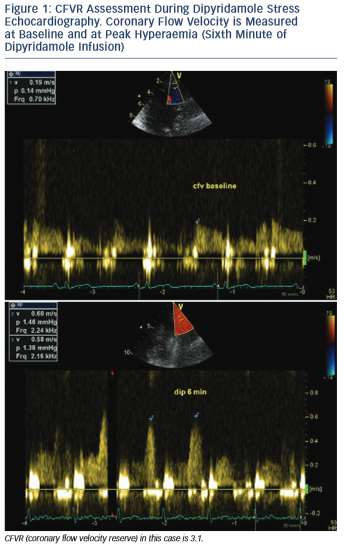
Coronary flow velocity reserve (CFVR) represents the ratio between maximal (stimulated) coronary blood flow, induced by using a coronary vasodilator, and baseline (resting) blood flow (see Figure 1). As a ratio it is a dimensionless variable. It could be measured with different tools – some of them, such as intracoronary Doppler flow wire and coronary sinus thermodilution, are invasive methods and therefore associated with certain risks, radiation exposure, increased cost and ethical considerations.1 Other methods, such as cardiac magnetic resonance imaging and cardiac nuclear imaging, are non-invasive and useful for clinical research, but with limited clinical application because they are complex, time-consuming, with limited availability and expensive.2,3
Transthoracic Doppler echocardiography (TDE) as a tool to measure CFVR has the advantages of being non-invasive, widely available, easily performed at bedside, without radiation exposure, inexpensive and not so time-consuming (mean time to complete a CFVR test is around 15 minutes; when it is combined with a cold-pressor test – see below, the duration is prolonged by 5 more minutes). However, CFVR assessment has a steep learning curve and operator experience is important. This review focuses on the technical details for CFVR assessment and major clinical applications.
Technical Details
All three coronary arteries could be visualised with TDE and CFVR could be assessed. The left anterior descending (LAD) coronary artery has been the most commonly interrogated, followed by the posterior descending artery (PDA). Technical feasibility to investigate LAD is high with more than 90 % in experienced hands4–6 and reaches nearly 100 % with the use of intravenous contrast agents.7 The feasibility of CFVR assessment in PDA is lower – in the range between 54 and 86 %.4,5,8 Left circumflex coronary artery (LCx) is most challenging of the three due to the particular anatomy of the artery and the poor resolution of the lateral wall.2
Interobserver and intraobserver variability of CFVR measurements have been assessed in various studies and both are in the range of 5 %.9,10 Intra-individual variability has also been shown to be low.10
Settings
The appropriate setting of the echo scanner is an important prerequisite for CFVR assessment. LAD is visualised either with a high-frequency transducer (4–8 MHz) or with transthoracic low-frequency probe (3.5–5 MHz) with a second harmonic capability.2,11 PDA is situated more deeply in the chest and a low frequency transducer is needed to assess coronary flow.11,12 Color Doppler pulse repetition frequency should be 15–25 cm/s, wall filters set high and pulse Doppler filters should be low. Pulse wave Doppler sample volume should be 3–4 mm.2
Proximal or Distal to a Stenosis?
The best way to assess the functional significance of a stenosis is to evaluate the coronary flow in the distal tract of the artery according to the lesion. Proximal to the stenosis, CFVR could be normal because there are usually side branches between the sampling site and the stenosis with preserved perfusion in adjacent territories. At the site of the stenosis, the flow accelerates to compensate for lumen loss.2,14
Considering the fact that CFVR is measured most commonly in the distal LAD and PDA, while the majority of relevant stenoses are located in the proximal to middle part of LAD and in the proximal right coronary artery (RCA) before the crux cordis, CFVR usually provides post-stenotic values.3
Echocardiographic Views
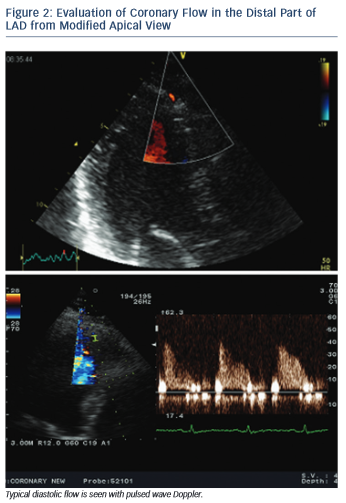
All three LAD segments (proximal, mid and distal) are visible with the new technical applications in TDE. CFVR is usually assessed in the distal and sometimes middle LAD segment. Distal LAD segment is evaluated from an apical view, somewhere between the classic two- and three-chamber view where the anterior interventricular groove runs, and near left-ventricular apex (see Figure 2). The mid- to-distal LAD segment is visualised in a modified left parasternal view with the patient in the left lateral decubitus position and the transducer moved lower and more lateral in order to visualise the anterior interventricular groove.15
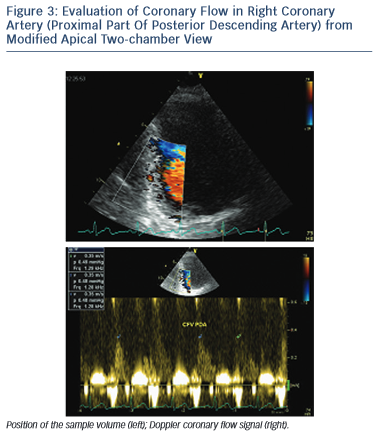
PDA is assessed from a modified apical two-chamber view showing the posterior interventricular groove and adjacent to the ostium of the coronary sinus (see Figure 3).12 The distal LCx is searched at the basal and mid-portion of left ventricular lateral wall in an apical four- chamber view.13
When the appropriate position is achieved, the respective artery is searched for using color Doppler flow mapping and predominantly diastolic signal. Blood flow velocity is measured using pulsed wave Doppler echocardiography. Angle correction is not necessary since CFVR is a ratio between baseline and hyperaemic flow velocity and is not affected by the absolute value of flow velocity. Nevertheless, angle should be kept as low as possible (below 40°).2,15
Systole or Diastole?
Coronary flow is biphasic with diastolic predominance. The blood supply to cardiac myocytes is largely diastolic due to the typical function of heart muscle – contracting in systole with generation of high intramural pressure, which impedes perfusion. Due to the translational motion of coronary arteries during the cardiac cycle it is sometimes difficult to obtain a complete Doppler signal throughout the cardiac cycle. This is not a problem, since only the diastolic flow is usually needed to assess baseline and hyperaemic coronary flow and calculate CFVR.2
Coronary flow velocities can be measured online or offline. Maximal flow velocity (averaging three cardiac cycles) at baseline and during hyperaemia is considered, although mean flow velocity could be used as well without influencing the final CFVR value, which represents the ratio between baseline and hyperaemic velocities. It should be emphasised that during administration of a vasodilating agent the probe must be kept in the same position and machine settings must not be changed compared with baseline.
Vasodilators
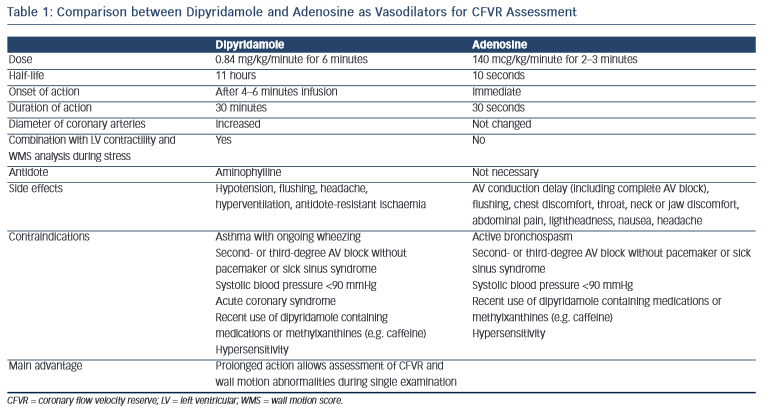
The most commonly used vasodilators are dipyridamole and adenosine. A comparison between modes of application, and advantages and disadvantages of both methods is presented in Table 1.
CFVR could also be assessed during dobutamine stress echocardiography. However, it is not widely used since dobutamine increases coronary flow via different mechanisms compared with dipyridamole and adenosine.11 Both exercise and dobutamine are submaximal stimuli for coronary flow reserve (CFR) and technically more demanding for imaging of CFVR compared with dipyridamole and adenosine.3
Non-invasive or Invasive CFVR
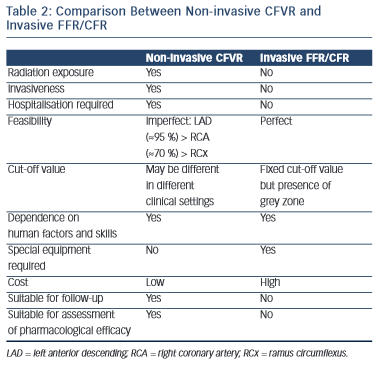
A comparison between non-invasive (with transthoracic echocardiography) CFVR and invasive (during cardiac catheterisation and coronary angiography) FFR/CFR assessment is presented in Table 2.
Learning Curve
CFVR assessment is an advanced echo tool requiring time and devotion. A detailed anatomical and technical knowledge is required in order to begin training. A period of supervision by a physician with considerable skills and experience in CFVR measurement is highly recommended. As with other techniques implicating technical skills, there is a learning curve and feasibility of CFVR measurement increases gradually in time.
Cold Pressor Test
It should be noted that both adenosine and dipyridamole induce a hyperaemic stimulus that relaxes vascular smooth muscle cells in coronary arteries in a fashion only partially dependent on endothelial function. The cold pressor test (CPT) is a well-validated, sympathetic stimulus able to induce hyperaemic vasodilation that depends totally on the endothelial release of nitric oxide (NO).16,17
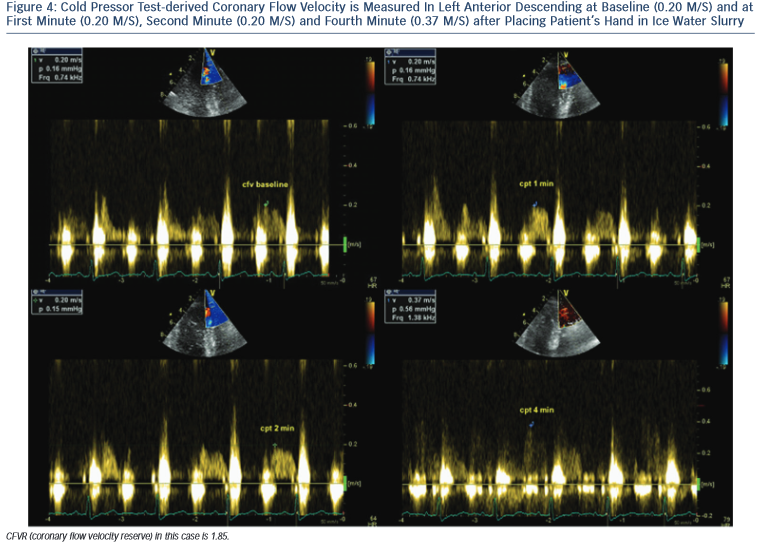
CPT is performed according to a standardised protocol,18 by placing the subject’s hand and distal part of the forearm in ice-water slurry for 3 minutes. CPT-derived CFVR is measured as the ratio between coronary diastolic peak flow velocities at rest and during maximal hyperaemia (see Figure 4).
Pitfalls
There are several possible ways to make mistakes during CFVR assessment. Errors occur more often at the beginning of the learning curve and diminish significantly as operators gain experience. Common pitfalls include loss of flow signal during investigation, mapping different coronary artery tracts during the same study, misinterpretation of coronary arteries (e.g. diagonal or intermediate branches for LAD, or recurrent distal part of LAD for PDA) or misinterpretation of wall noise or epicardial space due to mild pericardial effusion and investigating right ventricular flow.
It should be noted that CFVR as a stand-alone technique can not distinguish between microvascular and macrovascular disease – the reason for a decrease in coronary reserve could be either epicardial coronary artery stenosis, or microvascular dysfunction, or both.
Normal Values
If a normal value for CFVR should be defined, then the cut-off value of 2 must be accepted, because it has been demonstrated in various studies that CVFR <2 detects epicardial coronary artery stenosis and predicts myocardial ischaemia in the underlying territory.7,19,20 The sensitivity and specificity for the cut-off value of <2 CFVR to detect significant LAD stenosis are both more than 90 %.
In the setting of normal epicardial coronary arteries CFVR assesses coronary microcirculatory function and in this setting ‘normal’ CFVR values vary significantly according to the studied population,21–23 presence and extent of atherosclerostic risk factors,24,25 concomitant therapy,21,26 etc. Ageing also affects CFVR – baseline flow velocity increases with age, while maximal hyperaemic flow does not change and therefore CFVR value decreases with advancing age.27
Therefore in a clinical setting and in a study population a more useful way to interpret CFVR values is to compare CVFR before and after an event or therapeutic intervention, or to a control group, instead of using pre-defined cut-off values.
Clinical Application
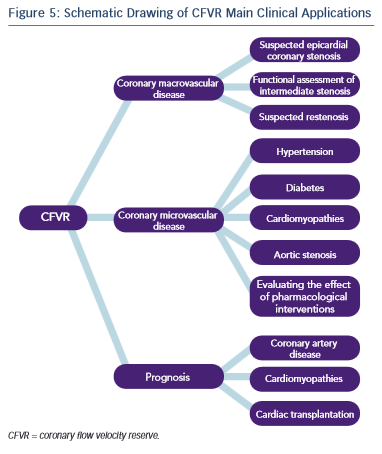
Given the physiological basis of CFVR measurement the method has two major areas of application: evaluation of epicardial coronary artery stenosis and assessing microvascular myocardial function in the absence of epicardial stenosis (see Figure 5).
CFVR could be useful as a diagnostic and prognostic tool in different clinical situations, such as the diagnosis of functionally significant coronary stenosis, evaluation of patients with intermediate coronary stenosis, follow-up after percutaneous coronary intervention (PCI), coupling left ventricular function with perfusion during stress echocardiography, evaluation of coronary microcirculation in the setting of hypertension, diabetes and other conditions, assessment of the effectiveness of certain therapeutic intervention and risk stratification in patients with dilated cardiomyopathy, after heart transplantation and other diseases.
Focusing the attention on patients with suspected or proved coronary artery disease, a practical guide to the application of CFVR is as follows:3
1.Before coronary angiography
a.Suspected epicardial coronary stenosis (CFVR combined with wall motion score).
b.Suspected microvascular abnormalities (CFVR in LAD).
2.After coronary angiography
a.Abnormal coronary angiogram – functional assessment of intermediate stenosis (CFVR combined with wall motion score).
b.Normal coronary angiogram – confirmation or exclusion of microvascular dysfunction (CFVR in LAD).
3.Follow-up after initial coronary angiogram
a.Follow-up of functional significance of intermediate stenosis (CFVR combined with wall motion score).
b.Patients with suspected restenosis (CFVR combined with wall motion score).
c.Verification of beneficial effect of pharmacological interventions (CFVR in LAD).
Coronary Artery Stenosis
Evaluation of patients with coronary stenosis in the range of 50–70 % is challenging. CFVR is a useful tool to assess the functional significance of the stenosis. When CFVR is <2 revascularisation could be safely deferred given the high negative predictive value of CFVR to detect ischaemia.20,28 The diagnostic accuracy of CFVR (adenosine) in three major coronary arteries for detecting ischaemia has been compared with FFR in a prospective study in 172 vessels of 140 patients with at least one ≥50 % stenosis in a major epicardial artery. A CFVR cut-off of 2.2 demonstrated high sensitivity and specificity to predict FFR ≤0.75.29
Percutaneous Coronary Interventions
Immediately after a PCI CFVR could be measured invasively with intracoronary Doppler. Surprisingly, however, these early (immediate) measurements have shown a high rate of impaired CFVR even in the absence of any residual angiographic stenosis.3 This could be explained by microvascular stunning due to microembolisation, thrombogenicity (thrombin release) and vasoconstriction (endothelin release), or to temporary reactive hyperaemia, which masks normal reserve. Therefore, invasive immediate-after-PCI CFVR measurement is not a reliable baseline reference value, which could serve for follow- up of patients and monitoring for restenosis. It is better to measure CFVR at least several days after PCI and here comes the role of the non-invasive, repeatable, inexpensive and accessible transthoracic Doppler echocardiography.
CFVR value <2 in LAD after PCI predicts the presence of restenosis with high sensitivity (from 78 to 89 %) and specificity (from 90 to 93 %).30–32
Using a cut-off CFVR value of 2 is useful in the setting of intermediate coronary stenosis or after PCI but a more sensitive way to follow-up the progression of an intermediate lesion or to detect restenosis is to evaluate the evolution of CFVR over time and to compare current values with a reference value established for the individual patient.2
The introduction of drug-eluting stents (DES) in the field of interventional cardiology has significantly reduced the rate of restenosis after PCI. DES, however, are associated with delayed healing, which could lead to vasodilator dysfunction and late stent thrombosis. It is of interest therefore to dispose of a reliable, repeatable, non-invasive and inexpensive method to monitor vasodilator function in this setting. In a recent small study in 24 patients with acute coronary syndrome and PCI with DES in LAD, 3 months after the index procedure CFVR measured with transthoracic Doppler echocardiography and with invasive thermodilution method showed good agreement, suggesting that the non-invasive CFVR measurement is a feasible and reliable method for assessment of vasodilator dysfunction after DES implantation33.
Microcirculatory Dysfunction
More than 20 % of patients referred for coronary angiography because of chest pain have no angiographic evidence of coronary artery stenosis. According to a recent study, however, more than 75 % of these patients have occult coronary abnormalities, mostly endothelial dysfunction and microvascular impairment.34
Microvascular dysfunction could develop before the occurrence of atherosclerotic epicardial artery involvement and it could also coexist with angiographically significant coronary artery disease. Coronary microvasculature cannot be visualised directly and CFVR represents a useful tool to assess microcirculatory function. Many risk factors and clinical conditions have been proved to be associated with microcirculatory impairment. Patients with type 2 diabetes, for example, have reduced CFVR compared with healthy controls, and diabetics with CFVR ≤2 have worse prognosis compared with those with CFVR >2, despite the fact that both groups have preserved left ventricular ejection fraction, normal wall motion score analysis during dipyridamole stress test and absence of angiographically significant coronary stenoses.35
In patients with chronic kidney disease in the absence of obstructive coronary artery disease, the presence of microvascular dysfunction, defined as CFVR <2, was associated with worse cardiovascular outcomes, independent of traditional cardiovascular risk factors.36
Stress Echocardiography
According to the European Association of Cardiovascular Imaging Expert consensus statement for performing stress echocardiography from 2008 wall motion analysis should be combined with perfusion assessment (CFVR) in order to provide dual imaging vasodilator stress echocardiography.37 Wall motion abnormalities are more specific for inducible ischaemia while perfusion changes are more sensitive and may occur in the absence of ischaemia. CFVR and wall motion analysis offer complementary information during stress echo, combining flow and function together. Wall motion abnormality is more efficient to include coronary artery disease, while a normal CFVR is more efficient to exclude it (CFVR has higher negative predictive value).
In a study of 1,660 patients with chest pain and no wall motion abnormalities at rest and during dipyridamole stress echocardiography, decreased CFVR on LAD was associated with significantly increased 4-year event rate both in women and men.38
Although some authors have reported successful application of three-vessel CFVR assessment during vasodilator stress test,28 dual imaging vasodilator stress echocardiography at present utilises LAD- only CFVR evaluation. A three-coronary approach would probably be more fruitful but it remains too technically challenging. Moreover, microvascular dysfunction, which is the mainstay of perfusion abnormalities detected with LAD CFVR measurement during stress echocardiography, is a global phenomenon and could be adequately assessed with Doppler interrogation of the distal LAD segment.
Athletes’ Heart
CFVR could be used to differentiate between physiological left ventricular hypertrophy (typical for endurance athletes) and pathological hypertrophy in the setting of hypertrophic cardiomyopathy (CMP) and hypertensive heart disease. In a group of 29 male endurance athletes CFVR has been found to be supranormal (mean value 5.9) and significantly higher compared with healthy controls despite the presence of left ventricular hypertrophy in the former group.39
Aortic Stenosis
Aortic stenosis induces a pressure overload of the left ventricle, leading eventually to concentric left ventricular remodelling and hypertrophy, and increase in left ventricular mass. In order to provide an adequate blood supply to an increased muscle mass at rest coronary arteries dilate. This baseline vasodilation leads in turn to a reduced capacity to increase coronary flow during exercise (or after pharmacological challenge with adenosine or dipyridamole) and therefore to a reduction in CFVR.
Decreased CFVR in patients with haemodynamically significant aortic stenosis in the absence of epicardial coronary artery stenosis has been repeatedly demonstrated and also the prognostic value of CFVR has been shown in this population. In the SummariZation of long-tErm prognostic siGnificance of coronary flow rEserve in special Disorders (SZEGED) study 49 aortic stenosis patients were followed-up for nearly 9 years after baseline CFVR assessment. Univariate and multivariate regression analysis showed that CFVR was an independent predictor of cardiovascular morbidity and mortality. The authors found that CFVR cut-off value of 2.13 had the highest accuracy in predicting cardiovascular outcome.40
In a larger study of 127 asymptomatic patients with moderate and severe aortic stenosis with preserved ejection fraction and without obstructive epicardial coronary disease followed-up for nearly 3 years, CFVR was shown to bear an independent prognostic significance of total mortality. A CFVR cut-off value of 1.85 had the highest accuracy in predicting death.41
After aortic valve replacement, CFVR increases together with a decrease in left ventricular mass. This has been demonstrated in a study with 39 aortic stenosis patients evaluated before and 6 months after aortic valve replacement: CFVR increased from 1.76±0.5 to 2.61±0.7, which paralleled a decrease in left ventricular mass index from 154±21 to 134±21g/m2.42
Cardiomyopathy
In patients with hypertrophic CMP CFVR is markedly lower compared with healthy controls. Abnormal CFVR values were more common in symptomatic compared with asymptomatic subjects and in those with left ventricular outflow tract obstruction. Impaired CFVR was a strong and independent predictor of outcome in hypertrophic CMP patients.43
In 132 patients with idiopathic dilated CMP with angiographically normal coronary arteries and left ventricular ejection fraction <40 % CFVR values were abnormal (<2) in nearly two-thirds of the participants and were associated with a worse prognosis during 2-year follow-up.44
Prognostic Value
Recently, low CFVR values have been shown to have prognostic significance in different clinical situations. In octogenarians (369 subjects) a reduced CFVR in LAD in the setting of a stress echo negative for wall motion abnormalities helps to risk stratify the subset at higher risk of mortality and major adverse cardiac events (MACE). The best CFVR cut-off predicting untoward cardiac events in this population was 1.93.45
In nearly 400 patients with angiographically normal coronary arteries, normal wall motion during stress and chest pain (microvascular angina), those with CFVR value >2 showed significantly better outcome during almost 5-year follow-up compared with the group with impaired CFVR.46 In more than 300 subjects with known or suspected coronary artery disease but with negative stress echocardiography (by wall motion criteria), CFVR ≤1.92 with dipyridamole is an independent predictor of worse prognosis.47 The 3-year event-free survival is 68 % versus 98 % in groups with reduced and preserved CFVR, respectively. In the setting of intermediate coronary stenosis (50–70 %) a CFVR value >2 predicts good prognosis during a mean follow-up of 15 months.48
Reduced CFVR (<2 with dipyridamole) is an independent predictor of unfavourable outcome in patients with non-ischaemic dilated cardiomyopathy during 22 months of follow-up.23 After heart transplantation CFVR <2.6, using adenosine, is the main independent predictor of MACE for a period of almost 2 years49.
In the largest study so far on CFVR assessment – 4,313 patients with known or suspected coronary artery disease – 4-year mortality was markedly higher in subjects with CFVR ≤2 than in those with CFR >2, both considering the group with ischaemia and the group without ischaemia at stress echocardiography. CFVR was also an independent predictor of mortality along with inducible ischaemia during stress echocardiography, resting wall motion score, left bundle branch block, age, male gender and diabetes mellitus.50
Clinical Utilisation
Considering the multiple areas of clinical application of CFVR measurement, the reasonable question arises why CFVR has not become a routine diagnostic test and a standard part of non- invasive echocardiographic assessment in patients suspected of or at increased risk of epicardial or microvascular coronary artery disease? A meaningful explanation for the lack of more widespread utilisation of CFVR measurement is that this method requires considerable anatomical and technological knowledge. A specific setting of the echo scanner is a prerequisite in order to be able to assess coronary flow. Also, there is a learning curve and initially a lot of time has to be dedicated to technical aspects and to acquiring necessary skills.
Conclusions
Transthoracic Doppler echocardiography is a reliable way to study CFVR with the advantage of being non-invasive, available and inexpensive. It is used to measure flow reserve in both stenosed and normal epicardial coronary arteries (every one of the three major coronary arteries can be evaluated although most of the experience is with CFVR measurement in LAD). In the presence of coronary artery stenosis CFVR is useful to detect a significant stenosis, to assess the functional significance of intermediate stenosis and to monitor for restenosis during follow-up after coronary revascularisation. In patients with anatomically normal epicardial coronary arteries impaired CFVR is a marker of microvascular dysfunction in different clinical settings.