-
PDF
- Split View
-
Views
-
Cite
Cite
Iki Adachi, Takayoshi Ueno, Yumiko Hori, Yoshiki Sawa, Alterations in the medial layer of the main pulmonary artery in a patient with longstanding Fontan circulation, Interactive CardioVascular and Thoracic Surgery, Volume 11, Issue 5, November 2010, Pages 682–684, https://doi.org/10.1510/icvts.2010.240879
Close - Share Icon Share
Abstract
In the past, pulmonary arterial (PA) structure has been extensively investigated with the aim of providing an insight into operative indication for patients with congenital heart disease (CHD). Although PA histological analysis is applied less frequently in the current era, demographic changes of CHD patients require a refocussing of attention. With an exponential increase in the number of adult CHD patients, it is important to realise how structural changes evolve long after previous procedures as a certain proportion of such cases necessitate surgical or interventional manipulation on their PAs. Herein we present our findings on main PA tissues obtained from a 35-year-old woman who had been palliated with a classic Fontan operation 23 years earlier. Immunohistological analysis showed severe alterations, especially in the medial layer; not only attenuation of muscular component but also disarray and fragmentation of elastic fibres were remarkable, which should represent the adaptive response to longstanding diminished lung perfusion. To our knowledge, these observations have not been well described in the literature, presumably because previous studies were conducted primarily with respect to ‘increased’ pulmonary flow, and hence little is known regarding structural alterations in response to ‘decreased’ perfusion. Our findings are provided with a review of the literature.
1. Introduction
Previous studies have scrutinised alterations in medial smooth muscles of a pulmonary arterial (PA) wall in response to elevated vessel wall stress resulting from an increased pulmonary flow [1]. By contrast, little is known regarding the impact of diminished lung perfusion onto the integrity of PA wall structure. We report herein immunohistological findings of the main PA that had been incorporated in the Fontan circulation where PA flow was decreased and even non-pulsatile.
2. Case
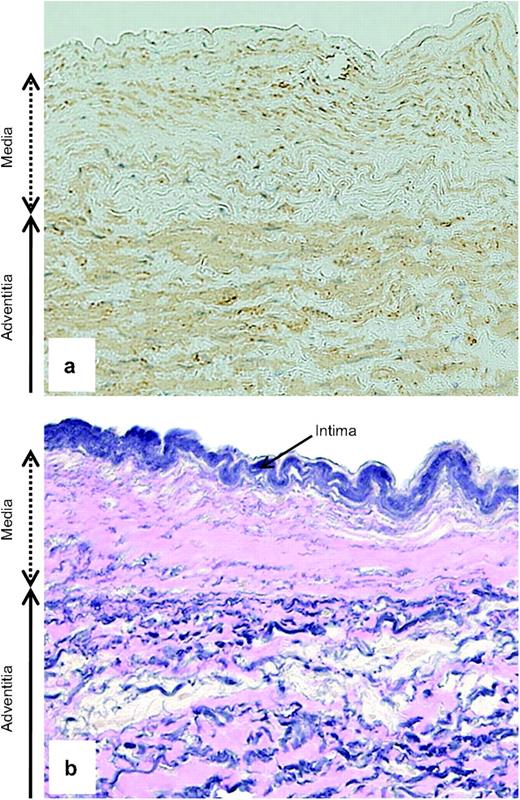
A 35-year-old female with a dominant right ventricle was scheduled to undergo revision of the Fontan pathway. In early infancy, her pulmonary bed was protected owing to the presence of moderate pulmonary valvar stenosis. She did not require any interventions until she was four years of age when she had a classic Blalock–Taussig shunt on the left. Subsequently, she was palliated with the Fontan operation by means of an atrio-pulmonary connection at the age of 12. A catheterisation study before revision showed a mean PA pressure of 9 mmHg and pulmonary vascular resistance of 1.3 Wood unit m2, as well as a marked decrease in cardiac index of 1.5 l/min/m2. Upon obtaining her consent, we resected a small piece of the main PA tissue when a prosthetic conduit was anastomosed to the PA. Microscopic examination revealed marked abnormalities in the PA wall. In particular, the medial layer was remarkably reduced in thickness, with both the muscular and elastic components being attenuated (Fig. 1 ).
Stains with α-SMA (a) and Elastica–van Gieson (b) of the main pulmonary arterial wall (original magnification, ×200). (a) The medial layer is decreased in thickness, with its muscular component (light brown) being remarkably attenuated. (b) Of particular note is the disarray and fragmentation of the medial elastin (dark blue) as being replaced with collagenous tissue (pink).
3. Discussion
Medial smooth muscles have been the focus of previous investigations that evaluated the structural changes of a PA wall in response to an abnormal haemodynamic state associated with congenital heart disease (CHD) [2]. Nonetheless, little is known regarding the adaptive response of medial elastin to diminished lung perfusion because vast majority of previous studies dealt with cases of increased pulmonary flow and examined intra-acinar, ‘muscular’ arteries, which contained few elastic fibres. Structural changes of elastic component would certainly be of clinical relevance as it determines not only mechanical but also functional properties of proximal, ‘elastic’ PAs. To augment such a paucity of information, we examined the main PA tissue from a Fontan patient having decreased PA flow for a prolonged period.
As anticipated, we observed remarkable thinning of the medial layer with its muscular component being reduced in amount, which would presumably evolve as an adaptive reaction to the longstanding Fontan circulation. This observation is in accordance with existing limited evidence that suggests attenuation of medial smooth muscles in response to a decreased pulmonary flow [2]. We did not predict, however, the concomitant attenuation of the medial elastin with a relative and probably compensatory increase of collagenous tissues. This might potentially be secondary to the attenuation of smooth muscles as the muscle cells play a crucial role in the maintenance of elastic fibres by secreting a complex extracellular matrix containing elastin. Irrespective of pathogenesis, histological alteration in proximal PAs itself deserves attention since it is this portion of the PA tree that often requires surgical or interventional manipulation. Moreover, in as much as distensibility and capacitive effects of proximal PAs are largely dependent on medial elastin, structural modifications of elastic fibres lead to functional changes of arterial properties, affecting arterial pressure through altered vessel compliance and wave transmission properties [3]. Previous experimental studies have shown that elastic insufficiency of proximal PAs predisposes to an elevated PA pressure in the setting of a normal pulsatile PA flow [4]. If elastic insufficiency secondary to changes in medial elastin is prevalent amongst patients with longstanding Fontan circulation, the Fontan survivors may be predisposed to unexpectedly elevated PA pressure with the restoration of normal pulsatile PA flow by means of heart transplant. Nonetheless, such functional insufficiency would not be evident when the pulmonary circulation is maintained by a constant, low-energy flow as in the Fontan circulation. Our speculation seems to be in line with the interesting observation by Mitchell and co-workers [5] who reported an elevation of PA pressure as well as significantly increased transpulmonary gradient and vascular resistance after heart transplantation for failing Fontan circulation. The authors attributed these somehow counterintuitive findings to the unreliability in estimation of preoperative pulmonary blood flow in this group of patients. They concluded that transpulmonary gradient increased to a pathological level after transplantation because of the presence of occult pulmonary vascular disease that was not identified with the Fontan state but became evident with the restoration of normal pulsatile PA flow. Besides their plausible explanation, our observation may provide an additional insight into this intriguing phenomenon. We hope the present study will stimulate further investigations on this currently unreported topic.