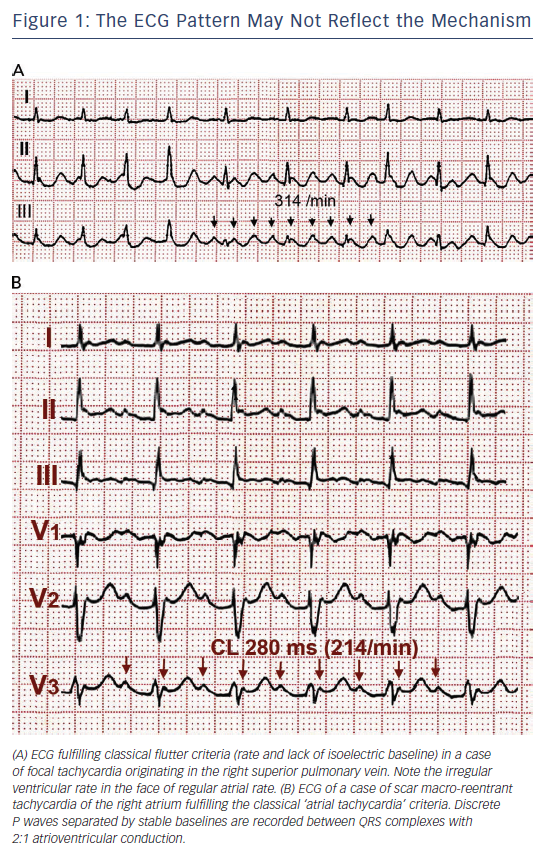
The term ‘flutter’ was coined to designate the visual and tactile rapid, regular atrial contraction induced by faradic stimulation in animal hearts, in contrast with irregular, vermiform contraction in atrial fibrillation (AF).1,2 On the ECG, flutter was a regular continuous undulation between QRS complexes at a cycle length (CL) of ≤250 ms (≥240 bpm). Slower tachycardias displaying discrete P waves, separated by isoelectric baselines, were called ‘atrial tachycardia’. Early studies suggested that flutter had a re-entrant mechanism3–5 but others attributed flutter to focal discharge.6,7 Later human studies left the door open for a focal mechanism.8 This was not a significant consideration when digitalis and very few antiarrhythmic drugs (AADs) were the only therapeutic armamentarium, but determining the mechanism involved in flutter has become crucial for the design and application of catheter and surgical ablation techniques. Modern electrophysiology (EP) has confirmed the re-entrant mechanism of typical flutter, and has opened wide the spectrum of mechanisms of macro-re-entrant tachycardias (MRTs), prompting a new, more open view of clinical ECG-based classification (see Figure 1A and 1B).9
Typical Atrial Flutter
The Re-entrant Mechanism
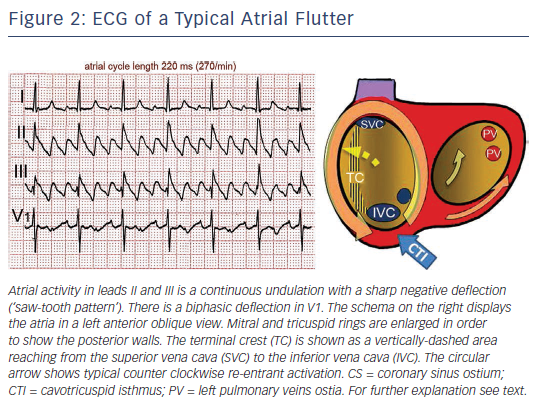
Typical flutter is the type of MRT most frequently found in the clinical setting. The mechanism is a large re-entrant circuit contained in the right atrium (RA) with passive activation of the left atrium (LA).10 Activation courses superoinferiorly in the anterior and lateral RA and inferosuperiorly in the septal RA, with a critical inferior turning point between the tricuspid ring and inferior vena cava (IVC) known as the cavotricuspid isthmus (CTI) (see Figure 2). An area of transverse conduction block in the posterior RA related to anisotropic conduction at the terminal crest11–14 and other structures15 forces activation toward the high RA so that the upper turning point can be at the RA roof or high in the posterior RA, depending of the size of the area of block.16–18 In either case, the CTI remains an obligatory passage for activation in the inferior RA.
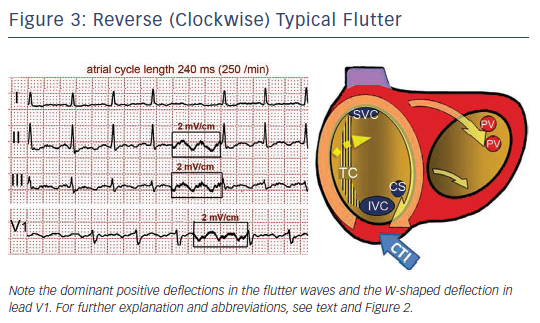
Either spontaneously or after programmed stimulation, re-entry may occur in the opposite (clockwise) direction – i.e. superoinferior in the septal wall and inferosuperior in the anterolateral wall – with the same zones of block in the posterior RA and obligatory passage through the CTI (see Figure 3).19 This reverse typical flutter is much less common clinically than the counter clockwise form, but the clinical manifestations are indistinguishable.
The ECG Patterns
Typical (counter clockwise) flutter is associated with the ‘common’ flutter pattern20,21 (see Figure 2): a regular continuous undulation with dominant negative deflections in inferior leads II, III and aVF, often described also as a ‘saw tooth pattern’, and flat atrial deflections in leads I and aVL. Atrial deflections in V1 can be positive, biphasic or negative. The CL is usually 250–170 ms (rates 240–350/min). Reverse typical flutter (see Figure 3) usually shows rounded or bimodal positive deflections in inferior leads II, III and aVF, and a very characteristic bimodal negative wave in the shape of a W is seen in lead V1.21,22
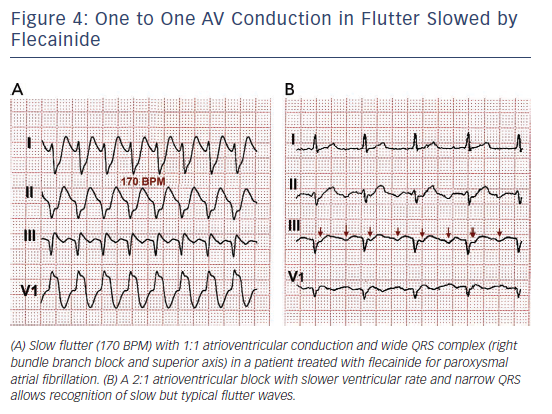
One frequent presentation of flutter is in patients treated with class IC AADs for AF. Flutter rate may be slowed by the AAD to ≤200/min, facilitating 1:1 atrioventricular (AV) conduction that due to the effect of the ADD results in aberrant intraventricular conduction and a wide QRS complex tachycardia (see Figure 4).
CTI-dependent MRT is also frequent in patients with previous surgical atriotomies or atrial baffle procedures, or after LA ablation for the treatment of AF.23,24 In these cases ECG patterns are often atypical. Conversely, a typical flutter ECG may be generated by atypical re-entry circuits, independent of the CTI, including LA circuits.25 Flutter wave morphology can be determined by activation outside the re-entry circuit, which would explain the often-difficult correlation between mechanism and ECG pattern.26,27
It should be emphasised that ECG diagnosis is based on atrial deflections and not on ventricular (QRS) rate and rhythm. An irregular ventricular rhythm may be caused by changing degrees of AV nodal block (see Figures 1A and 3), including Wenckebach cycles. In doubtful cases it is essential to document atrial activity dissociated from ventricular activity by increasing AV block by vagal manoeuvres or intravenous adenosine. However adenosine can produce a rebound increase in AV conduction to 1:128,29 and in some cases it can precipitate AF,30 therefore it should only be used if necessary for diagnosis and resuscitation equipment should be readily available.
Pathogenesis of Typical Flutter
About 80 % of flutter patients are male,31,32 otherwise flutter occurs in clinical contexts very much like those observed in AF (in old age, hypertension, diabetes, chronic obstructive lung disease, excessive alcohol consumption33 or during endurance sports practice).34 In many cases flutter episodes alternate with fibrillation episodes.32,35 Of those initially presenting with flutter as the only arrhythmia, 50 % develop fibrillation during long-term follow-up.36 This figure is not far from the proportion of patients developing fibrillation in the long term after CTI ablation for the treatment of typical flutter.37,38
The thickness of the terminal crest39,40 and its capacity to block transverse conduction41–44 are increased in cases of flutter compared to AF. EP studies have shown areas of low-voltage electrograms45 and slow conduction in the RA – particularly at the CTI46–48 – to be a sign of arrhythmogenic myocardial remodelling. LA dilatation and abnormalities in its reservoir function have been described as predictors of the incidence of atrial flutter or fibrillation.49
Clinical Presentation
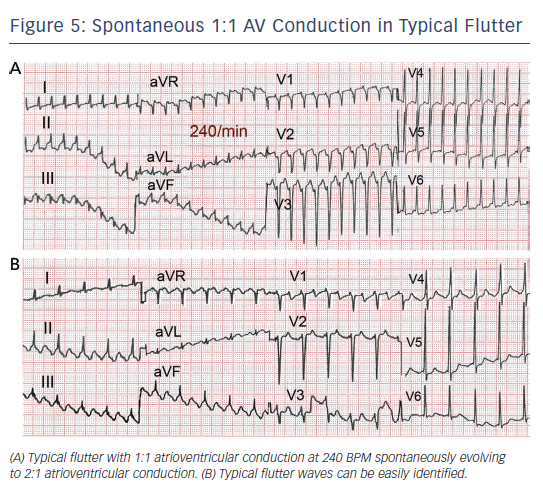
Flutter can be paroxysmal or persistent. Clinical presentation will depend in large part on the ventricular rate, which is most often around 120–150 due to 2:1 AV conduction, but in some cases 1:1 AV conduction leads to extremely high rates with poor clinical tolerance often requiring immediate intervention (see Figure 5). As in AF, loss of effective atrial contraction synchronised to ventricular contraction and rapid ventricular rates may result in hypotension, angina, heart failure, syncope or a feeling of palpitation making the patient seek medical attention.50 Occasionally flutter can be asymptomatic for weeks or months and the sustained tachycardia can lead to systolic ventricular dysfunction and heart failure (tachycardiomyopathy).51,52 Ventricular function and atrial dilatation may recover after return to sinus rhythm,53 but arrhythmia recurrence can again precipitate dysfunction with a risk of sudden death.54
LA appendage thrombi, spontaneous echo contrast and low appendage emptying velocities have been detected in cases of flutter submitted to cardioversion,55 although to a lesser extent than in AF,56 and normalisation can occur days after return to sinus rhythm.57 The frequency of systemic embolism in flutter is about one-third that in AF,36,58,59 but this difference disappears when both flutter and fibrillation occur in the same patient.
Management of Atrial Flutter
Rate control should be the first treatment step in symptomatic patients with a rapid ventricular rate. This is often a difficult goal in flutter, and even associations of the AV node blocking drugs (digoxin, beta-blockers and calcium antagonists) may fail, making cardioversion to sinus rhythm necessary. Dofetilide and ibutilide, pure class III AADs, are effective for interrupting flutter with a small risk of QT prolongation and torsade de pointes. Class IA and IC AADs are relatively ineffective or have no effect60–65 and can be problematic if they cause a slow atrial flutter rate ≤200/min with 1:1 AV conduction and QRS widening that mimics ventricular tachycardia (see Figure 4).66,67 Amiodarone may not be very effective at re-establishing sinus rhythm in the acute setting but it does help control ventricular rate.68,69
Rhythm Control: Cardioversion
The poor results of rhythm control strategies in AF may not apply in flutter because of a lower recurrence rate after cardioversion in flutter, making a strategy of repeated cardioversions supported with AADs a clinically applicable option.70,71 Transthoracic direct-current cardioversion, under short-lasting sedation, is the quickest and most effective method to recover sinus rhythm in patients with flutter, with a lower energy delivery and higher success rate than in AF.72,73
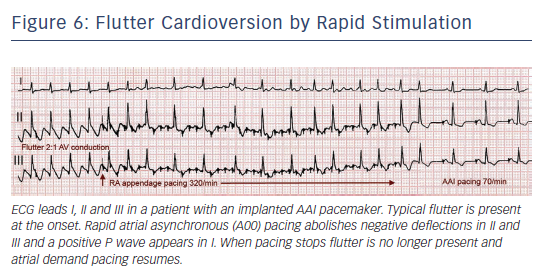
In 50–80 % of cases flutter interruption can be achieved by atrial pacing above the flutter rate through a transvenous catheter, through epicardial electrodes placed during cardiac surgery8 or by programming fast atrial rates in patients with atrial or dual-chamber pacemakers.74 Pacing runs of 20–30 s are started at a rate 10 bpm higher than flutter, increasing in 10 bpm steps up to 400 bpm or until flutter is interrupted and sinus (or paced) atrial rhythm is established (see Figure 6). Pacing may induce AF or a faster flutter (type II flutter),75 probably as an expression of functional re-entry76 that tends to return to baseline flutter or change to AF. AF induced by pacing usually results in a lower ventricular rate and, not infrequently, terminates spontaneously into sinus rhythm.
Flutter cardioversion by pacing is painless and can be done without sedation or anaesthesia.77 It may be more effective in postoperative flutter78 and in younger patients without structural heart disease or heart failure79 and it may be facilitated by class I AADs.80,81 Atrial pacing can be applied from the oesophagus82 but the higher output stimulation necessary may be painful83 and can occasionally induce ventricular arrhythmias.84
The patient should be anticoagulated if cardioversion is planned, be it by direct current shock or pacing, whenever the duration of flutter is >48 h. Patients with flutter of longer duration should be anticoagulated for 3–4 weeks before cardioversion or LA thrombi should be ruled out by transoesophageal echocardiography. Following cardioversion, anticoagulation should be maintained for a minimum of 3–4 weeks in patients with low embolic risk. If high embolic risk is present, anticoagulation should be continued indefinitely, unless prolonged follow-up monitoring demonstrates an absence of recurrence.85
Catheter Ablation
Radiofrequency catheter ablation of the CTI has become a standard treatment for typical flutter.86 The full thickness of the CTI must be ablated along a line reaching from the tricuspid ring (TR) to the IVC. Radiofrequency can be applied point-by-point, keeping the catheter tip stable for 45–60 s at each site or by dragging the catheter tip slowly from the TR to IVC during continuous radiofrequency delivery.87 The endpoint of the procedure is complete, bidirectional CTI conduction block that has to be checked by recordings along the ablation line88,89 and differential pacing manoeuvres.90 CTI block can be transient, so an observation period of 20–30 min is necessary to confirm success.91,92 Only when this endpoint is reached is flutter recurrence post ablation reduced to ≤10 %. At mid-term (months) conduction may still resume in 15 % of cases, even in the absence of flutter recurrence.93
For radiofrequency ablation large tipped (8 mm electrode length)94 or irrigated tip catheters95 are more effective than standard tip (4 mm electrode length) catheters. Supporting sheaths may be used to obtain good contact force on the CTI. Radiofrequency application to the CTI can be quite painful and moderate sedation is often needed during the procedure. Cryoablation can also be effective for CTI ablation and has the advantage of being painless.96 Resumption of CTI conduction at mid-term is more common after cryoablation than after radiofrequency ablation.93
Complications are infrequent (around 1 %)97 and are usually limited to vascular access; however, extension of ablation to the septal RA can result in AV block when using radiofrequency98,99 and cryoablation.96 Damage to the right coronary artery is rare but can result in myocardial infarction in some cases with pre-existing coronary atherosclerotic lesions.100,101 Cardiac perforation secondary to tissue disruption by boiling with audible ‘pops’ can occur when high energy is delivered with large-tip catheter-electrodes.102,103 A one in 1,000 incidence of cerebrovascular accidents is reported.97
The recurrence rate of typical flutter is ≤10 % after successful CTI ablation and definitive flutter suppression can be attained by a second procedure in recurrent cases. The main problem is the incidence of AF after ablation, which can be 30–50 % in the long term (>3 years).38,104–106 More recent reports have reported even higher incidences of AF.107 AF is more likely in patients who have had AF episodes before flutter ablation and in those with dilated LA.
The efficacies of CTI ablation and AADs have been compared for the treatment of typical flutter in two randomised studies.71,108 CTI ablation proved to be advantageous in terms of better quality of life, less hospitalisation and lower flutter recurrence, but the incidence of AF did not improve in both studies. In patients with flutter appearing during AAD treatment of AF, CTI ablation may help stabilise sinus rhythm109 at the same time allowing the use of class IC AADs without the risk of slow flutter with 1:1 AV conduction. Direct AF ablation has been proposed by some groups as a complement to CTI ablation in patients with both arrhythmias,110 and even in those with only flutter,111 to reduce the later incidence of AF. CTI ablation of typical flutter is associated with a favourable prognosis; however, given the higher incidence of severe complications in AF ablation, patients should be carefully selected for this strategy.112
There have been no randomised studies published on the risk–benefit ratio of anticoagulation after successful ablation of typical flutter with no associated AF. Prolonged monitoring of atrial rhythm under anticoagulation would appear to be indicated in patients with a high embolic risk score before anticoagulation is discontinued.
Summary: Long-term Strategy
A first and well-tolerated episode of flutter terminated spontaneously or by electrical cardioversion or AAD can be followed clinically with or without AAD coverage. A recurrence rate of around 50 % can be expected in these patients. Amiodarone, dronedarone or sotalol are indicated to prevent recurrences after cardioversion, while class IC AADs should be used cautiously or avoided. Catheter ablation is more effective for the prevention of recurrence and is a better alternative than maintenance AAD, especially in patients with depressed systolic ventricular function. A rate control strategy could be adequate for asymptomatic elderly patients with no deterioration of systolic ventricular function; however, cardioversion in active patients without apparent functional limitation will often improve a patient’s well-being and functional capacity. Chronic anticoagulation should be considered on the bases of embolic and haemorrhagic risk scores, along the same lines as for AF.85
Progression to AF after successful CTI ablation for typical flutter underlines the presence of an atrial arrhythmogenic substrate that can evolve in many cases, even in the absence of flutter recurrence. The diagnosis of flutter should thus be complemented with a clinical profile of AF risk factors that could guide ‘upstream therapy’. Recent reports have shown that physical fitness programmes and vigorous treatment of obesity, metabolic syndrome and sleep apnoea can result in a significant reduction in AF recurrence in patients whether or not they undergo AF ablation,113–116 and this may be applicable to flutter given the very similar risk factor profiles.
Atrial or AV pacing may be necessary in patients in whom conversion to sinus rhythm reveals sick sinus syndrome. In these cases, a device capable of overdrive atrial pacing should be implanted.
In the absence of direct evidence of the risk–benefit ratio of chronic anticoagulation in flutter, present recommendations for anticoagulation are the same as for AF, carefully balanced against bleeding risk scores.85
Atypical Flutter/Macro-re-entrant Tachycardia
The term atypical has been applied to rapid atrial tachycardias with ECG patterns differing from the typical and reverse typical flutter described above, and also to re-entrant tachycardias with circuit configuration different from the typical RA flutter circuit, even if they have an ECG pattern similar to typical flutter. ECG waveform can be determined by activation of the atrial myocardium outside the re-entry circuit26,27 and the precise mechanism generating atypical ECG flutter patterns can only be determined by mapping and pacing EP studies.117 Atypical flutter is often associated with structural heart disease, especially in patients that have undergone cardiac surgery or extensive catheter ablation for the treatment of AF. In these cases focal (centrifugal) mechanisms can coexist with MRT with indistinguishable ECG patterns, making EP study the only way to unveil the mechanisms causing the arrhythmia and plan ablation when clinically indicated.118,119
Right Atrial Macro-re-entrant Tachycardias
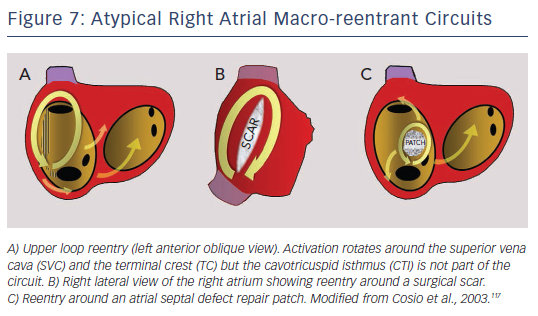
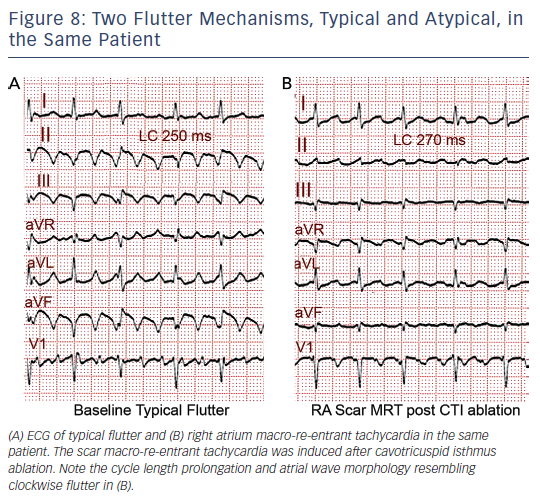
MRT circuits turning around the superior vena cava and part of the terminal crest, not involving the CTI, can occasionally be found in patients without surgical atriotomy (see Figure 7). In patients with RA surgical atriotomy, the scar can become the centre of the MRT, but the small incisions used to cannulate the superior vena cava and IVC are rarely arrhythmogenic by themselves. Lateral wall, superoinferior atriotomy is a frequent cause of atypical (non- CTI-dependent) MRT. ECG pattern may or may not be atypical (see Figures 1B and 8) and it is not unusual for two or more ECG patterns to alternate, as CTI-dependent typical flutter often coexists with the scar MRT (see Figure 8).23 A patch closing an interatrial septal defect can also become the centre of a MRT circuit (see Figure 7). Atypical flutter or MRT related to surgery often occurs years after the procedure, suggesting that an atrial remodelling process is necessary to make re-entry stable around the surgical obstacles in many cases.
In patients not subjected to cardiac surgery or AF ablation, unexcitable areas of low voltage, most often located in the lateral RA,120,121 can become the central obstacle sustaining atypical MRT. These areas are probably related to chronic atrial overload or cardiomyopathy and they are often considered to be fibrotic myocardium but there is no direct evidence of their histology. Low-voltage areas are most prevalent in the RA after a Fontan procedure,119 leading to the difficult management of recurrent MRTs in these cases.
Left Atrial Macro-re-entrant Tachycardias
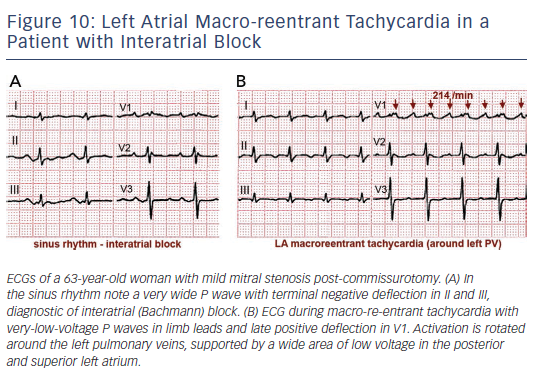
Surgical atriotomy scars are a well-known cause of MRT of the LA122–124 often combined with re-entry around low-voltage, inexcitable areas not related to atriotomy. In recent years the incidence of atypical flutter/MRT has become epidemic, with a wide variety of re-entry circuits following extensive LA ‘substrate ablation’ for the treatment of persistent AF (see Figure 9). A ‘maturation’ process appears to be necessary to make a MRT circuit stable, as tachycardia inducibility at the end of an AF ablation procedure does not predict later clinical occurrence.125,126 Recovery of slow conduction across ablation lines in the mid-term appears to be the arrhythmogenic mechanism in most cases.127,128 An ECG pattern of interatrial (Bachmann) block is often associated with atypical flutter/MRT based in the LA.129,130 This ECG pattern may be associated too with inexcitable, low-voltage areas in the LA (see Figure 10).131
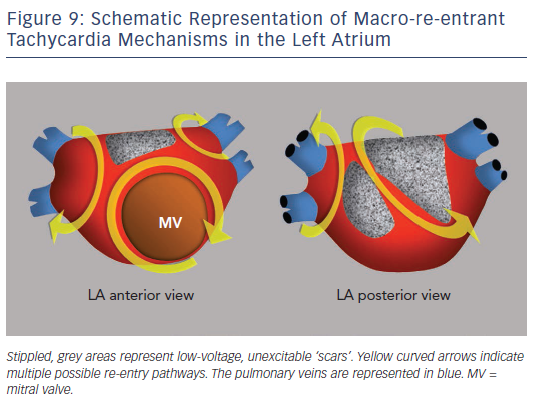
EP studies with RA and LA activation mapping and the response to pacing are necessary to reveal the mechanism in order to guide catheter or surgical ablation. MRT involving the interatrial septum is particularly difficult to treat and success rates are lower than in MRT based on the free atrial walls.24,132 Ablation of all inducible tachycardias is the accepted objective; however, the significance of non-clinicallyinducible tachycardias is not well known. Long-term recurrences can occur despite repeat ablation.133,134 Some authors describe better results by targeting areas of focal activity as possible triggers than with ablation of re-entry circuits.135
MRT can occur after surgical ‘maze’ procedures for the treatment of AF on the basis of re-established slow conduction across suture lines.136,137 Heart transplantation with atrial-to-atrial suture is practically an experimental model of flutter.5,138 Knowledge acquired by mapping and ablation-scar-related MRT should help the surgeons and electrophysiologists function as a team to devise non-arrhythmogenic incisions, avoiding surgical approaches that have proved arrhythmogenic, such as the superior transeptal approach to the LA.139
Management of Atypical Flutter/Macro-re-entrant Tachycardia
Management of atypical flutter does not differ from that of typical flutter, but the more frequent association with structural heart disease and the multiple possible mechanisms causing an atypical ECG pattern are important factors to consider before making therapeutic decisions. There is very little specific evidence regarding indications for anticoagulation in patients with atypical flutter/MRT and the same indications as in AF are generally recommended.85
When atypical flutter/MRT is poorly tolerated and is not controlled with AADs, catheter ablation should be considered. There is no set rule for catheter ablation of atypical MRT tachycardia circuits. Mapping and entrainment studies are necessary to define the focal (centrifugal spread) or MRT mechanism and localise the focal sources or the target isthmus or isthmuses. These procedures may be complicated by the induction of multiple MRT circuits that are not clinically documented. Ablation success is lower than in typical flutter and the recurrence rate is higher, especially in circuits located in the paraseptal areas.24,132–134 On the other hand, CTI-dependent flutter is a frequent finding in patients with atrial tachycardia and surgical or ablation scars.24 In cases with multiple MRT circuits, CTI ablation may make ablation success easier by stabilising the atypical MRT circuit and thus making mapping and ablation possible. In cases of atypical MRT of the RA, CTI ablation could be considered even if typical flutter is not documented in order to prevent the later appearance of typical flutter.
Prognosis in these complex cases is difficult to predict24,128,132–135 but long remissions of tachycardias can be attained in many cases of free wall RA and LA scar. Indications for ablation should be established, taking into account the underlying pathology, quality of life and limitations in functional capacity.
Postoperative Atrial Flutter
The incidence of atrial arrhythmias in the early postoperative period (days) after cardiac surgery is 20–30 %.140 This high incidence is related to inflammatory changes in the atrial myocardium,141 not unlike the experimental pericarditis animal models,142 and it may be prevented by anti-inflammatory corticosteroid treatment.143,144 AF is the most commonly reported arrhythmia but flutter can also occur in this setting,8,35,78 although its frequency in relation to AF is not clear. There are very few data on the long-term follow-up of this postoperative flutter, but the incidence of AF in such cases is reported to be around 30 %.145 If this incidence is extrapolated to flutter, it would appear reasonable to consider that in the early postoperative period after cardiac surgery flutter is an acute, one-time event in the majority of patients and ablation treatment should not be contemplated unless recurrences are documented.