Abstract
Since William James (1890) first distinguished primary from secondary memory, equivalent to short- and long-term memory, respectively, it has been assumed that short-term memory processes are in charge of cognition while long-term memory is being consolidated. From those days a major question has been whether short-term memory is merely a initial phase of long-term memory, or a separate phenomena. Recent experiments have shown that many treatments with specific molecular actions given into the hippocampus and related brain areas after one-trial avoidance learning can effectively cancel short-term memory without affecting long-term memory formation. This shows that short-term memory and long-term memory involve separate mechanisms and are independently processed. Other treatments, however, influence both memory types similarly, suggesting links between both at the receptor and at the post-receptor level, which should not be surprising as they both deal with nearly the same sensorimotor representations. This review examines recent advances in short- and long-term memory mechanisms based on the effect of intra-hippocampal infusion of drugs acting upon neurotransmitter and signal transduction systems on both memory types.
Short-term memory; long-term memory; hippocampus; PKA; PKC; MAPK
Short- and Long-term memory: Differential involvement of neurotransmitter systems and signal transduction cascades* ** This paper is dedicated to the memory of Prof. Carlos Chagas Filho. * Invited paper Member of the Academia Brasileira de Ciências Correspondence to: Dr. Iván Izquierdo E-mail: izquier@zaz.com.br
MÔNICA R.M. VIANNA1, LUCIANA A. IZQUIERDO1, DANIELA M. BARROS1,
ROGER WALZ1, JORGE H. MEDINA2 and IVÁN IZQUIERDO1
1Centro de Memoria, Departamento de Bioquímica, ICBS,
Universidade Federal do Rio Grande do Sul - 90035-003 Porto Alegre, RS, Brasil
2Instituto de Biología Celular y Neurociencia "Eduardo De Robertis'',
Facultad de Medicina, Universidad de Buenos Aires, Buenos Aires, Argentina.
Manuscript received on May 16, 2000; accepted for publication on May 19, 2000;
contributed by IVÁN IZQUIERDO** ** This paper is dedicated to the memory of Prof. Carlos Chagas Filho. * Invited paper Member of the Academia Brasileira de Ciências Correspondence to: Dr. Iván Izquierdo E-mail: izquier@zaz.com.br
ABSTRACT
Since William James (1890) first distinguished primary from secondary memory, equivalent to short- and long-term memory, respectively, it has been assumed that short-term memory processes are in charge of cognition while long-term memory is being consolidated. From those days a major question has been whether short-term memory is merely a initial phase of long-term memory, or a separate phenomena. Recent experiments have shown that many treatments with specific molecular actions given into the hippocampus and related brain areas after one-trial avoidance learning can effectively cancel short-term memory without affecting long-term memory formation. This shows that short-term memory and long-term memory involve separate mechanisms and are independently processed. Other treatments, however, influence both memory types similarly, suggesting links between both at the receptor and at the post-receptor level, which should not be surprising as they both deal with nearly the same sensorimotor representations. This review examines recent advances in short- and long-term memory mechanisms based on the effect of intra-hippocampal infusion of drugs acting upon neurotransmitter and signal transduction systems on both memory types.
Key words: Short-term memory, long-term memory, hippocampus, PKA, PKC, MAPK.
INTRODUCTION
Declarative memories are not immediately established as long-term memories (LTMs), this process takes 3 to 6 hours and involves a sequence of specific molecular processes in the CA1 area of the hippocampus and its connections (see Izquierdo & Medina 1997). In 1890, William James proposed that while LTM formation is taking place, one or more short-term memory (STM) systems are in charge of cognition. This concept was further developed by others (Carew 1996, Gold & McGaugh 1975, Mansuy et al. 1998, Markowitsch 1997, McGaugh 1966), but a key question remained unanswered until recently: is STM just a step towards LTM, or are both separate processes (Gold & McGaugh 1975, Izquierdo 1989, Izquierdo & Medina 1998, Squire 1992)? To answer this question undoubtedly it would be necessary to demonstrate that STM can be suppressed without affecting LTM of the same learning experience in the same animal, or that this is utterly impossible (Izquierdo 1989, Izquierdo et al. 1998a, 1999).
Here we review the effect on STM and LTM of drugs known to affect specific steps of LTM formation when given into CA1 region of rats hippocampal area (Ardenghi et al. 1997, Bernabeu et al. 1997a, b, Bevilaqua et al. 1997, Cammarota et al. 1997, Izquierdo & Medina 1998, Izquierdo & Medina 1995). The drugs used exert specific effects upon neurotransmitter receptors or enzymes activities crucial to plastic processes (Bernabeu et al. 1997a, Cammarota et al. 1998, Izquierdo & Medina 1997, Izquierdo & Medina 1995).
MEMORY TYPES
Memories are classified according to their content (declarative or explicit, procedural or implicit) (Squire 1992, Markowitsch 1997), according to their duration (STM, LTM) (Fuster 1998, Markowitsch 1997), and according to their nature: archival (STM, LTM) as opposed to transient, moment-to-moment (WM) (Goldman-Rakic 1992, 1996). The present article deals with STM and LTM of one-trial inhibitory or passive avoidance (Gold 1986, Izquierdo & Medina 1997). This might be viewed as an explicit memory, to the point that terms such as ``declarative'' or ``explicit'' can be applied to experiments using rodents (Bures 1998). Putative subdivisions of STM and LTM (see Markowitsch 1997 and Squire 1992) will be ignored, inasmuch as they are irrelevant to the findings discussed here, and there is no tangible biological basis to substantiate any such subdivision (Izquierdo et al. 1999). Here, therefore, we will restrict ourselves to McGaugh's (1968) concept of ``three memory trace systems: one for immediate memory, one for short-term memory (which develops in a few seconds or minutes and lasts for several hours), and one which consolidates slowly and is relatively permanent'' (see also McGaugh 2000)
SHORT- AND LONG-TERM MEMORY
Early attempts to extricate STM from LTM (see Cherkin 1997, Gold & McGaugh 1975, McGaugh 1966, Sara 1974) failed because the treatments used to block memory of one or the other type (protein synthesis inhibitors, electroconvulsive shock, hypoxia, etc.) were inappropriate and severely affected subjects performance. Over the years, many treatments were found to preserve STM but to cancel LTM (eg, Bourtchouladze et al. 1994, Yin & Tully 1996). These experiments are uninformative as to whether the former is a step towards the latter or not. Those who have studied short-term potentiation (STP) and long-term potentiation (LTP) have confronted a similar problem. Treatments that spare the former but block the latter are ambiguous as to whether STP and LTP are separate or sequential.
In 1993, Emptage & Carew found that the non-specific 5HT antagonist cyproheptadine blocks the short- (2-6 min) but not the long-lasting (24 h) facilitation of a monosynap-tic response in Aplysia induced by 5HT. This was the first demonstration of a mechanistic separation between short- and long-lasting forms of plasticity. We decided to extend this finding to STM and LTM of one-trial step-down inhibitory avoidance in rats, using pharmacological approaches to interfere with cellular mechanisms known to be relevant to plastic events involved in learning and memory.
METHODOLOGICAL CONCERNS
Recent data have substantially enhanced understanding of the molecular basis of LTM formation of the one-trial avoidance task in rats (Izquierdo & Medina 1995, Izquierdo & Medina 1997) and other forms of plasticity (Yin & Tully 1996, Colley & Routtenberg 1993). Thus, the question of the relation between STM and LTM can now be posed in cellular and molecular terms.
There is no direct way to extricate the biochemistry of the first 3-6 h of LTM from that of STM, since both occur simultaneously. Lesions, surgical or genetic, are particularly inadequate for such an endeavor since both their effect and the recovery from their effect are very long lasting and will contaminate any memory studied after them, be it short or long (Izquierdo & Medina 1998). The only way to address this question is to use drugs known to affect those biochemical events and whose action is rapidly reversible. We used immediate and prolonged posttraining infusions into definite brain regions of drugs of known biochemical effects whose influence on LTM had been well-established (Izquierdo & Medina 1995, Izquierdo & Medina 1997).
In our experiments we did not measure STM at periods shorter than 1.5 h, not only in order to avoid a superposition with WM, but also to avoid confounds by the lingering presence of the drug at the infusion sites or by the infusion procedure itself. Using similar infusion procedures (0.5  l given over 30 sec), drugs take between 0.5 to 1 h to diffuse away from the infusion sites (Martin 1991, Walz et al. 2000a).
l given over 30 sec), drugs take between 0.5 to 1 h to diffuse away from the infusion sites (Martin 1991, Walz et al. 2000a).
We chose to use a one-trial step-down inhibitory (passive) avoidance task in rats for several reasons. First, its rapid acquisition facilitates the analysis of the time of occurrence of post-training events (Gold 1996, Izquierdo & Medina 1995). Second, it depends on the integrated activity of a well-studied neural circuit, i.e., the hippocampal CA1 region, the entorhinal cortex and the posterior parietal cortex (Ardenghi et al. 1997, Izquierdo et al. 1997) modulated early on by the amygdala and the medial septum (Bianchin et al. 1999, Cahill & McGaugh 1998, Izquierdo et al. 1992). Third, it is the task whose pharmacology and molecular basis has been most extensively studied, particularly in CA1 and the entorhinal cortex (Izquierdo & Medina 1995, Izquierdo & Medina 1997). Fourth, years of work have established that using a 0.3-0.4 mA training shock one can obtain retention test latencies far enough from a floor or a ceiling, and therefore easily amenable to the comparative analysis of stimulant and depressant posttraining treatments (see Izquierdo & Medina 1997, Izquierdo et al. 1999). Finally, it has been reliably shown to depend on the actual inhibition of one particular response (stepping down with the four paws on the grid) and not of others (rearing, exploration, sticking the head out, placing just the forepaws on the grid).
Our methodology consisted in testing animals twice: first at 1.5 h from training, in order to measure STM, and then again at 24 h, in order to measure LTM (see Izquierdo et al. 1999). One concern was whether testing the animals twice might alter LTM either by extinction or by a reminder effect. This was ruled out by two facts: First, there were no significant differences in control groups between STM and LTM performance in any of our studies (Figures 1-4). Second, it was recently shown that repeated testing over the first 6 h after training does not lead to extinction, whereas repeated testing between 9 and 96 h does (Medina et al. 1999).
EFFECT OF TREATMENTS GIVEN INTO THE HIPPOCAMPUS ON SHORT- AND LONG-TERM MEMORY
The drugs used, their doses and the results obtained on STM and LTM are shown in Figures 1 - 4. The whole data is published elsewhere (Izquierdo et al. 1998a, b, c, 1999, 2000, Vianna et al. 1999, 2000a, b, Walz et al. 1999). Results shown in Figure 1 demonstrate the involvement of the different glutamatergic receptor subtypes, and of the  , colinergic muscarinic, dopaminergic D1 and of the serotonergic
, colinergic muscarinic, dopaminergic D1 and of the serotonergic  hippocampal receptors on the early events of STM and LTM processsing. The subsequent data (Figures 2-4) demonstrate the selective contribution of different signal transduction cascades to the prolonged postraining period of STM and LTM simultaneous processing.
hippocampal receptors on the early events of STM and LTM processsing. The subsequent data (Figures 2-4) demonstrate the selective contribution of different signal transduction cascades to the prolonged postraining period of STM and LTM simultaneous processing.
IMMEDIATE INVOLVEMENT OF NEUROTRANSMITTER SYSTEMS
The drugs used were: the glutamate NMDA receptor antagonist, AP5; the glutamate AMPA receptor blocker, CNQX; the glutamate metabotropic receptor antagonist, MCPG; the GABAA receptor agonist, muscimol; the muscarinic receptor antagonist, scopolamine; the dopamine  receptor agonist, SKF38393; the
receptor agonist, SKF38393; the  antagonist, SCH23390; noradrenaline; the
antagonist, SCH23390; noradrenaline; the  -adrenoceptor antagonist, timolol; the
-adrenoceptor antagonist, timolol; the  receptor agonist, 8-HO-DPAT; the
receptor agonist, 8-HO-DPAT; the  antagonist, NAN-190.
antagonist, NAN-190.
Several of the treatments blocked both STM and LTM: AP5, CNQX, MCPG, muscimol and scopolamine. This suggests a link between STM and LTM at the glutamate receptor level and at the level of modulation by GABAA and muscarinic receptors.
Interestingly, some treatments selectively affected one but not the other memory type. When given immediately after training, SCH23390 enhanced whereas SKF38393 and 8-HO-DPAT cancelled STM retention without affecting LTM. Oppositely, noradrenaline facilitated LTM retention without affecting STM. These results, specially those obtained with the infusion of  and
and  receptor agonists, clearly demonstrates the independence of both memory types.
receptor agonists, clearly demonstrates the independence of both memory types.
THE PKA SIGNAL TRANSDUCTION CASCADE
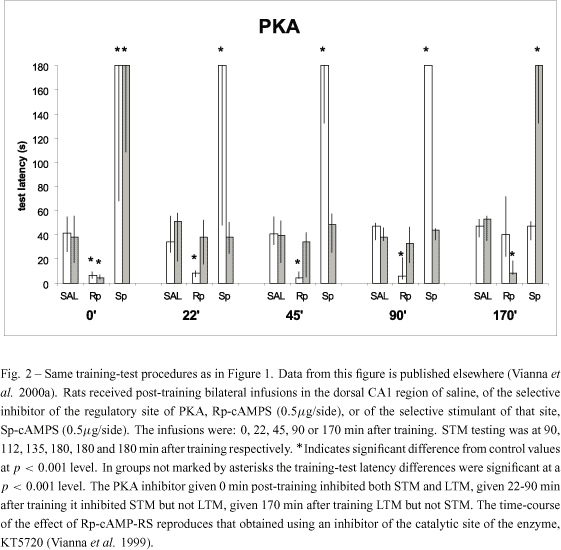
We used drugs known to specifically influence AMPc-dependent protein kinase (PKA) activity: the inhibitor of the regulatory subunit of PKA, Rp-cAMPS, and the selective activator of that subunit, Sp-cAMPS. Infusion of Rp-cAMPS in CA1 at 0 or 170 min after training cancelled LTM, when the drugs were given 22, 45 or 90 min posttraining, they blocked STM but not LTM. The opposite effect was obtained when the specific stimulator of the regulatory subunit of PKA, SpcAMPS is used: when given into CA1 0 or 170 min posttraining it enhanced LTM, when given 22, 45 or 90 min posttraining, it enhanced STM selectively (Figure 2).
THE PKC SIGNAL TRANSDUCTION CASCADE
To measure the involvement of calcium-dependent protein kinase (PKC) and the relative contribution of different PKC isoforms we used two inhibitors: one selective to the calcium-dependent isoforms  and
and  I, Gö 6976, and other unspecific as to PKC isoforms, Gö 7874. As shown in Figure 3, STM was suppressed by Gö 6976 given into the CA1 region of the hippocampus 10 min before or 50 min after training. The two compounds given 10 min before, or 50 or 110 min after training canceled LTM.
I, Gö 6976, and other unspecific as to PKC isoforms, Gö 7874. As shown in Figure 3, STM was suppressed by Gö 6976 given into the CA1 region of the hippocampus 10 min before or 50 min after training. The two compounds given 10 min before, or 50 or 110 min after training canceled LTM.
THE MAPK SIGNAL TRANSDUCTION CASCADE
Finally, to evaluate the contribution of the mitogen-activated protein kinase (MAPK) signalling pathway we used the selective inhibitor of MAPKK (mitogen activated protein kinase kinase), an upstream activator of MAPK, PD098059. As shown in figure 4, STM was cancelled when PD098059 was given immediatelly after training whereas LTM was sensitive to the inhibitor only when administration occurred three hours later.
NEUROBIOLOGICAL SEPARATION OF SHORT- AND
LONG- TERM MEMORY
As shown in Figures 1-4, we were indeed able to replicate and considerably extend the separation of short- and long-term plastic events demonstrated by Emptage and Carew (1993). Our results clearly demonstrate that STM and LTM are in a great degree independent phenomena, recruiting cellular and molecular events in a separate manner. Several of the neurotransmitter systems and signal transduction cascades studied were demonstrated to be selectively involved in STM and LTM processing, contributing at diferent time-windows of the posttraining period. Moreover, the abolishment of STM without interference on LTM retention by several drugs definitively points to the independence of both memory types.
Of course, in addition, there are links between the mechanisms of STM and of LTM at the receptor and at the post-receptor level. The links are to be expected, after all, both STM and LTM do deal with the same basic sensorimotor representation. Frey and Morris (1998) have suggested various possible mechanisms of 'synaptic tagging' in order to explain the links between STP and LTP, or between the very early (0 min) and the much later (3-6 h) molecular events that determine persistence of the plastic change over long periods of time. The persistence of LTP or LTM for more than 3-6 h requires gene activation and protein synthesis, which distinguishes it from STP or STM (Bernabeu et al. 1997a, Frey & Morris 1998, Izquierdo & Medina 1997, Quevedo et al. 1999).
INKLINGS INTO THE BIOCHEMISTRY OF SHORT - TERM MEMORY
Several of the treatments provide hints on a separation of the biochemistry of STM and that of LTM, particularly in CA1. STM, like LTM (Izquierdo & Medina 1995, Izquierdo & Medina 1997), is dependent immediately after training on the integrity of AMPA, NMDA and metabotropic glutamate receptors, and on their presumable modulation by cholinergic muscarinic,  -noradrenergic (stimulant) and
-noradrenergic (stimulant) and  receptors (inhibitory).
receptors (inhibitory).
PKA clearly has separate influences on the two memory types. It is in charge of STM between 0 and 90 min after training, and it is in charge of LTM at 0 and again at 180 min after training, but not in the period between these two peaks (Vianna et al. 1999, 2000a, b). The role of PKA in LTM formation involves phosphorylation of the nuclear transcription factor  (Bernabeu et al. 1997a) twice: first immediately, and again 3-6 h after training. There are peaks of PKA activity and
(Bernabeu et al. 1997a) twice: first immediately, and again 3-6 h after training. There are peaks of PKA activity and  in CA1 at both periods (Bernabeu et al. 1997a, Vianna et al. 2000a), and both are necessary for LTM formation (Bernabeu et al. 1997a, Vianna et al. 1999, 2000a). The role of PKA in STM presumably uses other substrates, inasmuch as
in CA1 at both periods (Bernabeu et al. 1997a, Vianna et al. 2000a), and both are necessary for LTM formation (Bernabeu et al. 1997a, Vianna et al. 1999, 2000a). The role of PKA in STM presumably uses other substrates, inasmuch as  levels are low between those two peaks (Bernabeu et al. 1997a), while STM is highly dependent on PKA activity between 22 and 90 min after acquisition (Vianna et al. 1999, 2000a) (Figure 2).
levels are low between those two peaks (Bernabeu et al. 1997a), while STM is highly dependent on PKA activity between 22 and 90 min after acquisition (Vianna et al. 1999, 2000a) (Figure 2).
Results obtained with the PKC inhibitors extend the STM and LTM separation, demonstrating it occurs even at the isoform level. Whereas LTM apparently does not discriminate among the PKC isoforms, depending on their activity during almost two hours after training, STM selectively recruits the  and
and  I isoforms in a more restricted time-window (Figure 3). The findings on the effect of the two PKC inhibitors on LTM formation are very similar in nature and time course to those previously reported for the generic PKC inhibitors staurosporin and CGP 41231 (Jerusalinsky et al. 1994). This also correlates with the report by Paratcha et al. (2000) showing a specific learning-induced increase of the activity of
I isoforms in a more restricted time-window (Figure 3). The findings on the effect of the two PKC inhibitors on LTM formation are very similar in nature and time course to those previously reported for the generic PKC inhibitors staurosporin and CGP 41231 (Jerusalinsky et al. 1994). This also correlates with the report by Paratcha et al. (2000) showing a specific learning-induced increase of the activity of  I-PKC within the first few min after inhibitory avoidance training. The findings on STM may also agree with those of Bourtchouladze et al. (1990) in which they described an amnesic effect of the unspecific PKC inhibitors mellitin and H7 on memory of inhibitory avoidance in the chick measured 3 h after training.
I-PKC within the first few min after inhibitory avoidance training. The findings on STM may also agree with those of Bourtchouladze et al. (1990) in which they described an amnesic effect of the unspecific PKC inhibitors mellitin and H7 on memory of inhibitory avoidance in the chick measured 3 h after training.
Finally, the separation on STM and LTM also occurs in relation to the MAPK signaling pathway (Figure 4). This pathway, of which MAPKK is part, probably plays a rather complex regulatory role in plastic events, since it is linked at various different levels with the PKC, CaMKII and PKA cascades (Bhalla & Iyengar 1999, Lisman & Fallon 1999), all of which are crucial for LTP and LTM (Izquierdo & Medina 1997). As recently reported inhibitory avoidance LTM for depends on the activation of hippocampal MAPKK on the late period (3-6 h) of memory consolidation (Walz et al. 2000a, b) whereas it is important in the induction of STM (Walz et al. 1999).
Taken together these biochemical characteristics point to similarities between STM and STP (Bliss & Collingridge 1993), as well as, once more (Izquierdo & Medina 1995, 1997), between LTP and LTM in CA1. It is tempting to suggest that some substrate of the studied kinases may be involved in the tagging of synapses during the early phase of memory formation in CA1, during which STM runs its full course (Vianna et al. 1999, 2000a, b, Walz et al. 1999), as has indeed been suggested for the early phase of LTP, during which STP runs it course (Frey & Morris 1998).
FINAL COMMENTS
The results not only show that the mechanisms of STM and LTM are essentially distinct, but also, suggest links between STM and LTM in CA1 both at the receptor level and at the post-receptor level: i.e., in signal transduction cascades known to be related to glutamate receptor stimulation (Vianna et al. 1999, 2000a, b, Walz et al. 1999).
The relative importance or intervention of one or other link, or one or other modulatory mechanism, would be expected to vary with the nature of the task, and with the relation of each task to others (Izquierdo 1989, Medina et al. 1999) or to other on-going physiological events (Morris 1998). Therefore, it might be judicious at this stage to refrain from postulating theoretical connections or disconnections among memory types. Further research will no doubt contribute to this, and will eventually find out the extent to which some or all of the many hypothetical constructs on cognition (Gold 1986, McGaugh 1968, Squire 1992) are right or wrong. The popular concept that STM is just a passageway to LTM certainly is wrong, as is the idea that WM may in any way constitute a sort of STM.
At this stage it is safe to say that STM and LTM pertain to and are regulated by separate subsystems of the brain, which belong in some cases to the same and in others to different brain structures (see Izquierdo et al. 1999), and involve a great variety of molecular mechanisms at the receptor and post-receptor level, some of which may be linked. This fits with modern concepts of memory organisation (Fuster 1998, Izquierdo & Medina 1997, Izquierdo et al. 1997), which supersede old phrenological concepts based on lesion studies (i.e., ``hippocampal'' as opposed to, say ``amygdala-dependent'' tasks, see references (Fuster 1998). All types of memory depend on the integrated activity of various brain sites and involve more than one receptor or post-receptor mechanism (Izquierdo & Medina 1997, Izquierdo et al. 1999, 2000).
From a biological standpoint, it clearly sounds reasonable to search for integrative mechanisms for the various memory types among those proposed for ``synaptic tagging'' (Frey & Morris 1998), looking perhaps very particularly at substrates of the studied kinases. From a clinical point of view, the monoaminergic pathways that regulate STM and LTM (Ardenghi et al. 1997, Bevilacqua et al. 1997, Izquierdo et al. 1998c) may be the most interesting to examine. All these pathways are physiologically related to cAMP/PKA-mediated signaling in CA1, the entorhinal cortex and the parietal cortex (Ardenghi et al. 1997, Bevilaqua et al. 1997, Bernabeu et al. 1997a).
- ARDENGHI P, BARROS D, IZQUIERDO LA, BEVILAQUA L, SCHRÖDER N, QUEVEDO J, RODRIGUES C, MADRUGA M, MEDINA JH & IZQUIERDO I. 1997. Late and prolonged memory modulation in entorhinal and parietal cortex by drugs acting on the cAMP/protein kinase A signalling pathway. Behav Pharmacol 8: 745-751.
- BERNABEU R, BEVILAQUA L, ARDENGHI P, BROMBERG E, SCHMITZ PK, BIANCHIN M, IZQUIERDO I & MEDINA JH. 1997a. Involvement of hippocampal D1/D5 receptor - cAMP signaling pathways in a late memory consolidation phase of an aversively-motivated task in rats, Proc Natl Acad Sci USA 94: 7041-7046.
- BERNABEU R, SCHRÖDER N, QUEVEDO J, CAMMAROTA M, IZQUIERDO I & MEDINA JH. 1997b. Further evidence for the involvement of a hippocampal Cgmp/Cgmp-dependent protein kinase cascade in memory consolidation, NeuroReport 8: 2221-2224.
- BEVILAQUA L, ARDENGHI P, SCHRÖDER N, BROMBERG E, SCHMITZ PK, SCHAEFFER E, QUEVEDO J, BIANCHIN M, WALZ R, MEDINA JH & IZQUIERDO I. 1997. Drugs acting upon the protein kinase A/CREB pathway modulate memory consolidation when given late after training into rat hippocampus but not amygdala. Behav Pharmacol 8: 331-338.
- BHALLA M & IYENGAR R. 1999. Emergent properties of networks of biological signalling pathways. Science 283: 381-387.
- BIANCHIN M, MELLO E SOUZA T, MEDINA JH & IZQUIERDO I. 1999. The amygdala is involved in the modulation of long-term memory but not in working or short-term memory. Neurobiol Learn Mem 71: 127-31.
- BLISS TVP & COLLINGRIDGE GR. 1993. A synaptic model of memory long-term potentiation. Nature 361: 31-39.
- BOURTCHOULADZE R, POTTER J & ROSE SPR. 1990. Memory formation in the chick depends on memory bound protein kinase C. Brain Res 535: 131-138.
- BOURTCHOULADZE R, FRENGUELLI B, CIOFFI FD, SCHULTZ G & SILVA AJ. 1994. Deficient long-term memory in mice with a targeted mutation of the cAMP-responsive element binding protein. Cell 79: 59-68.
- BURES J. 1998. How declarative can be a rat's memory? Proc IV Europ Conf: Neural Mechanisms of Learning and Memory, SJ SARA & Y DUDAI (Chairpersons), Acqua-fredda di Maratea, 5 p.
- CAHILL L & MCGAUGH JL. 1998. Mechanisms of emotional arousal and lasting declarative memory. Trends in Neurosci 21: 294-249.
- CAMMAROTA M, PARATCHA G, LEVI DE STEIN M, BERNABEU R, IZQUIERDO I & MEDINA JH. 1997. B-50/GAP-43 Phosphorylation and PKC activity are increased in rat hippocampal synaptosomal membranes after an inhibitory avoidance training. Neurochem Res 22: 499-505.
- CAMMAROTA M, BERNABEU R, LEVI DE STEIN M, IZQUIERDO I & MEDINA JH. 1998. Learning-specific, time-dependent increases in hippocampal Ca2+ / calmodulin-dependent protein kinase II activity and AMPA GluR1 subunit immunoreactivity. Europ J Neurosci 10: 2669-2676.
- CAREW TJ. 1996. Molecular enhancement of memory formation. Neuron 16: 5-8.
- CHERKIN A. 1997. Kinetics of memory consolidation: role of amnesic treatment parameters. Proc Natl Acad Sci USA 63: 1094-1101.
- COLLEY PA & ROUTTENBERG A. 1993. Long-term potentiation as a synaptic dialogue. Brain Res Rev 18: 115-122.
- EMPTAGE NJ & CAREW TJ. 1993. Long-term facilitation in the absence of short-term facilitation in Aplysia neurons, Science 262: 253-256.
- FREY U & MORRIS GM. 1998. Synaptic tagging: implications for late maintenance of hippocampal long-term potentiation. Trends Neurosci 21: 181-187.
- FUSTER JM. 1998. Distributed memory for both short- and long-term. Neurobiol Learn Mem 70: 268-274.
- GOLD PE. 1986. The use of inhibitory avoidance training in studies of modulation of memory storage. Behav neural Biol 46: 87-98.
- GOLD PE & MCGAUGH JL. 1975. A single-trace, two-process view of memory storage processes. In: D DEUTSCH & JA DEUTSCH, (Eds), Short-term Memory, Academic Press, New York, 1975. 355-378 p.
- GOLDMAN-RAKIC P. 1992. Prefrontal cortical dysfunction in schixophrenia: the relevance of working memory. In: BJ CARROLL & JE BARRETT (Eds), Psychopathology and the Brain, Raven Press, New York. 1-23 p.
- GOLDMAN-RAKIC P. 1996. Regional and cellular fractionation of working memory. Proc Natl Acad Sci USA, 93: 13473-13480.
- IZQUIERDO I. 1989. Different forms of posttraining memory processing, Behav Neural Biol 51: 171-202.
- IZQUIERDO I & MEDINA JH. 1995. Correlation between the pharmacology of long-term potentiation and the pharmacology of memory, Neurobiol Learn Mem 63: 19-32.
- IZQUIERDO I & MEDINA JH. 1997. Memory formation: the sequence of biochemical events in the hippocampus and its connections to activity in other brain structures, Neurobiol Learn Mem 68: 285-316.
- IZQUIERDO I & MEDINA JH. 1998. On brain lesions, the milkman and Sigmunda, Trends Neurosci 21: 423-426.
- IZQUIERDO I, DA CUNHA C, ROSAT R, FERREIRA MBC, JERUSALINSKY D & MEDINA JH. 1992. Neurotransmitter receptors involved in memory processing by the amygdala, medial septum and hippocampus of rats. Behav Neural Biol 58: 16-25.
- IZQUIERDO I, QUILFELDT J, ZANATTA MS, QUEVEDO J, SCHAEFFER E, SCHMITZ PK & MEDINA JH. 1997. Sequential role of hippocampus, amygdala, entorhinal and pariental cortex in formation and retrieval of memory for inhibitory avoidance in rats, Eur J Neurosci 9: 786-793.
- IZQUIERDO I, BARROS DM, MELLO E SOUZA T, DE SOUZA MM, IZQUIERDO LA & MEDINA JH. 1998a. Mechanisms for memory types differ, Nature 393: 635-636.
- IZQUIERDO I, IZQUIERDO LA, BARROS DM, MELLO E SOUZA T, DE SOUZA MM, QUEVEDO J, RODRIGUES C, SANT'ANNA MK, MADRUGA M & MEDINA JH. 1998b. Differential involvement of cortical receptor mechanisms in working, short- and long-term memory, Behav Pharmacol 9: 421-427.
- IZQUIERDO I, MEDINA JH, IZQUIERDO LA, BARROS DM, DE SOUZA M & MELLO E SOUZA T. 1998c. Short- and long-term memory are differentially regulated by mono-aminergic systems in the rat brain. Neurobiol Learn Mem 69: 219-224.
- IZQUIERDO I, MEDINA JH, VIANNA MRM, IZQUIERDO LA & BARROS DM. 1999. Separate mechanisms for short- and long term memory. Behav Brain Res 103: 1-11.
- IZQUIERDO I, VIANNA MRM, BARROS DM, MELLO E SOUZA T, ARDENGHI P, SANT'ANNA MK, RODRIGUES C, MEDINA JH, IZQUIERDO I. 2000. Short- and long-term memory are differentially affected by metabolic inhibitors given into hippocampus and entorhinal cortex. Neurobiol Learn Mem 73: 141-149.
- JAMES W. 1890. The Principles of Psychology, New York, Holt.
- JERUSALINSKY D, QUILLFELDT JA, WALZ R, DA SILVA R, MEDINA JH & IZQUIERDO I. 1994. Post-training intrahippocampal infusion of PKC inhibitors causes retrograde manesia in rats. Behav Neural Biol 61: 107-109.
- LISMAN JE & FALLON JR. 1999. What maintain memories? Science 283: 339-347.
- MANSUY IM, MAYFORD M, JACOB B, KANDEL ER & BACH ME. 1998. Restricted and regulated overexpression reveals calcineurin as a key component in the transition from short-term to long-term memory. Cell 92: 39-49.
- MARKOWITSCH HJ. 1997. Varieties of memory systems, structures, mechanisms of disturbance. Neurol Psychiat Brain Res 2: 49-68.
- MARTIN JH. 1991. Autoradiographic estimation of the extent of reversible inactivation produced by microinjection of lidocaine and muscimol in the rat. Neurosci Lett 127: 160-164.
- MCGAUGH JL. 1966. Time-dependent processes in memory storage. Science 153: 1351-1359.
- MCGAUGH JL. 1968. A multi-trace view of memory storage processes. Accademia Nazionale dei Lincei 109: 13-28.
- MCGAUGH JL. 2000. Memory- a Century of Consolidation, Science 287: 248-251.
- MEDINA JH, SCHRODER N & IZQUIERDO I. 1999. Behavioural differences between short- and long-term memory. Rev Argent Neurosci 3: 10-15.
- MORRIS RGM. 1998. Down with novelty. Nature 394: 834-835.
- PARATCHA G, FURMAN M, BEVILAQUA L, CAMMAROTA M, VIANNA MRM, LEVI DE STEIN M, IZQUIERDO I & MEDINA JH. 2000. Involvement of hippocampal PKCbI isoform in the early phase of memory formation. Brain Res 855: 199-205.
- QUEVEDO J, VIANNA MRM, DE PARIS F, IZQUIERDO I & ROSE SPR. 1999. Two time windows of anisomycin-induced amnesia for inhibitory avoidance training in rats: protection from amnesia by pretraining but not pre-exposure to the task apparatus. Learn Mem 6: 600-607.
- SARA SJ. 1974. Delayed development of amnestic behavior after hypoxia. Physiol Behav 13: 689-696.
- SQUIRE LR. 1992. Memory and the hippocampus: a synthesis of findings with rats, monkeys and humans, Psychol Rev, 99: 195-221.
- VIANNA MRM, IZQUIERDO LA, BARROS DM, MEDINA JH & IZQUIERDO I. 1999. Intrahippocampal infusion of an inhibitor of protein kinase A separates short- from long-term memory. Behav Pharmacol 10: 223-227.
- VIANNA MRM, IZQUIERDO LA, BARROS DM, ARDENGHI P, PEREIRA P, RODRIGUES C, MOLETA B, MEDINA JH & IZQUIERDO I. 2000a. Differential role of hippocampal cAMP-dependent protein kinase in short- and long-term memory. Neurochem Res, 25: 621-626.
- VIANNA MRM, BARROS DM, SILVA T, CHOI H, MADCHE C, RODRIGUES C, MEDINA JH & IZQUIERDO I. 2000b. Pharmacological demonstration of the differential involvement of protein kinase C isoforms in short- and long-term memory. Psychopharmacology, 150: 77-84.
- WALZ R, ROESLER R, QUEVEDO J, SANT'ANNA MK, MADRUGA M, RODRIGUES C, GOTTFRIED C, MEDINA JH & IZQUIERDO I. 1999. Effects of post-training infusions of a mitogen-activated protein kinase kinase inhibitor into the hippocampus or entorhinal cortex on short- and long-term retention of inhibitory avoidance. Behav Pharmacol 10: 723-730.
- WALZ R, LENZ G, ROESLER R, VIANNA MRM, MARTINS V, BRENTANI R, RODNIGHT R & IZQUIERDO I. 2000a. Time-dependent enhancement of inhibitory avoidance retention and MAPK activation by post-training infusion of nerve growth factor into CA1 region of hippocampus of adult rats. Eur J Neurosci 12: 2185-2189.
- WALZ R, ROESLER R, QUEVEDO J, SANT'ANNA MK, MADRUGA M, RODRIGUES C, GOTTFRIED C, MEDINA JH & IZQUIERDO I. 2000b. Time-dependent impairment of inhibitory avoidance retention in rats by postraining infusion of mitogen-activated protein kinase kinase into cortical and limbic structures. Neurobiol Learn Mem 73: 11-20.
- YIN JCP & TULLY T. 1996. CREB and the formation of long-term memory. Curr Opin Neurobiol 6: 264-268.
Publication Dates
-
Publication in this collection
05 Oct 2000 -
Date of issue
Sept 2000
History
-
Accepted
19 May 2000 -
Received
16 May 2000