mortality ranges from 45 to 100% [3] in older series, and in recent data from 0 to 17% [4–7]. During the neonatal period, high pulmonary vascular resistance and pulmonary artery pressure ensure antegrade flow from pulmonary artery into the anomalous left coronary artery. As the pulmonary vascular resistance gradually decreases, left to right shunting into pulmonary artery increases. Consequently, left ventricular perfusion becomes dependent on intercoronary collateral circulation from an enlarged right coronary artery. Coronary steal results in ischemia and subsequent infarction of left ventricular myocardium. Ischemia of the anterolateral papillary muscle leads to mitral regurgitation. Two types of ALCAPA have been documented: ‘infantile-type’ with poorly developed collateral coronary circulation and ‘adult-type’ with more developed coronary collaterals [3]. ALCAPA is usually seen as an isolated lesion. Operation should be undertaken as soon as the diagnosis has been confirmed to construct a two-coronary system to minimize the extent of permanent damage to the left ventricular myocardium. Surgical strategy to construct a two-coronary system for patients with ALCAPA has evolved with time and limited long-term follow-up data are available in these children. We report a population-based follow-up experience in patients with ALCAPA treated with coronary repair. We assessed the surgical outcomes, postoperative recovery of cardiac function, and New York Heart Association (NYHA) classification.
2. Materials and methods
Surgery for ALCAPA started in Finland in 1979. By the end of 2006, 29 patients have been operated on for ALCAPA by seven experienced pediatric cardiac surgeons. In this study, the medical records of all 29 patients operated on for ALCAPA were reviewed for demographic and anatomical characteristics, echocardiographic and catheterization findings and surgery. This study was approved by the hospital Ethics Committee.
At the time of the diagnosis, median age was 2.25 (range 0.01–12.5) years and median weight 12.3 (3–54) kg. All children had cardiac symptoms ranging from poor exercise tolerance in 25 (86%), reduced weight gain in 19 (66%), tachypnea in 18 (62%) and chest pain in 6 (21%) to cardiogenic shock in 12 (41%) patients. Four children (14%) had suspected respiratory infection at the time of diagnosis. The diagnosis of ALCAPA was confirmed by echocardiography using two-dimensional, M-mode, and Doppler imaging, and finally with aortic root angiography. Associated anomalies were seen in six children (21%): one premature infant was diagnosed with a patent ductus arteriosus, two patients with tetralogy of Fallot, one with a hemitruncus, one with a ventricular septal defect and one with a coarctation of the aorta and congenital mitral stenosis. Preoperative findings and symptoms are presented in Tables 1 and 2 .
Patient characteristics at the time of the diagnosis
| Patient | Year of | Age | Weight | Height | BSA | Associated | Diagnostic | Procedure | Follow-up |
| diagnosis | (years) | (kg) | (cm) | defects | delay | (months) | |||
| (months) | |||||||||
| 1 | 1979 | 4.31 | 13.6 | 112 | 0.67 | 0 | 0 | DRI | 325 |
| 2 | 1983 | 1.15 | 8.14 | 76 | 0.4 | 1 | 5 | DRI | 280 |
| 3 | 1986 | 1.29 | 6.94 | 71 | 0.36 | 0 | 0 | DRI | 239 |
| 4 | 1987 | 1.98 | 12.4 | 93 | 0.56 | 0 | 24 | DRI | 237 |
| 5 | 1988 | 0.31 | 6.22 | 64 | 0.32 | 0 | 0 | DRI | 216 |
| 6 | 1988 | 0.54 | 5.78 | 68 | 0.32 | 0 | 0 | IPT | D |
| 7 | 1989 | 0.54 | 5.95 | 69 | 0.33 | 0 | 0 | IPT | D |
| 8 | 1991 | 5.87 | 17.2 | 108 | 0.72 | 0 | 0 | DRI | 187 |
| 9 | 1991 | 1.64 | 16.9 | 103 | 0.69 | 3 | 18 | DRI | 180 |
| 10 | 1993 | 0.32 | 0 | 0 | IPT | D | |||
| 11 | 1994 | 12.5 | 54.1 | 164 | 1.58 | 0 | 0 | DRI | 144 |
| 12 | 1994 | 0.34 | 5.00 | 60 | 0.28 | 0 | 0 | IPT | 149 |
| 13 | 1995 | 0.46 | 6.70 | 67 | 0.34 | 0 | 0 | DRI | 136 |
| 14 | 1995 | 0.38 | 4.18 | 3 | 1 | DRI | D | ||
| 15 | 1995 | 0.23 | 0 | 0 | DRI | 136 | |||
| 16 | 1996 | 0.13 | 3.20 | 52 | 0.21 | 3 | 0 | DRI | 125 |
| 17 | 1997 | 0.01 | 4.08 | 52 | 0.23 | 0 | 0 | DRI | 111 |
| 18 | 1997 | 0.55 | 6.10 | 66 | 0.32 | 0 | 0 | DRI | 115 |
| 19 | 1997 | 7.14 | 25.0 | 124 | 0.93 | 0 | 0 | DRI | 112 |
| 20 | 1998 | 0.42 | 5.82 | 62 | 0.31 | 0 | 0 | DRI | 100 |
| 21 | 1998 | 3.39 | 17.3 | 108 | 0.72 | 0 | 0 | EPAT | 97 |
| 22 | 2000 | 0.24 | 4.70 | 57 | 0.26 | 0 | 0 | EPAT | 81 |
| 23 | 2001 | 1.82 | 12.6 | 88 | 0.54 | 0 | 0 | DRI | 60 |
| 24 | 2002 | 7.15 | 21.0 | 120 | 0.84 | 4 | 84 | DRI | 56 |
| 25 | 2003 | 0.19 | 5.20 | 62 | 0.29 | 0 | 0 | DRI | 38 |
| 26 | 2003 | 8.09 | 35.5 | 136 | 1.18 | 0 | 96 | DRI | 37 |
| 27 | 2004 | 2.18 | 12.6 | 88 | 0.54 | 5 | 0 | EPAT | 23 |
| 28 | 2004 | 0.45 | 6.13 | 66 | 0.33 | 0 | 0 | EPAT | 28 |
| 29 | 2005 | 1.70 | 10.0 | 84 | 0.47 | 0 | 0 | EPAT | 9 |
| Patient | Year of | Age | Weight | Height | BSA | Associated | Diagnostic | Procedure | Follow-up |
| diagnosis | (years) | (kg) | (cm) | defects | delay | (months) | |||
| (months) | |||||||||
| 1 | 1979 | 4.31 | 13.6 | 112 | 0.67 | 0 | 0 | DRI | 325 |
| 2 | 1983 | 1.15 | 8.14 | 76 | 0.4 | 1 | 5 | DRI | 280 |
| 3 | 1986 | 1.29 | 6.94 | 71 | 0.36 | 0 | 0 | DRI | 239 |
| 4 | 1987 | 1.98 | 12.4 | 93 | 0.56 | 0 | 24 | DRI | 237 |
| 5 | 1988 | 0.31 | 6.22 | 64 | 0.32 | 0 | 0 | DRI | 216 |
| 6 | 1988 | 0.54 | 5.78 | 68 | 0.32 | 0 | 0 | IPT | D |
| 7 | 1989 | 0.54 | 5.95 | 69 | 0.33 | 0 | 0 | IPT | D |
| 8 | 1991 | 5.87 | 17.2 | 108 | 0.72 | 0 | 0 | DRI | 187 |
| 9 | 1991 | 1.64 | 16.9 | 103 | 0.69 | 3 | 18 | DRI | 180 |
| 10 | 1993 | 0.32 | 0 | 0 | IPT | D | |||
| 11 | 1994 | 12.5 | 54.1 | 164 | 1.58 | 0 | 0 | DRI | 144 |
| 12 | 1994 | 0.34 | 5.00 | 60 | 0.28 | 0 | 0 | IPT | 149 |
| 13 | 1995 | 0.46 | 6.70 | 67 | 0.34 | 0 | 0 | DRI | 136 |
| 14 | 1995 | 0.38 | 4.18 | 3 | 1 | DRI | D | ||
| 15 | 1995 | 0.23 | 0 | 0 | DRI | 136 | |||
| 16 | 1996 | 0.13 | 3.20 | 52 | 0.21 | 3 | 0 | DRI | 125 |
| 17 | 1997 | 0.01 | 4.08 | 52 | 0.23 | 0 | 0 | DRI | 111 |
| 18 | 1997 | 0.55 | 6.10 | 66 | 0.32 | 0 | 0 | DRI | 115 |
| 19 | 1997 | 7.14 | 25.0 | 124 | 0.93 | 0 | 0 | DRI | 112 |
| 20 | 1998 | 0.42 | 5.82 | 62 | 0.31 | 0 | 0 | DRI | 100 |
| 21 | 1998 | 3.39 | 17.3 | 108 | 0.72 | 0 | 0 | EPAT | 97 |
| 22 | 2000 | 0.24 | 4.70 | 57 | 0.26 | 0 | 0 | EPAT | 81 |
| 23 | 2001 | 1.82 | 12.6 | 88 | 0.54 | 0 | 0 | DRI | 60 |
| 24 | 2002 | 7.15 | 21.0 | 120 | 0.84 | 4 | 84 | DRI | 56 |
| 25 | 2003 | 0.19 | 5.20 | 62 | 0.29 | 0 | 0 | DRI | 38 |
| 26 | 2003 | 8.09 | 35.5 | 136 | 1.18 | 0 | 96 | DRI | 37 |
| 27 | 2004 | 2.18 | 12.6 | 88 | 0.54 | 5 | 0 | EPAT | 23 |
| 28 | 2004 | 0.45 | 6.13 | 66 | 0.33 | 0 | 0 | EPAT | 28 |
| 29 | 2005 | 1.70 | 10.0 | 84 | 0.47 | 0 | 0 | EPAT | 9 |
Associated cardiac defects included ventricular septal defect (1), hemitruncus arteriosus (2), tetralogy of Fallot (3), coarctation of the aorta with congenital mitral stenosis (4) and patent ductus arteriosus (5). The time delay in months from the beginning of the symptoms to the diagnosis, the type of the procedure and the follow-up time in years are presented. The lack of the follow-up data (D) indicates early (<30 days) postoperative mortality. BSA, body surface area; DRI, direct reimplantation; EPAT, extrapulmonary analogous tube; IPT, intrapulmonary tunnel.
Patient characteristics at the time of the diagnosis
| Patient | Year of | Age | Weight | Height | BSA | Associated | Diagnostic | Procedure | Follow-up |
| diagnosis | (years) | (kg) | (cm) | defects | delay | (months) | |||
| (months) | |||||||||
| 1 | 1979 | 4.31 | 13.6 | 112 | 0.67 | 0 | 0 | DRI | 325 |
| 2 | 1983 | 1.15 | 8.14 | 76 | 0.4 | 1 | 5 | DRI | 280 |
| 3 | 1986 | 1.29 | 6.94 | 71 | 0.36 | 0 | 0 | DRI | 239 |
| 4 | 1987 | 1.98 | 12.4 | 93 | 0.56 | 0 | 24 | DRI | 237 |
| 5 | 1988 | 0.31 | 6.22 | 64 | 0.32 | 0 | 0 | DRI | 216 |
| 6 | 1988 | 0.54 | 5.78 | 68 | 0.32 | 0 | 0 | IPT | D |
| 7 | 1989 | 0.54 | 5.95 | 69 | 0.33 | 0 | 0 | IPT | D |
| 8 | 1991 | 5.87 | 17.2 | 108 | 0.72 | 0 | 0 | DRI | 187 |
| 9 | 1991 | 1.64 | 16.9 | 103 | 0.69 | 3 | 18 | DRI | 180 |
| 10 | 1993 | 0.32 | 0 | 0 | IPT | D | |||
| 11 | 1994 | 12.5 | 54.1 | 164 | 1.58 | 0 | 0 | DRI | 144 |
| 12 | 1994 | 0.34 | 5.00 | 60 | 0.28 | 0 | 0 | IPT | 149 |
| 13 | 1995 | 0.46 | 6.70 | 67 | 0.34 | 0 | 0 | DRI | 136 |
| 14 | 1995 | 0.38 | 4.18 | 3 | 1 | DRI | D | ||
| 15 | 1995 | 0.23 | 0 | 0 | DRI | 136 | |||
| 16 | 1996 | 0.13 | 3.20 | 52 | 0.21 | 3 | 0 | DRI | 125 |
| 17 | 1997 | 0.01 | 4.08 | 52 | 0.23 | 0 | 0 | DRI | 111 |
| 18 | 1997 | 0.55 | 6.10 | 66 | 0.32 | 0 | 0 | DRI | 115 |
| 19 | 1997 | 7.14 | 25.0 | 124 | 0.93 | 0 | 0 | DRI | 112 |
| 20 | 1998 | 0.42 | 5.82 | 62 | 0.31 | 0 | 0 | DRI | 100 |
| 21 | 1998 | 3.39 | 17.3 | 108 | 0.72 | 0 | 0 | EPAT | 97 |
| 22 | 2000 | 0.24 | 4.70 | 57 | 0.26 | 0 | 0 | EPAT | 81 |
| 23 | 2001 | 1.82 | 12.6 | 88 | 0.54 | 0 | 0 | DRI | 60 |
| 24 | 2002 | 7.15 | 21.0 | 120 | 0.84 | 4 | 84 | DRI | 56 |
| 25 | 2003 | 0.19 | 5.20 | 62 | 0.29 | 0 | 0 | DRI | 38 |
| 26 | 2003 | 8.09 | 35.5 | 136 | 1.18 | 0 | 96 | DRI | 37 |
| 27 | 2004 | 2.18 | 12.6 | 88 | 0.54 | 5 | 0 | EPAT | 23 |
| 28 | 2004 | 0.45 | 6.13 | 66 | 0.33 | 0 | 0 | EPAT | 28 |
| 29 | 2005 | 1.70 | 10.0 | 84 | 0.47 | 0 | 0 | EPAT | 9 |
| Patient | Year of | Age | Weight | Height | BSA | Associated | Diagnostic | Procedure | Follow-up |
| diagnosis | (years) | (kg) | (cm) | defects | delay | (months) | |||
| (months) | |||||||||
| 1 | 1979 | 4.31 | 13.6 | 112 | 0.67 | 0 | 0 | DRI | 325 |
| 2 | 1983 | 1.15 | 8.14 | 76 | 0.4 | 1 | 5 | DRI | 280 |
| 3 | 1986 | 1.29 | 6.94 | 71 | 0.36 | 0 | 0 | DRI | 239 |
| 4 | 1987 | 1.98 | 12.4 | 93 | 0.56 | 0 | 24 | DRI | 237 |
| 5 | 1988 | 0.31 | 6.22 | 64 | 0.32 | 0 | 0 | DRI | 216 |
| 6 | 1988 | 0.54 | 5.78 | 68 | 0.32 | 0 | 0 | IPT | D |
| 7 | 1989 | 0.54 | 5.95 | 69 | 0.33 | 0 | 0 | IPT | D |
| 8 | 1991 | 5.87 | 17.2 | 108 | 0.72 | 0 | 0 | DRI | 187 |
| 9 | 1991 | 1.64 | 16.9 | 103 | 0.69 | 3 | 18 | DRI | 180 |
| 10 | 1993 | 0.32 | 0 | 0 | IPT | D | |||
| 11 | 1994 | 12.5 | 54.1 | 164 | 1.58 | 0 | 0 | DRI | 144 |
| 12 | 1994 | 0.34 | 5.00 | 60 | 0.28 | 0 | 0 | IPT | 149 |
| 13 | 1995 | 0.46 | 6.70 | 67 | 0.34 | 0 | 0 | DRI | 136 |
| 14 | 1995 | 0.38 | 4.18 | 3 | 1 | DRI | D | ||
| 15 | 1995 | 0.23 | 0 | 0 | DRI | 136 | |||
| 16 | 1996 | 0.13 | 3.20 | 52 | 0.21 | 3 | 0 | DRI | 125 |
| 17 | 1997 | 0.01 | 4.08 | 52 | 0.23 | 0 | 0 | DRI | 111 |
| 18 | 1997 | 0.55 | 6.10 | 66 | 0.32 | 0 | 0 | DRI | 115 |
| 19 | 1997 | 7.14 | 25.0 | 124 | 0.93 | 0 | 0 | DRI | 112 |
| 20 | 1998 | 0.42 | 5.82 | 62 | 0.31 | 0 | 0 | DRI | 100 |
| 21 | 1998 | 3.39 | 17.3 | 108 | 0.72 | 0 | 0 | EPAT | 97 |
| 22 | 2000 | 0.24 | 4.70 | 57 | 0.26 | 0 | 0 | EPAT | 81 |
| 23 | 2001 | 1.82 | 12.6 | 88 | 0.54 | 0 | 0 | DRI | 60 |
| 24 | 2002 | 7.15 | 21.0 | 120 | 0.84 | 4 | 84 | DRI | 56 |
| 25 | 2003 | 0.19 | 5.20 | 62 | 0.29 | 0 | 0 | DRI | 38 |
| 26 | 2003 | 8.09 | 35.5 | 136 | 1.18 | 0 | 96 | DRI | 37 |
| 27 | 2004 | 2.18 | 12.6 | 88 | 0.54 | 5 | 0 | EPAT | 23 |
| 28 | 2004 | 0.45 | 6.13 | 66 | 0.33 | 0 | 0 | EPAT | 28 |
| 29 | 2005 | 1.70 | 10.0 | 84 | 0.47 | 0 | 0 | EPAT | 9 |
Associated cardiac defects included ventricular septal defect (1), hemitruncus arteriosus (2), tetralogy of Fallot (3), coarctation of the aorta with congenital mitral stenosis (4) and patent ductus arteriosus (5). The time delay in months from the beginning of the symptoms to the diagnosis, the type of the procedure and the follow-up time in years are presented. The lack of the follow-up data (D) indicates early (<30 days) postoperative mortality. BSA, body surface area; DRI, direct reimplantation; EPAT, extrapulmonary analogous tube; IPT, intrapulmonary tunnel.
Preoperative clinical symptoms and findings in children with anomalous origin of left coronary artery from pulmonary artery (n=29)
| Preoperative symptoms | Incidence (%)/ |
| median (range) | |
| Poor exercise tolerance | 25/29 (86%) |
| Poor weight gain | 19/29 (66%) |
| Tachypnea | 18/29 (62%) |
| Circulatory shock | 12/29 (41%) |
| Chest pain | 6/29 (21%) |
| Anxiety | 4/29 (14%) |
| Suspected respiratory infection | 4/29 (14%) |
| Preoperative findings | |
| Electrocardiogram abnormalities | 26/29 (90%) |
| Myocardial infarction/ischemia | 20/29 (70%) |
| Left atrial enlargement | 11/29 (38%) |
| Left ventricular hypertrophy | 18/29 (62%) |
| Chest X-ray | |
| Cardiomegaly | 23/29 (80%) |
| Echocardiography | |
| Left ventricular end diastolic | 2.0 (1–4) |
| Z-score diameter (S.D.) | |
| Fractional shortening (%) | 20 (5–38) |
| Mitral regurgitation | 26/29 (90%) |
| Mitral prolapse | 5 (17%) |
| Left ventricular fibroelastosis | 7 (24%) |
| Left ventricular aneurysm | 2 (7%) |
| Blood samples | |
| Lactate (mmol/l) | 1.0 (0.9–1.6) |
| Catheterization | |
| Performed in | 28/29 (97%) |
| No collaterals | 4/29 (14%) |
| Preoperative symptoms | Incidence (%)/ |
| median (range) | |
| Poor exercise tolerance | 25/29 (86%) |
| Poor weight gain | 19/29 (66%) |
| Tachypnea | 18/29 (62%) |
| Circulatory shock | 12/29 (41%) |
| Chest pain | 6/29 (21%) |
| Anxiety | 4/29 (14%) |
| Suspected respiratory infection | 4/29 (14%) |
| Preoperative findings | |
| Electrocardiogram abnormalities | 26/29 (90%) |
| Myocardial infarction/ischemia | 20/29 (70%) |
| Left atrial enlargement | 11/29 (38%) |
| Left ventricular hypertrophy | 18/29 (62%) |
| Chest X-ray | |
| Cardiomegaly | 23/29 (80%) |
| Echocardiography | |
| Left ventricular end diastolic | 2.0 (1–4) |
| Z-score diameter (S.D.) | |
| Fractional shortening (%) | 20 (5–38) |
| Mitral regurgitation | 26/29 (90%) |
| Mitral prolapse | 5 (17%) |
| Left ventricular fibroelastosis | 7 (24%) |
| Left ventricular aneurysm | 2 (7%) |
| Blood samples | |
| Lactate (mmol/l) | 1.0 (0.9–1.6) |
| Catheterization | |
| Performed in | 28/29 (97%) |
| No collaterals | 4/29 (14%) |
Preoperative clinical symptoms and findings in children with anomalous origin of left coronary artery from pulmonary artery (n=29)
| Preoperative symptoms | Incidence (%)/ |
| median (range) | |
| Poor exercise tolerance | 25/29 (86%) |
| Poor weight gain | 19/29 (66%) |
| Tachypnea | 18/29 (62%) |
| Circulatory shock | 12/29 (41%) |
| Chest pain | 6/29 (21%) |
| Anxiety | 4/29 (14%) |
| Suspected respiratory infection | 4/29 (14%) |
| Preoperative findings | |
| Electrocardiogram abnormalities | 26/29 (90%) |
| Myocardial infarction/ischemia | 20/29 (70%) |
| Left atrial enlargement | 11/29 (38%) |
| Left ventricular hypertrophy | 18/29 (62%) |
| Chest X-ray | |
| Cardiomegaly | 23/29 (80%) |
| Echocardiography | |
| Left ventricular end diastolic | 2.0 (1–4) |
| Z-score diameter (S.D.) | |
| Fractional shortening (%) | 20 (5–38) |
| Mitral regurgitation | 26/29 (90%) |
| Mitral prolapse | 5 (17%) |
| Left ventricular fibroelastosis | 7 (24%) |
| Left ventricular aneurysm | 2 (7%) |
| Blood samples | |
| Lactate (mmol/l) | 1.0 (0.9–1.6) |
| Catheterization | |
| Performed in | 28/29 (97%) |
| No collaterals | 4/29 (14%) |
| Preoperative symptoms | Incidence (%)/ |
| median (range) | |
| Poor exercise tolerance | 25/29 (86%) |
| Poor weight gain | 19/29 (66%) |
| Tachypnea | 18/29 (62%) |
| Circulatory shock | 12/29 (41%) |
| Chest pain | 6/29 (21%) |
| Anxiety | 4/29 (14%) |
| Suspected respiratory infection | 4/29 (14%) |
| Preoperative findings | |
| Electrocardiogram abnormalities | 26/29 (90%) |
| Myocardial infarction/ischemia | 20/29 (70%) |
| Left atrial enlargement | 11/29 (38%) |
| Left ventricular hypertrophy | 18/29 (62%) |
| Chest X-ray | |
| Cardiomegaly | 23/29 (80%) |
| Echocardiography | |
| Left ventricular end diastolic | 2.0 (1–4) |
| Z-score diameter (S.D.) | |
| Fractional shortening (%) | 20 (5–38) |
| Mitral regurgitation | 26/29 (90%) |
| Mitral prolapse | 5 (17%) |
| Left ventricular fibroelastosis | 7 (24%) |
| Left ventricular aneurysm | 2 (7%) |
| Blood samples | |
| Lactate (mmol/l) | 1.0 (0.9–1.6) |
| Catheterization | |
| Performed in | 28/29 (97%) |
| No collaterals | 4/29 (14%) |
2.1. Cardiac function
All patients underwent echocardiographic examination at the time of the diagnosis, at home discharge, and at postoperative follow-up time points of 3 months, 6 months, 12 months, 2 years, 3 years, 7 years, 10 years and 15 years. In M-mode echocardiography performed from the parasternal long-axis view, left ventricular end-diastolic and end-systolic dimensions were measured and left ventricular shortening fraction (FS) calculated. The Z-score of left ventricular end-diastolic dimension (LVEDD-Z) was determined [8]. NYHA classification was determined at the time of the last follow-up in all patients. We compared the results of the infants diagnosed before the age of one year (n=15) to those of the children diagnosed after the age of one year (n=14).
2.2. Surgical management
After a transverse incision in the pulmonary trunk, the left coronary/left anterior descending artery ostium was precisely identified. The pulmonary sinuses were identified as right-hand, left-hand and anterior sinuses according to the view obtained from posterior commissure looking anteriorly. The site of the anomalous coronary origin from the pulmonary artery was the right sinus of Valsalva in one and the left sinus of Valsalva in 23, 15 of them originating far left in the sinus, higher in the main pulmonary artery in one and at the bifurcation of the pulmonary artery in two. The origin of left coronary artery was non-specified in two patients. The type of the operation was decided when the origin of the anomalous coronary artery was identified.
All patients underwent aortic reimplantation of the anomalous coronary artery with hypothermic cardiopulmonary bypass. The most common form of repair used in 20 (69%) children was direct aortic implantation, the earliest done in 1979 in our series. Direct implantation was performed by mobilizing a large button of the pulmonary artery wall around the anomalous coronary ostium, often removing the whole sinus and creating a simple hole or a trap-door flap in the aorta [9, 10]. Seven patients underwent additional surgery due to associated cardiac anomalies (Tables 1 and 2). In five (17%) patients, the anomalous coronary artery was repaired using a modified tubular extension technique in which an extrapulmonary autologous tension free tube was created either from the posterior wall of the transected segment of pulmonary artery (four patients) and posterolateral wall of the aorta (four patients) or using two long flaps of pulmonary artery tissue (one patient) [9]. Pulmonary artery was adequately mobilized and reconstructed with a patch. In four patients (14%), an intrapulmonary baffling technique was used to create an aortopulmonary window [11]. This technique was used before coronary reimplantation was commonly performed (latest 1994).
2.3. Statistical analysis
Analyses were performed with the SPSS version 17.0 (SPSS Inc, Chicago, IL, USA) and SAS software, release 9.1.3 (SAS Institute, Cary, NC). For patient characteristics and variables derived from echocardiograms, mean and standard deviation (S.D.) or mean and range were calculated when appropriate. The repeated measures analysis of variance (RM ANOVA) test served for statistical analysis of echocardiographic data between the patients diagnosed before one year of age and those diagnosed after one year of age. Due to missing data in the measures after two years follow-up (range 1–5 measures per each child), the mean of the five measures between 2 and 15 years after diagnosis were used at the two years time point for all patients in the RM ANOVA models. Student's two-tailed t-test with Bonferroni correction was used for further analysis of intergroup differences, when appropriate. The differences in the complication data between the patients treated with different operation techniques and the differences in NYHA classification and incidence of more than trivial mitral valve regurgitation between different age groups were tested using Cochran–Mantel–Haenzel Method χ2-test. The level of significance was chosen at P<0.05.
3. Results
The incidence of ALCAPA in the Finnish population from 1979 to 2006 was 0.0017%, representing 29 cases out of 1,719,602 infants born over the study period [12].
3.1. Early results
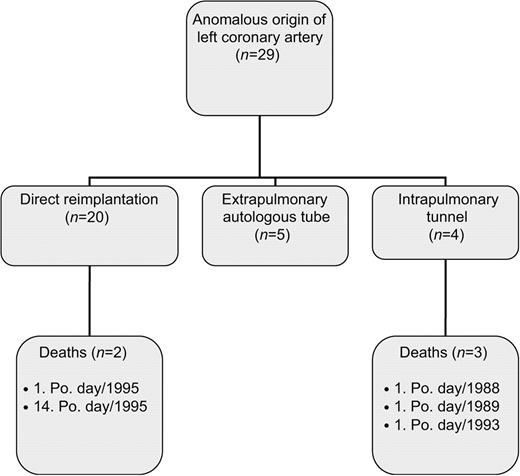
Early postoperative overall mortality (<30 days) was 5/29 (17%): 10% (2/20) for direct aortic reimplantation, 75% (3/4) for intrapulmonary tunnel and 0% (0/5) for extrapulmonary autologous tube (Fig. 1 ). All deaths occurred due to low cardiac output before the end of the year 1995. Since then, 15 patients were operated on with no early or late mortality. The median duration of intensive care treatment was eight days (range 1–24 days). The morbidity and complications encountered during the early postoperative period in a total of 16 (55%) patients included need for cardiopulmonary resuscitation in 6 (21%), rhythm disorders in 5 (7%), septicemia in 5 (7%), need for peritoneal dialysis in 4 (14%), neurologic disorders in 3 (10%), tracheitis in 2 (7%), need for pericardial drainage in 2 (7%), pancreatitis in 1 (3%), mediastinitis in 1 (3%) and paresis of the peroneus nerve in 1 (3%) patient. The intrapulmonary tunnel technique was related to increased early mortality (P=0.005) but no other differences in the complication rate were detected between the different operation techniques (P>0.2, data not shown). Mean duration of hospital stay was 29 (range 9–85) days (Table 3 ).
Flow diagram of overall management strategies and early mortality (n=5) for all patients with anomalous origin of coronary artery at the Hospital for Children and Adolescents, University of Helsinki, Finland from 1979 to 2006. Po. day, postoperative day.
Surgical characteristics in children with anomalous origin of left coronary artery from pulmonary artery (n=29)
| Surgical techniques | Incidence (%)/ |
| median (range) | |
| Direct implantation with a button | 20 (69%) |
| Extrapulmonary tunnel | 5 (17%) |
| Intrapulmonary tunnel | 4 (14%) |
| Associated surgery | |
| Mitral valve primary operation | |
| Mitral valve plasty | 1 (3%) |
| Mitral valve prosthesis-mechanical | 1 (3%) |
| Mitral valve bioprosthesis | 1 (3%) |
| Repair of tetralogy of Fallot | 2 (7%) |
| Repair of hemitruncus | 1 (3%) |
| Repair of aortic coarctation | 1 (3%) |
| Closure of ventricular septal defect | 2 (7%) |
| Aneurysm resection | 2 (7%) |
| Peri-operative data | |
| Cardiopulmonary bypass time (min) | 140 (31–317) |
| Aortic cross-clamp time (min) | 73 (36–139) |
| Circulatory arrest (n) | 6 (21%) |
| Duration of circulatory arrest (min) | 22 (6–42) |
| Left ventricular assist device (n) | 2 (7%) |
| ECMO (n) | 1 (3%) |
| Sternal closure | |
| Immediate | 25 (86%) |
| Delayed for 2–6 days | 3 (10%) |
| Surgical techniques | Incidence (%)/ |
| median (range) | |
| Direct implantation with a button | 20 (69%) |
| Extrapulmonary tunnel | 5 (17%) |
| Intrapulmonary tunnel | 4 (14%) |
| Associated surgery | |
| Mitral valve primary operation | |
| Mitral valve plasty | 1 (3%) |
| Mitral valve prosthesis-mechanical | 1 (3%) |
| Mitral valve bioprosthesis | 1 (3%) |
| Repair of tetralogy of Fallot | 2 (7%) |
| Repair of hemitruncus | 1 (3%) |
| Repair of aortic coarctation | 1 (3%) |
| Closure of ventricular septal defect | 2 (7%) |
| Aneurysm resection | 2 (7%) |
| Peri-operative data | |
| Cardiopulmonary bypass time (min) | 140 (31–317) |
| Aortic cross-clamp time (min) | 73 (36–139) |
| Circulatory arrest (n) | 6 (21%) |
| Duration of circulatory arrest (min) | 22 (6–42) |
| Left ventricular assist device (n) | 2 (7%) |
| ECMO (n) | 1 (3%) |
| Sternal closure | |
| Immediate | 25 (86%) |
| Delayed for 2–6 days | 3 (10%) |
Surgical characteristics in children with anomalous origin of left coronary artery from pulmonary artery (n=29)
| Surgical techniques | Incidence (%)/ |
| median (range) | |
| Direct implantation with a button | 20 (69%) |
| Extrapulmonary tunnel | 5 (17%) |
| Intrapulmonary tunnel | 4 (14%) |
| Associated surgery | |
| Mitral valve primary operation | |
| Mitral valve plasty | 1 (3%) |
| Mitral valve prosthesis-mechanical | 1 (3%) |
| Mitral valve bioprosthesis | 1 (3%) |
| Repair of tetralogy of Fallot | 2 (7%) |
| Repair of hemitruncus | 1 (3%) |
| Repair of aortic coarctation | 1 (3%) |
| Closure of ventricular septal defect | 2 (7%) |
| Aneurysm resection | 2 (7%) |
| Peri-operative data | |
| Cardiopulmonary bypass time (min) | 140 (31–317) |
| Aortic cross-clamp time (min) | 73 (36–139) |
| Circulatory arrest (n) | 6 (21%) |
| Duration of circulatory arrest (min) | 22 (6–42) |
| Left ventricular assist device (n) | 2 (7%) |
| ECMO (n) | 1 (3%) |
| Sternal closure | |
| Immediate | 25 (86%) |
| Delayed for 2–6 days | 3 (10%) |
| Surgical techniques | Incidence (%)/ |
| median (range) | |
| Direct implantation with a button | 20 (69%) |
| Extrapulmonary tunnel | 5 (17%) |
| Intrapulmonary tunnel | 4 (14%) |
| Associated surgery | |
| Mitral valve primary operation | |
| Mitral valve plasty | 1 (3%) |
| Mitral valve prosthesis-mechanical | 1 (3%) |
| Mitral valve bioprosthesis | 1 (3%) |
| Repair of tetralogy of Fallot | 2 (7%) |
| Repair of hemitruncus | 1 (3%) |
| Repair of aortic coarctation | 1 (3%) |
| Closure of ventricular septal defect | 2 (7%) |
| Aneurysm resection | 2 (7%) |
| Peri-operative data | |
| Cardiopulmonary bypass time (min) | 140 (31–317) |
| Aortic cross-clamp time (min) | 73 (36–139) |
| Circulatory arrest (n) | 6 (21%) |
| Duration of circulatory arrest (min) | 22 (6–42) |
| Left ventricular assist device (n) | 2 (7%) |
| ECMO (n) | 1 (3%) |
| Sternal closure | |
| Immediate | 25 (86%) |
| Delayed for 2–6 days | 3 (10%) |
3.2. Late results
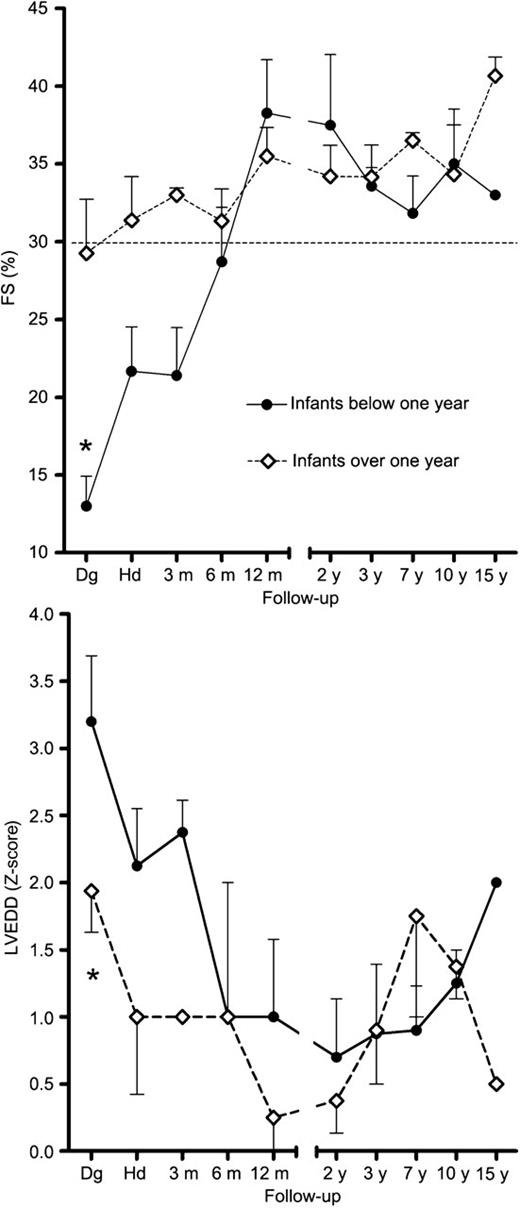
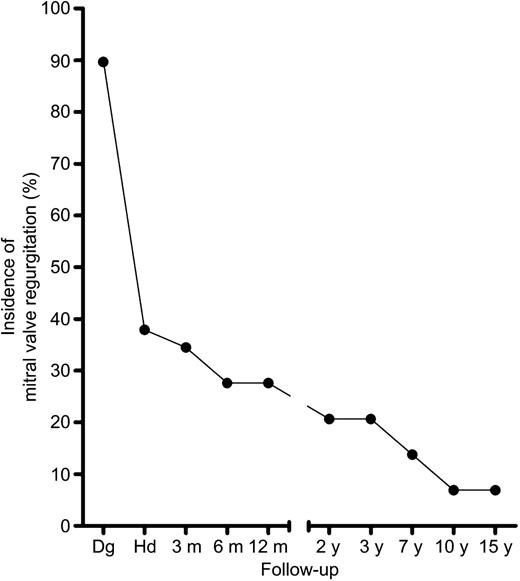
No late mortality (>30 days) was detected during the median follow-up of 11 years (range 10 months–27 years). Global left ventricular function, as assessed by echocardiography, improved in all patients. At the time of the diagnosis, the mean left ventricular FS 15% (±9.4%, S.D.) was significantly lower in patients diagnosed before the age of one year when compared with FS 28% (±9.9%) in patients diagnosed after the age of one year (P=0.003). The mean LVEDD-Z of +3.2 (±1.1) was also significantly larger in patients diagnosed before the age of one year when compared with the mean Z-score of +1.9 (±0.8) in patients diagnosed after the age of one year (P=0.03). However, both measurements were within normal limits in all patients one year after operation (Fig. 2 ). Significant decrease in the incidence of more than trivial mitral valve regurgitation was detected over the study period (P<0.001 Fig. 3 ). Eighty percent of patients were classified in functional class NYHA I and twenty percent in NYHA II at the time of the last examination. No patients were in NYHA classes III/IV. Late sequelae included mitral valve regurgitation in one patient (3%) treated with mitral valve replacement, ventricular tachycardia in one (3%), mild supravalvar pulmonary stenosis in two (7%), coronary fistula in one (3%), coronary artery dilation in one (3%) and tendency to atrial flutter in one (3%).
Left ventricular fractional shortening (FS) and Z-score of left ventricular end diastolic diameter (LVEDD) in patients with ALCAPA diagnosed before (n=15) and after (n=14) the age of one year. Data presented in mean and S.E.M. At the time of the diagnosis, FS was lower and LVEDD Z-score higher in infants diagnosed below one year of age compared with those infants diagnosed over one year of age. RM ANOVA time*group interaction P=0.03 and P=0.02 for FS and LVEDD, respectively; measures after two years follow-up were combined for the analysis due to missing data in follow-up measures. Dg, the time of the diagnosis; Hd, the time of discharge.
Incidence of more than trivial mitral valve regurgitation (%) decreased significantly over the follow-up period (P<0.001 for differences between time points). Dg, the time of the diagnosis; Hd, the time of discharge.
4. Discussion
This study demonstrates excellent survival of the patients with ALCAPA treated with current operation techniques. The overall mortality after dual coronary repair in our series was 17%, which is comparable with recent results from other pediatric centres (0–17%) [4–7]. In our centre, there has been no mortality after ALCAPA operations since 1995.
Our surgical technique, like that of other groups, has been developed through increasing experience in coronary transfer from the arterial switch operation. We currently prefer to use aortic implantation of the anomalous artery by creating a tension free autologous tube made of aortic and pulmonary artery flaps. The tube is of viable tissue and has a potential to grow with the child: this technique with its minor modifications can be used in any type of ALCAPA [10]. We no longer prefer intrapulmonary baffling technique due to the increased incidence of pulmonary artery stenosis in other series [13] and increased mortality encountered in our series.
Mitral regurgitation is a very common finding in infants with ALCAPA [14]. It is related to left ventricular dilatation and ischemic damage of the papillary muscles. It has been suggested that mitral valve should not be interfered with at the initial operation because it can be very difficult to repair and increase the operative risk [4, 15] and further, because it almost always decreases after successful revascularisation of the myocardium and normalization of LV size. However, in some patients, regurgitation can persist due to the irreversible ischemic papillary muscle damage even after successful revascularization, and additional interventions may be required. In our series, only one child underwent mitral valve reoperation. This is comparable with previous observations in children [16]. Also the incidence of other late postoperative complications was low in our series. No differences in the incidence of late complications were detected between the operative techniques.
This study confirms that the normalization of the left ventricular size and systolic function is achieved irrespective of the preoperative measurements [6]. The finding suggests hibernating ability of a child's myocardium in the presence of ALCAPA. At the time of the diagnosis, infants younger than one year had larger LVEDD as well as lower FS as compared with those diagnosed in older age. However, both measurements were within normal limits in all patients one year after operation. ALCAPA repair in younger (<12 months) symptomatic infants offers best potential for left ventricular recovery despite worse initial presentation.
Although recent encouraging results achieved with cardiac mechanical support in the postoperative period [17], pediatric cardiac assist devices still carry high risk for mortality and morbidity. These techniques should, however, be considered, when weaning from cardiopulmonary bypass under stable hemodynamical condition is not possible. In the present study, extracorporeal membrane oxygenation was used for one patient, who unfortunately died of complications soon after weaning. We agree with others in that the indication for cardiac assistance should be carefully discussed individually; it can be avoided in most cases with successful peri-operative myocardial support strategies [4, 18].
In conclusion, excellent results with good long-term outcome can be achieved in infants with ALCAPA using coronary artery reimplantation techniques. Normalization of cardiac function is expected within the first year in all operative survivors with a patent dual coronary system.
Manuscript was supported by grants from the Finnish Cultural Foundation, Finnish-Foundation for Cardiovascular Research, Finnish Pediatric Research Foundation, and Paavo Nurmi Association.
The authors appreciate the assistance of Ms Maiju Saarinen M.SSc. for the statistical analysis at the Cardiorespiratory Research Unit, University of Turku, Finland.