-
PDF
- Split View
-
Views
-
Cite
Cite
Ali N. Azadani, Nicolas Jaussaud, Peter B. Matthews, Liang Ge, Timothy A.M. Chuter, Elaine E. Tseng, Transcatheter aortic valves inadequately relieve stenosis in small degenerated bioprostheses, Interactive CardioVascular and Thoracic Surgery, Volume 11, Issue 1, July 2010, Pages 70–77, https://doi.org/10.1510/icvts.2009.225144
Close - Share Icon Share
Abstract
Objective: Transcatheter aortic valves (TAVs) are a promising treatment for high risk surgical patients suffering from degeneration of previously implanted bioprostheses (valve-in-valve therapy). However, unlike native stenosed aortic valves which have accommodated Edwards SAPIEN transcatheter valves after valvuloplasty, rigid bioprostheses may prevent full TAV stent expansion and disrupt leaflet function. We hypothesized that current 23 mm TAVs would not completely relieve severe stenosis in small bioprosthetic valves. The objective of this study was to study the hemodynamics of TAVs in degenerated bioprostheses. Methods: Twelve TAVs designed to mimic the 23 mm SAPIEN valve were created. Using a pulse duplicator, hemodynamics of valve-in-valve implantation were measured within 19, 21, and 23 mm Carpentier–Edwards PERIMOUNT degenerated bioprostheses (n=6 each). Bioprosthetic degeneration was simulated using BioGlue to achieve a mean pressure gradient of 50 mmHg. Results: TAVs significantly reduced the mean pressure gradient (50.9±4.7–9.1±4.1 mmHg, P<0.001) and total energy loss (870.3±157.4–307.8±87.3 mJ, P<0.001) in 23 mm degenerated bioprostheses. In 21 mm bioprostheses, the pressure gradient (52.3±7.0–19.5±5.0 mmHg, P<0.001) and energy loss (785.5±128.1–477.8±123.2 mJ, P=0.007) were reduced significantly. However, no significant changes in the pressure gradient (57.1±4.3–46.5±9.3 mmHg, P=0.086) or energy loss (839.3±49.3–960.5±158.1 mJ, P=0.144) were obtained after TAVI implantation in 19 mm bioprostheses. Incomplete stent expansion resulted in leaflet distortion and central regurgitation when implanted in 19 and 21 mm bioprostheses. Conclusions: The bioprosthetic annulus and stent posts offered a suitable landing zone for TAVs. However, oversized transcatheter valves were constrained by the rigid bioprostheses resulting in inadequate resolution of bioprosthetic stenosis. Hemodynamics of valve-in-valve intervention was worse than comparable size surgical valve replacements, particularly in 19 and 21 mm valves. Small degenerated bioprostheses require modification of current TAV design to yield acceptable hemodynamics.
1. Introduction
Transcatheter aortic valve implantation (TAVI) via transfemoral or transapical approaches has been performed in high-risk patients with native aortic stenosis [1–3]. Since bioprostheses degenerate similarly to native valves, a valve-in-valve concept has been proposed in which transcatheter aortic valves (TAVs) would be implanted within degenerated surgical bioprostheses [4, 5]. The valve-in-valve concept may be a promising option for high-risk patients requiring reoperative valve replacement. A few successful valve-in-valve cases in both the aortic and mitral positions have been reported in the literature [6–11]. However, clinical experience remains limited, and crucial questions regarding hemodynamics remain unanswered.
Degenerated bioprostheses offer a well-distinguished circular landing zone with known dimensions for TAVI. However, unlike native stenosed aortic valves which have accommodated an oversized TAV after valvuloplasty, the rigid bioprosthesis may prevent full TAV stent expansion and disrupt TAV leaflet function. It is unknown whether all bioprosthetic sizes can be treated effectively with currently available TAVs of limited sizes, or if new TAVs are required to achieve better hemodynamics. We hypothesized that the oversized TAV inadequately relieves severe stenosis of small sized degenerated bioprostheses. The objective of this study was to determine if small sized bioprostheses adversely impacted TAV hemodynamics, resulting in transcatheter-bioprosthetic size mismatch.
2. Materials and methods
2.1. Transcatheter aortic valves
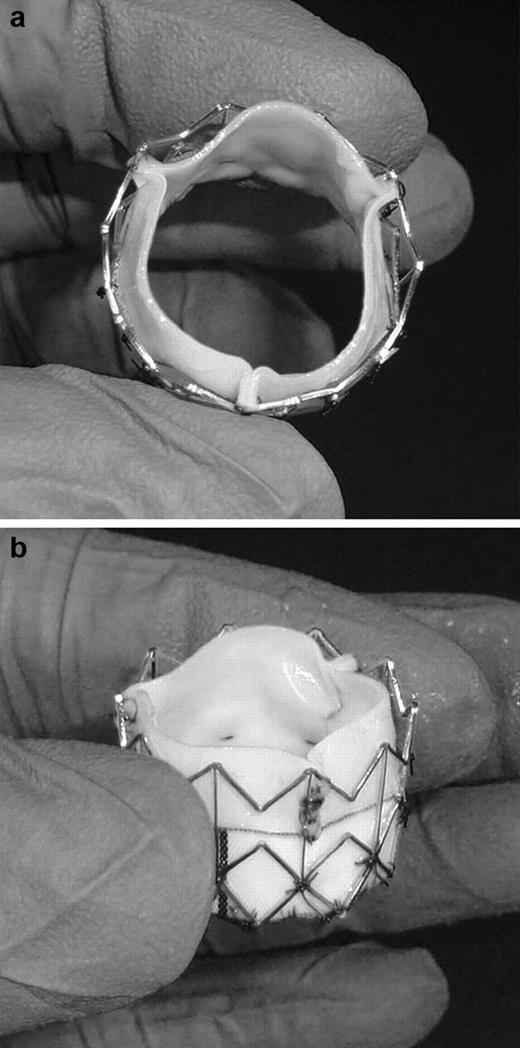
Our TAVs were created based on the Edwards SAPIEN valve design, currently being investigated in the PARTNER trial in the USA and CE Mark approved in Europe. Edwards Lifesciences, Inc was restricted from providing the SAPIEN for independent testing by FDA regulations until FDA approval; however, they did provide us with bioprostheses and bovine pericardium to create TAV leaflets. To study transcatheter-bioprosthetic valve size mismatch, twelve 23 mm TAVs were made for implantation within 19, 21, and 23 mm degenerated Carpentier–Edwards PERIMOUNT bioprostheses (n=6 each). Detailed description of our TAV has been previously described [12]. Briefly, three trapezoidal shaped leaflets were cut from a flat piece of bovine pericardium (Edwards Bovine Pericardial Patch, Edwards Lifesciences, Irvine, CA, USA). The lateral sides of the leaflets were sutured together and leaflets were then sutured at the base to a Dacron sheet. A customized cylindrical stainless steel stent (W.L. Gore & Associates, Inc, Fladstaff, AZ, USA) 15 mm in height was dilated to an external diameter of 23 mm to anchor the leaflets and Dacron sheet. Interrupted stitches were used at each intersection of the metal stent to attach the Dacron sheet to the stent (Fig. 1 ).
(a) Top and (b) side view of our 23 mm transcatheter aortic valve.
2.2. Degenerated bioprosthetic valves
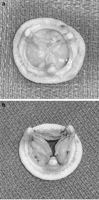
Acquiring explanted degenerated bioprostheses from patients would be unpredictable with respect to pressure gradients as well as bioprosthetic valve sizes, and it would be difficult to achieve sufficient quantity of each size for statistical analyses at one institution. Thus, a reproducible model simulating degeneration of normal bioprostheses was developed. The model provided consistent transvalvular pressure gradients and reflected in vivo pathology of the calcified valve. To simulate calcification, the most frequent mode of failure in pericardial bioprostheses, BioGlue was applied to leaflets of normal bioprostheses to stiffen leaflets and imitate calcification [13]. An additional sheet of pericardium was required to maintain BioGlue adherence and prevent dislodgement during balloon pre-dilatation of the bioprosthesis before TAVI. Therefore, three pieces of bovine pericardium (Edwards Bovine Pericardial Patch, Edwards Lifesciences, Irvine, CA, USA) were cut in half-circles the same diameter as that of the leaflet's free edge. One piece of pericardium was sutured to the aortic side of each bioprosthetic leaflet using polypropylene 5-0 running along the circular part of the piece, from the bottom of the bioprosthetic sinus to the commissure. No sutures were made on the leaflet free edge to create a pocket for BioGlue. Approximately 2–3 ml of BioGlue was then injected into the pocket to reach the desirable pressure gradient (Fig. 2 ). A mean bioprosthetic gradient of 50 mmHg was set as the goal based on echocardiographic data of degenerated aortic bioprostheses [14, 15]. The bioprosthetic degeneration model reproduced hemodynamics of a patient with severe bioprosthetic aortic stenosis.
(a) A 21 mm explanted degenerated bioprosthesis, and (b) matching sized bioprosthesis with simulated degeneration.
2.3. Pulse duplicator system
An in vitro study provides a consistent and well-controlled environment to examine valve-in-valve hemodynamics. Valves were tested at room temperature in a custom-built pulse duplicator system, developed for TAVI (Vivitro Systems, Inc, Victoria, Canada). A detailed description of the pulse duplicator has been previously described [12]. Heart rate, blood pressure, and cardiac output were used as control parameters for the waveform generator controlling a servo pump. Recirculating fluid of 36% by volume glycerin solution in normal saline solution was used as a blood analog fluid which mimicked blood viscosity at 37 °C when tested at room temperature. Pulse duplicator input parameters were used to match ISO 5840 and FDA standards for testing heart valves: heart rate of 70 beats/min, 35% systolic duration of cycle period, mean atrial and aortic pressures of 10 and 100 mmHg, and cardiac output 5 l/min [16, 17]. These hemodynamic parameters were maintained constant throughout the study.
2.4. Hemodynamic measurements
Valve hemodynamics were evaluated with four parameters: mean pressure gradient, effective orifice area, regurgitant volume, and transvalvular energy loss. Transvalvular energy allows assessment of valvular hemodynamics during the entire cardiac cycle and not just during forward flow. By this means, the ventricle becomes the focus of evaluation rather than the systolic function of the valve [18, 19]. Pressure was measured in the left atrium, left ventricle, left ventricular outflow tract, and ascending aorta with strain gauge pressure transducers (Cobe Laboratories, Inc, Lakewood, CO, USA). Effective orifice area within the TAV was calculated using the Gorlin equation. An electromagnetic flowmeter (Carolina Medical Electronics, Inc, NC, USA) was used to measure aortic valve flow rate and regurgitation volume by determining flows during systole and diastole. Subsequently, regurgitation fraction was calculated, defined as aortic retrograde flow divided by systolic ejection flow. Mild regurgitation was defined as regurgitant fraction <20%, and moderate as 20–40%. Furthermore, two-dimensional echocardiography (ACUSON Sequoia C256, Siemens Medical Solutions USA, Inc, Mountain View, CA, USA) was used to identify valvular leakage location and TAV opening and closing processes.
Transvalvular energy loss during forward, closing, and leakage flow was calculated using control volume analysis based on the principle of conservation of energy. Energy loss was assessed by the difference in energy flux entering and leaving the control volume during one cardiac cycle. A detailed description of energy loss calculation has been described [19]. Briefly, the energy loss (Φ) during forward flow, closing flow, and leakage flow was calculated separately by integrating the instantaneous flow (Qvalve) through the valve and the instantaneous pressure gradient (ΔP) during each time period:
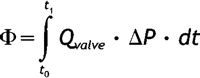
where t0=beginning and t1=the end of each time period. Total energy loss was the sum of energy loss during forward, closing, and leakage flow periods.
2.5. Data acquisition and analyses
To study efficacy of valve-in-valve implantation, first, TAVs were tested alone in the pulse duplicator before implantation. Data acquisition was run over 10 consecutive cardiac cycles and transvalvular pressure gradient, effective orifice area, regurgitant volume, and transvalvular energy loss were determined. Then, normal Carpentier–Edwards PERIMOUNT aortic heart valves were tested to obtain a hemodynamic baseline. Subsequently, the same size degenerated Carpentier–Edwards PERIMOUNT aortic valves were tested in the pulse duplicator to assess severity of bioprosthetic stenosis. Finally, after balloon pre-dilation of the degenerated bioprosthesis, the TAV was implanted within the bioprosthesis, and measurements were made to obtain valve-in-valve hemodynamics.
All measurements were repeated for the three bioprosthetic valve sizes (19, 21 and 23 mm) (n=6). A total of 12 TAVs were created, n=6 were tested within the 23 mm bioprostheses (n=6). It was found these TAVs could be recrimped and retested with no change in baseline hemodynamics (P=0.32). These TAVs were next studied within the 21 mm bioprostheses (n=6). However, due to the leaflet distortion within the 21 mm bioprostheses, these TAVs could no longer be recrimped and tested within the 19 mm bioprostheses. From the original 12 TAVs, the other n=6 created TAVs were tested within the 19 mm bioprostheses; their baseline hemodynamics prior to valve-in-valve implantation was not different than the TAVs used for the 21 mm bioprostheses (P=0.81). Lastly, an analysis of variance on TAVs used for all three sizes (TAVs before implantation within the 19, 21 and 23 mm bioprostheses) demonstrated no significant differences among the TAVs (P=0.59). Individual paired t-tests were utilized to compare measurements made in degenerated prostheses to those made after valve-in-valve for each of the valve sizes. To compare valve-in-valve therapy with surgical re-replacement of the bioprostheses, valve-in-valve measurements were compared to measurements made in normal prostheses for each valve size, using two-sample t-tests assuming unequal variances. In both cases, the results had a normal distribution determined by the Kolmogorov–Smirnov test. Furthermore, statistical power analysis showed that the used sample size was adequate to evaluate two-sided standardized differences >0.5 achieving a statistical power >0.80. Lastly, effect size calculations were performed comparing valve-in-valve results with those of normal bioprostheses. The percentile standing reflected the average percentile standing of the valve-in-valve relative to the average normal bioprosthesis. Reported values are quoted as mean±standard deviation (S.D.) and statistical analyses were performed using SPSS (version 17).
3. Results
3.1. Transvalvular mean pressure gradient
Twelve 23 mm intravalvular TAVs made in the laboratory demonstrated similar hemodynamics to Edwards SAPIEN valves which have a mean pressure gradient of 7.7±2.5 mmHg after implantation in patients [20]. Our TAVs had a mean pressure gradient of 5.9±1.8 mmHg when tested alone in the pulse duplicator. Bioprostheses with simulated degeneration achieved the desired mean pressure gradient (Table 1 ). After valve-in-valve implantation, the 23 mm TAVs demonstrated excellent hemodynamics within the 23 mm degenerated bioprostheses. A significant reduction in pressure gradient was seen in degenerated 23 mm (P<0.001) and 21 mm bioprostheses (P<0.001) after 23 mm TAVI (Table 1). However, a mean pressure gradient of the 19 mm degenerated bioprosthesis did not change significantly (P=0.09) after TAVI.
Transvalvular mean pressure gradient (mmHg) before and after TAVI compared with normal bioprosthesis
| Degenerated bioprosthesis | Valve-in-valve | Normal bioprosthesis | Cohen's d | Effect-size | Percentile standing (%) | |
| Mean±S.D. | Mean±S.D. | Mean±S.D. | correlation rYλ | |||
| 19 mm | 57.1±4.3 | 46.5±9.3 | 16.2±2.2† | 4.5 | 0.91 | >98 |
| 21 mm | 52.3±7.0 | 19.5±5.0* | 12.4±2.0† | 1.9 | 0.68 | 97 |
| 23 mm | 50.9±4.7 | 9.1±4.1* | 5.5±0.8 | 1.2 | 0.52 | 88 |
| Degenerated bioprosthesis | Valve-in-valve | Normal bioprosthesis | Cohen's d | Effect-size | Percentile standing (%) | |
| Mean±S.D. | Mean±S.D. | Mean±S.D. | correlation rYλ | |||
| 19 mm | 57.1±4.3 | 46.5±9.3 | 16.2±2.2† | 4.5 | 0.91 | >98 |
| 21 mm | 52.3±7.0 | 19.5±5.0* | 12.4±2.0† | 1.9 | 0.68 | 97 |
| 23 mm | 50.9±4.7 | 9.1±4.1* | 5.5±0.8 | 1.2 | 0.52 | 88 |
*P<0.001 between degenerated bioprostheses and valve-in-valve.
†P<0.02 between valve-in-valve and normal bioprosthesis.
Cohen's d, the effect-size correlation rYλ, and percentile standing reflect valve-in-valve vs. normal bioprosthesis.
TAVI, transcatheter aortic valve implantation; S.D., standard deviation.
Transvalvular mean pressure gradient (mmHg) before and after TAVI compared with normal bioprosthesis
| Degenerated bioprosthesis | Valve-in-valve | Normal bioprosthesis | Cohen's d | Effect-size | Percentile standing (%) | |
| Mean±S.D. | Mean±S.D. | Mean±S.D. | correlation rYλ | |||
| 19 mm | 57.1±4.3 | 46.5±9.3 | 16.2±2.2† | 4.5 | 0.91 | >98 |
| 21 mm | 52.3±7.0 | 19.5±5.0* | 12.4±2.0† | 1.9 | 0.68 | 97 |
| 23 mm | 50.9±4.7 | 9.1±4.1* | 5.5±0.8 | 1.2 | 0.52 | 88 |
| Degenerated bioprosthesis | Valve-in-valve | Normal bioprosthesis | Cohen's d | Effect-size | Percentile standing (%) | |
| Mean±S.D. | Mean±S.D. | Mean±S.D. | correlation rYλ | |||
| 19 mm | 57.1±4.3 | 46.5±9.3 | 16.2±2.2† | 4.5 | 0.91 | >98 |
| 21 mm | 52.3±7.0 | 19.5±5.0* | 12.4±2.0† | 1.9 | 0.68 | 97 |
| 23 mm | 50.9±4.7 | 9.1±4.1* | 5.5±0.8 | 1.2 | 0.52 | 88 |
*P<0.001 between degenerated bioprostheses and valve-in-valve.
†P<0.02 between valve-in-valve and normal bioprosthesis.
Cohen's d, the effect-size correlation rYλ, and percentile standing reflect valve-in-valve vs. normal bioprosthesis.
TAVI, transcatheter aortic valve implantation; S.D., standard deviation.
In order to compare valve-in-valve therapy with surgical re-replacement of the bioprostheses, TAVI hemodynamics for each bioprosthetic size were compared to hemodynamics of normal Carpentier–Edwards PERIMOUNT bioprostheses of equivalent size. For TAVI within the 23 mm degenerated bioprostheses, valve-in-valve pressure gradient was not significantly different from a normal 23 mm PERIMOUNT bioprosthesis (P=0.09) (Table 1). TAVI within a 21 mm degenerated bioprosthesis resulted in significantly higher pressure gradient than that of a normal 21 mm PERIMOUNT bioprosthesis (P=0.014). Valve-in-valve implantation did not improve hemodynamics within the 19 mm degenerated bioprostheses resulting in a significantly higher gradient than that of a normal 19 mm bioprosthesis (P<0.001). For all our comparisons of valve-in-valve results vs. normal bioprostheses, Cohen's d and effect size correlation r coefficients are presented within the tables along with the percentile standing interpretation. For example, TAVI in the 19 mm bioprostheses had an average transvalvular gradient that would be in greater than the 98th percentile of the average gradient for a normal 19 mm bioprostheses to reflect how significantly different these results are.
3.2. Effective orifice area
Prior to valve-in-valve implantation, 23 mm TAVs had an effective orifice area of 2.18±0.32 cm2. The TAV could not be dilated beyond the bioprosthetic annulus and was anchored inside the bioprosthesis. Effective orifice area significantly increased after TAVI in degenerated 23 mm (P=0.002) and 21 mm bioprostheses (P<0.001) (Table 2 ). However, in the 19 mm degenerated bioprosthesis, no significant change in effective orifice area was found (P=0.905) after TAVI.
Effective orifice area (cm2) before and after TAVI compared with normal bioprosthesis
| Degenerated bioprosthesis | Valve-in-valve | Normal bioprosthesis | Cohen's d | Effect-size | Percentile standing (%) | |
| Mean±S.D. | Mean±S.D. | Mean±S.D. | correlation rYλ | |||
| 19 mm | 0.75±0.17 | 0.76±0.09 | 1.28±0.10† | 5.5 | 0.94 | >98 |
| 21 mm | 0.72±0.04 | 1.17±0.14* | 1.49±0.13† | 2.4 | 0.76 | >98 |
| 23 mm | 0.65±0.06 | 1.81±0.48* | 2.20±0.15 | 1.1 | 0.48 | 86 |
| Degenerated bioprosthesis | Valve-in-valve | Normal bioprosthesis | Cohen's d | Effect-size | Percentile standing (%) | |
| Mean±S.D. | Mean±S.D. | Mean±S.D. | correlation rYλ | |||
| 19 mm | 0.75±0.17 | 0.76±0.09 | 1.28±0.10† | 5.5 | 0.94 | >98 |
| 21 mm | 0.72±0.04 | 1.17±0.14* | 1.49±0.13† | 2.4 | 0.76 | >98 |
| 23 mm | 0.65±0.06 | 1.81±0.48* | 2.20±0.15 | 1.1 | 0.48 | 86 |
*P<0.003 between degenerated bioprosthesis and valve-in-valve.
†P<0.003 between valve-in-valve and normal bioprosthesis.
Cohen's d, the effect-size correlation rYλ, and percentile standing reflect valve-in-valve vs. normal bioprosthesis.
TAVI, transcatheter aortic valve implantation; S.D., standard deviation.
Effective orifice area (cm2) before and after TAVI compared with normal bioprosthesis
| Degenerated bioprosthesis | Valve-in-valve | Normal bioprosthesis | Cohen's d | Effect-size | Percentile standing (%) | |
| Mean±S.D. | Mean±S.D. | Mean±S.D. | correlation rYλ | |||
| 19 mm | 0.75±0.17 | 0.76±0.09 | 1.28±0.10† | 5.5 | 0.94 | >98 |
| 21 mm | 0.72±0.04 | 1.17±0.14* | 1.49±0.13† | 2.4 | 0.76 | >98 |
| 23 mm | 0.65±0.06 | 1.81±0.48* | 2.20±0.15 | 1.1 | 0.48 | 86 |
| Degenerated bioprosthesis | Valve-in-valve | Normal bioprosthesis | Cohen's d | Effect-size | Percentile standing (%) | |
| Mean±S.D. | Mean±S.D. | Mean±S.D. | correlation rYλ | |||
| 19 mm | 0.75±0.17 | 0.76±0.09 | 1.28±0.10† | 5.5 | 0.94 | >98 |
| 21 mm | 0.72±0.04 | 1.17±0.14* | 1.49±0.13† | 2.4 | 0.76 | >98 |
| 23 mm | 0.65±0.06 | 1.81±0.48* | 2.20±0.15 | 1.1 | 0.48 | 86 |
*P<0.003 between degenerated bioprosthesis and valve-in-valve.
†P<0.003 between valve-in-valve and normal bioprosthesis.
Cohen's d, the effect-size correlation rYλ, and percentile standing reflect valve-in-valve vs. normal bioprosthesis.
TAVI, transcatheter aortic valve implantation; S.D., standard deviation.
3.3. Regurgitation fraction
A regurgitant fraction of 23 mm TAVs was determined initially in the pulse duplicator and found to be 12.0±1.7% at baseline, which was significantly greater than the 8.2±1.6% of 23 mm PERIMOUNT valves (P=0.002). After TAVI, the regurgitant fraction significantly increased (P<0.001) in the 19, 21 and 23 mm degenerated bioprostheses (Table 3 ). Leakage was both paravalvular and central, but intravalvular leakage was more pronounced after TAVI in the 19 and 21 mm bioprostheses. Furthermore, valve-in-valve regurgitant fraction was significantly higher than regurgitant fraction of normal bioprostheses in all the three sizes (P<0.001).
Regurgitation fraction (%) before and after TAVI compared with normal bioprosthesis
| Degenerated bioprosthesis | Valve-in-valve | Normal bioprosthesis | Cohen's d | Effect-size | Percentile standing (%) | |
| Mean±S.D. | Mean±S.D. | Mean±S.D. | correlation rYλ | |||
| 19 mm | 4.0±1.5 | 20.8±4.2* | 6.1±1.0† | 4.8 | 0.92 | >98 |
| 21 mm | 4.6±1.8 | 19.0±3.8* | 8.0±1.8† | 3.7 | 0.88 | >98 |
| 23 mm | 8.4±2.0 | 19.0±1.5* | 8.2±1.6† | 7.0 | 0.96 | >98 |
| Degenerated bioprosthesis | Valve-in-valve | Normal bioprosthesis | Cohen's d | Effect-size | Percentile standing (%) | |
| Mean±S.D. | Mean±S.D. | Mean±S.D. | correlation rYλ | |||
| 19 mm | 4.0±1.5 | 20.8±4.2* | 6.1±1.0† | 4.8 | 0.92 | >98 |
| 21 mm | 4.6±1.8 | 19.0±3.8* | 8.0±1.8† | 3.7 | 0.88 | >98 |
| 23 mm | 8.4±2.0 | 19.0±1.5* | 8.2±1.6† | 7.0 | 0.96 | >98 |
*P<0.001 between degenerated bioprosthesis and valve-in-valve.
†P<0.001 between valve-in-valve and normal bioprosthesis.
Cohen's d, the effect-size correlation rYλ, and percentile standing reflect valve-in-valve vs. normal bioprosthesis.
TAVI, transcatheter aortic valve implantation; S.D., standard deviation.
Regurgitation fraction (%) before and after TAVI compared with normal bioprosthesis
| Degenerated bioprosthesis | Valve-in-valve | Normal bioprosthesis | Cohen's d | Effect-size | Percentile standing (%) | |
| Mean±S.D. | Mean±S.D. | Mean±S.D. | correlation rYλ | |||
| 19 mm | 4.0±1.5 | 20.8±4.2* | 6.1±1.0† | 4.8 | 0.92 | >98 |
| 21 mm | 4.6±1.8 | 19.0±3.8* | 8.0±1.8† | 3.7 | 0.88 | >98 |
| 23 mm | 8.4±2.0 | 19.0±1.5* | 8.2±1.6† | 7.0 | 0.96 | >98 |
| Degenerated bioprosthesis | Valve-in-valve | Normal bioprosthesis | Cohen's d | Effect-size | Percentile standing (%) | |
| Mean±S.D. | Mean±S.D. | Mean±S.D. | correlation rYλ | |||
| 19 mm | 4.0±1.5 | 20.8±4.2* | 6.1±1.0† | 4.8 | 0.92 | >98 |
| 21 mm | 4.6±1.8 | 19.0±3.8* | 8.0±1.8† | 3.7 | 0.88 | >98 |
| 23 mm | 8.4±2.0 | 19.0±1.5* | 8.2±1.6† | 7.0 | 0.96 | >98 |
*P<0.001 between degenerated bioprosthesis and valve-in-valve.
†P<0.001 between valve-in-valve and normal bioprosthesis.
Cohen's d, the effect-size correlation rYλ, and percentile standing reflect valve-in-valve vs. normal bioprosthesis.
TAVI, transcatheter aortic valve implantation; S.D., standard deviation.
3.4. Energy loss
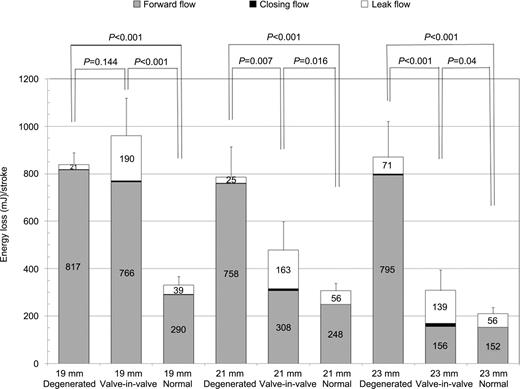
From an energy stand point, the 23 mm TAVs tested initially in the pulse duplicator had a total energy loss of 233.5±47.61 mJ/stroke (148.8±44.6 during forward, 4.7±1.4 during closing, and 79.9±20.6 mJ during leak flow) which was not significantly different than a normal 23 mm PERIMOUNT bioprosthesis (P=0.184). After the 23 mm TAVI within degenerated 23 mm bioprosthesis, the total energy loss was reduced significantly (P<0.001) (Table 4 ), but was still higher than that seen with a normal 23 mm PERIMOUNT bioprosthesis (P=0.040) and comparable to a normal 21 mm bioprosthesis (P=0.969) (Fig. 3 ). In a 21 mm degenerated bioprosthesis after TAVI, total energy loss was also significantly reduced (P=0.007); however, similar to the 23 mm degenerated bioprosthesis, the total valve-in-valve energy loss was significantly higher than that of the normal 21 mm bioprosthesis (P=0.017). In contrast to the 21 and 23 mm degenerated bioprostheses, the 23 mm TAVI within the 19 mm degenerated bioprosthesis failed to reduce a total energy loss (P=0.144), and imposed a relatively higher workload on the left ventricle, which was significantly higher than that of a normal 19 mm PERIMOUNT bioprosthesis (P<0.001).
Total energy loss mJstroke before and after TAVI compared with normal bioprosthesis
| Degenerated bioprosthesis | Valve-in-valve | Normal bioprosthesis | Cohen's d | Effect-size | Percentile standing (%) | |
| Mean±S.D. | Mean±S.D. | Mean±S.D. | correlation rYλ | |||
| 19 mm | 839.3±49.3 | 960.5±158.1 | 330.0±37.0† | 5.5 | 0.94 | >98 |
| 21 mm | 785.5±128.2 | 477.8±123.2* | 306.3±32.6† | 1.9 | 0.69 | 97 |
| 23 mm | 870.3±157.4 | 307.8±87.3* | 209.0±28.8† | 1.5 | 0.61 | 93 |
| Degenerated bioprosthesis | Valve-in-valve | Normal bioprosthesis | Cohen's d | Effect-size | Percentile standing (%) | |
| Mean±S.D. | Mean±S.D. | Mean±S.D. | correlation rYλ | |||
| 19 mm | 839.3±49.3 | 960.5±158.1 | 330.0±37.0† | 5.5 | 0.94 | >98 |
| 21 mm | 785.5±128.2 | 477.8±123.2* | 306.3±32.6† | 1.9 | 0.69 | 97 |
| 23 mm | 870.3±157.4 | 307.8±87.3* | 209.0±28.8† | 1.5 | 0.61 | 93 |
*P<0.01 between degenerated bioprosthesis and valve-in-valve.
†P<0.05 between valve-in-valve and normal bioprosthesis.
Cohen's d, the effect-size correlation rYλ, and percentile standing reflect valve-in-valve vs. normal bioprosthesis.
TAVI, transcatheter aortic valve implantation; S.D., standard deviation.
Total energy loss mJstroke before and after TAVI compared with normal bioprosthesis
| Degenerated bioprosthesis | Valve-in-valve | Normal bioprosthesis | Cohen's d | Effect-size | Percentile standing (%) | |
| Mean±S.D. | Mean±S.D. | Mean±S.D. | correlation rYλ | |||
| 19 mm | 839.3±49.3 | 960.5±158.1 | 330.0±37.0† | 5.5 | 0.94 | >98 |
| 21 mm | 785.5±128.2 | 477.8±123.2* | 306.3±32.6† | 1.9 | 0.69 | 97 |
| 23 mm | 870.3±157.4 | 307.8±87.3* | 209.0±28.8† | 1.5 | 0.61 | 93 |
| Degenerated bioprosthesis | Valve-in-valve | Normal bioprosthesis | Cohen's d | Effect-size | Percentile standing (%) | |
| Mean±S.D. | Mean±S.D. | Mean±S.D. | correlation rYλ | |||
| 19 mm | 839.3±49.3 | 960.5±158.1 | 330.0±37.0† | 5.5 | 0.94 | >98 |
| 21 mm | 785.5±128.2 | 477.8±123.2* | 306.3±32.6† | 1.9 | 0.69 | 97 |
| 23 mm | 870.3±157.4 | 307.8±87.3* | 209.0±28.8† | 1.5 | 0.61 | 93 |
*P<0.01 between degenerated bioprosthesis and valve-in-valve.
†P<0.05 between valve-in-valve and normal bioprosthesis.
Cohen's d, the effect-size correlation rYλ, and percentile standing reflect valve-in-valve vs. normal bioprosthesis.
TAVI, transcatheter aortic valve implantation; S.D., standard deviation.
Transvalvular energy loss during forward, closing, and leak flow of degenerated bioprosthesis (19, 21, 23 mm), valve-in-valve (after a 23 mm TAVI within degenerated bioprosthesis), and normal bioprostheses.
4. Discussion
In this study, we evaluated the hemodynamic performance of 23 mm TAVs within degenerated small size Carpentier–Edwards PERIMOUNT bioprostheses to assess the efficacy of valve-in-valve implantation. Our in vitro results demonstrated that the rigid annulus and stent posts of the bioprostheses constrained an oversized TAV and prevented full expansion of the stent. For 23 mm TAVs in 23 mm PERIMOUNT degenerated bioprostheses, the transvalvular pressure gradient dropped significantly after TAVI and the effective orifice area increased. Although the valve-in-valve pressure gradient was not significantly different from a normal 23 mm PERIMOUNT bioprosthesis, energy loss was significantly higher, matching that of a 21-mm bioprosthesis.
However, the 23 mm TAVI within the 19 mm PERIMOUNT bioprosthesis did not improve the transvalvular pressure gradient. Incomplete stent expansion resulted in excess pericardial tissue relative to stent orifice area and led to severe stenosis. Valve area was not increased and the total energy loss after valve-in-valve did not decrease. Use of a 23 mm TAV within the 19 mm degenerated bioprostheses does not yield adequate hemodynamic results and may be acutely detrimental in a clinical situation. A potential solution may be the development of a less obstructive TAV of smaller and matching size (i.e. 19 mm) or use of a supravalvular TAV to obtain better hemodynamics [21].
The 23 mm TAV implanted within the 21 mm degenerated bioprosthesis also significantly reduced pressure gradient (19.5 mmHg). However, this gradient was significantly higher than the standard surgical valve replacement using a 21 mm PERIMOUNT bioprosthesis (12.4 mmHg). These gradients corresponded to a valve-in-valve effective orifice area of 1.17 vs. 1.48 cm2 for surgical re-replacement with a 21 mm valve. The impact of such a size difference would depend on the patient's co-morbidities and body surface area to determine the risk/benefit ratio of open reoperation as compared to less invasive transcatheter therapy. Valve-in-valve implantation with current TAV size and technology in these circumstances may nonetheless benefit non-surgical and high-risk patients. Careful clinical judgment regarding surgical risks of reoperative valve replacement and severity of degenerated prosthetic dysfunction should be taken into account before decisions are made between surgery and valve-in-valve intervention in these instances. A smaller size matched TAV (21 mm) potentially may improve hemodynamics to the level of standard surgical aortic valve replacement.
4.1. Regurgitation and energy loss
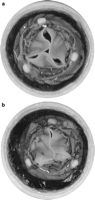
Mild to moderate regurgitation was observed in all three bioprosthetic sizes after deploying the oversized TAV and regurgitant volume was significantly increased after implantation. Two-dimensional echocardiography assessment of valve leakage showed paravalvular leak in the 23 mm bioprostheses most likely due to the lack of blood and coagulation factors in the in vitro circuit. However, both paravalvular and intravalvular leaks were observed in 19 and 21 mm bioprostheses. Intravalvular leakage was mainly due to incomplete coaptation of the leaflets. TAV leaflets were distorted after implantation within the 19 and 21 mm PERIMOUNT bioprostheses mainly due to excess of pericardial tissue relative to the stent orifice area (Fig. 4 ). In fact, even the 23 mm bioprosthesis with an internal diameter of 22 mm slightly constrained the full expansion of a 23-mm TAV.
(a) 23 mm TAVI within a 21 mm degenerated bioprosthesis. (b) 23 mm TAVI within a 19 mm degenerated bioprosthesis.
Transvalvular energy loss analysis demonstrated that TAVI within the 23 mm degenerated bioprosthesis significantly reduces the workload on the left ventricle. However, energy loss was significantly higher than normal 23 mm and comparable to the 21 mm PERIMOUNT bioprostheses. Furthermore, in the 21 mm degenerated bioprosthesis, the reduction in transvalvular energy loss observed after TAVI was significantly higher than that found in a normal 21 mm. However, in the 19 mm bioprosthesis, energy loss was unchanged after valve-in-valve implantation. Given such poor hemodynamics, the 23 mm TAVI would not be recommended for the 19 mm bioprostheses. Overall, TAVI with all three bioprosthetic sizes imposed higher workload on the left ventricle than the surgical re-replacement of the same sized valve.
4.2. Previous studies
In valve-in-valve implantation studies reported in the literature in either aortic or mitral position, TAVs were of the same or smaller size than the bioprostheses with few exceptions [7, 9]. The first animal study on valve-in-valve implantation was conducted by Walther et al. in pigs [4]. Seven 23 mm Edwards SAPIEN transcatheter valves were implanted within non-degenerated 23 mm aortic (n=5) and 25 mm mitral (n=2) Carpentier–Edwards porcine xenograft (Edwards Lifesciences Inc, Irvine, CA, USA). Hemodynamic function in all seven cases was good without any coronary obstruction and paravalvular or transvalvular leakage. The first successful valve-in-valve procedure in humans in an aortic position was reported by Wenaweser et al. [10]. A 21-F CoreValve was implanted percutaneously in a 23-mm Mitroflow degenerated aortic bioprosthesis (Sorin Group Canada Inc, Burnaby, BC, Canada). An immediate improvement in cardiac output was obtained. Furthermore, the one year follow-up showed acceptable TAV hemodynamics.
Recently, Walther et al. implanted a 23-mm Edwards SAPIEN valve in a 21-mm degenerated PERIMOUNT prosthesis (Edwards Lifesciences, Irvine, CA, USA) in an 82-year-old woman [9, 22]. Excellent hemodynamics were reported after TAVI with no incompetence and low gradients (maximum velocity, 2.1 m/s). Since the patient's cardiac output before and after TAVI was not reported, it is not possible to make a valid comparison to our in vitro results. However, we found that the 3 mm difference between the internal diameter of a 21 mm Carpentier–Edwards aortic valve (Edwards Lifesciences) and the 23 mm TAV distorted the TAV and disrupted TAV leaflet function, a phenomenon which was also described by Zegdi et al. [23]. Similarly, Klaaborg et al. described patient prosthetic mismatch when a 23-mm Edwards SAPIEN valve was implanted within a 21-mm Mitroflow valve with a peak gradient of 40 mmHg, valve area of 1.0 cm2, and mild central regurgitation. However, the patient nonetheless, benefited from the result [7]. Therefore, results of valve-in-valve within a 21 mm bioprostheses must be individualized to the type of bioprostheses, the hemodynamic expectations for the patient, and the patient's risk profiles.
4.3. Study limitations
The primary limitation was an inability to use the Edwards SAPIEN valve in this study. Our TAVs mimic the SAPIEN in size, shape, and hemodynamics. However, precise leaflet geometry and dimension are proprietary to the company which may affect valve function and the degree of intravalvular leakage from size mismatch. Our TAVs provided an acceptable pressure gradient and effective orifice area. However, regurgitant volume was slightly higher than with the Edwards SAPIEN valve. Two-dimensional Doppler echocardiography showed that the 23 mm TAV within the 23 mm bioprosthesis had a paravalvular leak, but no intravalvular leak. The paravalvular leak may be due to the circular annulus of the bioprosthesis and the recoil of the TAV after balloon expansion which does not allow a complete seal. Some leakage may also be from the suturing line between the leaflets and the Dacron sheet. Lastly, since the blood analog fluid does not have any coagulation properties, a higher regurgitant fraction is expected in our experiments than in the in vivo results.
The second limitation of the study was an inability to acquire and use real degenerated bioprostheses explanted from patients. It would be difficult to obtain sufficient numbers of explanted degenerated bioprostheses with consistent transvalvular pressure gradients for comparison. Our degenerated bioprosthetic model provided consistent gradients in different sizes. However, clinical TAVI within real degenerated bioprostheses may be complicated by irregular leaflet calcification, stent deformation, or pannus, which could not be addressed by our study. Although we cannot directly extrapolate our results to clinical practice, our in vitro study suggests that smaller degenerated bioprostheses must be carefully considered for valve-in-valve implantation. Based on our results, we would not recommend the use of the current 23 mm TAV for the 19 mm bioprostheses and careful consideration is required for the 21 mm bioprostheses. Design modifications of the TAV, such as the smaller 20 mm TAVs size matched to bioprosthetic size soon to be available or a supravalvular TAV may provide better hemodynamics than an oversized TAV [21].
5. Conclusions
Valve-in-valve intervention may be a promising option for elderly and high surgical risk patients suffering from structural valve degeneration of previously implanted bioprostheses. The rigid bioprosthetic annulus and stent posts offer a suitable landing zone for TAVs. However, the rigidity of the bioprostheses can constrain an oversized TAV and prevent full expansion of the stent. In this study, we investigated the hemodynamics of valve-in-valve treatment for small sized degenerated Carpentier–Edwards pericardial bioprostheses. We demonstrated that:
TAVI within a 23 mm degenerated bioprosthesis significantly reduced the pressure gradient, improved valve area similar to standard surgical valve replacement using a 23-mm PERIMOUNT valve, though total energy loss was greater.
TAVI within a 21 mm degenerated bioprosthesis also reduced significantly the pressure gradient; however, this gradient and total energy loss was higher than surgical aortic valve replacement using a 21 mm PERIMOUNT valve.
TAVI within the 19 mm bioprosthesis yielded unacceptable hemodynamics with no reduction in pressure gradient and energy loss.
Intravalvular regurgitation was observed in 19 and 21 mm bioprostheses after valve-in-valve implantation due to leaflet distortion.
Based on energy loss calculations, TAVI within all three bioprosthetic sizes imposed a higher workload on the left ventricle than surgical re-replacement of the valve.
Valve-in-valve hemodynamics in 19 and 21 mm degenerated bioprostheses may be improved by using a smaller size specific TAV to match bioprosthetic valve size or developing new TAV designs, such as supravalvular TAV [21]. Alternatively, given current technology, aortic enlarging procedures may be considered to allow implantation of 23 mm PERIMOUNT bioprostheses if future TAV implantation is a consideration.
Presented at the 59th International Congress of the European Society for CardioVascular Surgery, Izmir, Turkey, April 15–18, 2010.
Portion of work presented at the Transcatheter Therapeutics Conference 2009, September 21–25, 2009, San Francisco, CA, USA.
This work was supported by American Heart Association, Fédération/Société Française de Cardiologie, Société Française de Chirurugie Thoracique et Cardio-Vasculaire, and Northern California Institute for Research and Education. The study was not supported by Edwards Lifesciences, Inc and the authors did not have any financial arrangements or affiliation with the company. We thank Christine Frohlich, Edwards Lifesciences, Inc for providing bioprosthetic valves and bovine pericardium.