Abstracts
Botulinum toxin type A (BT-A) has been described as an important strategy to various types of pain such as cervical dystonia, myofascial pain syndrome and headache. Although BT-A efficacy has not been proven in tension type headache, its use in migraine continues controversial. In this open trial, we evaluated the efficacy of BT-A in refractory migraine. BT-A was injected in patients diagnosed with migraine who had previously used three classes of prophylactic drugs by at least one year with no response. The most important improvement was observed within 30 days, but pain intensity and frequency of headache had been decreased until the end of three months of follow up. Side effects of BT-A were mild and self limited. We conclude that BT-A seems to be a safe and effective treatment to refractory migraine patients.
botulinum toxin type A; migraine; pain; refractory pain
Toxina botulínica tipo A (TB-A) tem sido descrita como importante estratégia para diversos tipos de dor como cefaléia e dores relacionadas a distonia cervical ou síndrome miofascial. Embora a eficácia da TB-A não tenha sido demonstrada na cefaléia do tipo tensional, seu uso na enxaqueca continua controverso. Nesse estudo avaliamos a eficácia da TB-A na enxaqueca refratária. TB-A foi injetada em pacientes com enxaqueca que fizeram tratamento prévio com no mínimo três classes de medicamentos profiláticos, sem resultados satisfatórios. A melhora mais significativa dos pacientes foi observada após 30 dias de aplicação de TB-A, enquanto intensidade da dor e freqüência de cefaléia continuaram reduzidas até o final de três meses de seguimento. Os efeitos colaterais observados após a aplicação de TB-A foram moderados e auto-limitados. Os nossos dados mostram que TB-A parece ser um tratamento seguro e eficaz para pacientes com enxaqueca refratária.
toxina botulínica do tipo A; enxaqueca; dor; dor refratária
Carla Menezes; Bernardo Rodrigues; Elza Magalhães; Ailton Melo
Division of Neurology and Epidemiology Federal University of Bahia, Salvador BA, Brazil
ABSTRACT
Botulinum toxin type A (BT-A) has been described as an important strategy to various types of pain such as cervical dystonia, myofascial pain syndrome and headache. Although BT-A efficacy has not been proven in tension type headache, its use in migraine continues controversial. In this open trial, we evaluated the efficacy of BT-A in refractory migraine. BT-A was injected in patients diagnosed with migraine who had previously used three classes of prophylactic drugs by at least one year with no response. The most important improvement was observed within 30 days, but pain intensity and frequency of headache had been decreased until the end of three months of follow up. Side effects of BT-A were mild and self limited. We conclude that BT-A seems to be a safe and effective treatment to refractory migraine patients.
Key words: botulinum toxin type A, migraine, pain, refractory pain.
RESUMO
Toxina botulínica tipo A (TB-A) tem sido descrita como importante estratégia para diversos tipos de dor como cefaléia e dores relacionadas a distonia cervical ou síndrome miofascial. Embora a eficácia da TB-A não tenha sido demonstrada na cefaléia do tipo tensional, seu uso na enxaqueca continua controverso. Nesse estudo avaliamos a eficácia da TB-A na enxaqueca refratária. TB-A foi injetada em pacientes com enxaqueca que fizeram tratamento prévio com no mínimo três classes de medicamentos profiláticos, sem resultados satisfatórios. A melhora mais significativa dos pacientes foi observada após 30 dias de aplicação de TB-A, enquanto intensidade da dor e freqüência de cefaléia continuaram reduzidas até o final de três meses de seguimento. Os efeitos colaterais observados após a aplicação de TB-A foram moderados e auto-limitados. Os nossos dados mostram que TB-A parece ser um tratamento seguro e eficaz para pacientes com enxaqueca refratária.
Palavras-chave: toxina botulínica do tipo A, enxaqueca, dor, dor refratária.
Botulinum toxin type A (BT-A) has been used in several neurological diseases such as dystonia, spasticity, hyperhydrosis and sialorrhea1-5. In cervical dystonia, pain is relieved more satisfactorily than torticollis, and tipically it is perceived before muscle relaxation is observed6.
Experimental studies demonstrate that BT-A interferes with neurotransmitters and pain modulators such as P substance, bradykinin and CGRP (calcitonin gene-related peptide)7-9. Although many open trials have demonstrated improvement in patients with chronic tension type headache10,11, the controlled double blind trials did not support these results12-14.
In this paper we study the efficacy of BT-A in refractory chronic migraine.
METHOD
This study was approved by the Ethical Comitee of the Federal University of Bahia and was performed according to the 1964 Declaration of Helsinki. From June to November 2006, all patients with a diagnosis of migraine were consecutively evaluated in the clinic of cranio-facial pain of the Hospital Universitário Professor Edgard Santos - Federal University of Bahia. Patients with the diagnosis of migraine were submitted to a standardized questionnaire and neurologic examination in order to identify co-morbidities and drugs already used. If the patient were classified as refractory to three classes of drugs, the physician would suggest the use of BT-A.
After informed consent, patients were trained to fill a headache standardized diary, with objective questions about frequency of pain and the use of analgesics. In addiction, Visual Analogic Scale (VAS) was used to measure the intensity of pain.
Migraine was defined according IHS criteria15. Patients were considered refractory after using three classes of prophylactic drugs by at least 1 year with no improvement.
BT-A (Dysport®) was administered in standardized doses of 250 U, distributed in 15 sites according Figure 1. Injections were applied by the same neurologist after specific trainning. Patients were reevaluated 15, 30, 60 e 90 days after BT-A injection following the same protocol.
Statistical analysis was performed using Wilcoxon test by means of SPSS 9.0.
RESULTS
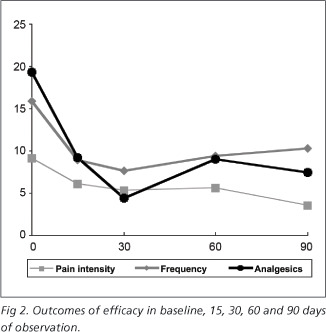
From a total of 15 patients, 13 were evaluated (12 female and 1 male patients, mean age of 41.54 years and 19.23 years of migraine) and followed over 4 months (two patients were lost in the follow up). The progression of pain is shown in Figure 2.
The most significant improvement was observed within 30 days (p<0.05), while pain intensity (VAS) decreased until the end of follow up (p=0.03).
The subjective analisys of pain performed by the patients were coincident with those performed by the physician, and showed improvement in 9 patients (p<0.05).
In the observed period of time the frequency of pain was reduced in 35%, use of analgesics decreased in 61%, and pain intensity in 62%.
Hematoma in the site of injection was observed in 1 patient, itching in the head in 1 patient, and two patients complained of pain. All side effects were transitory and did not interfere with the patients activities.
DISCUSSION
In our study, BT-A proved to be a safe and well tolerated profilatic treatment for patients with refractory migraine. Although being an open label study, the use of objective techniques allowed the impartial evaluation of the patients, and it was clear the improvement in the majority of them, as we could observe from the decrease of analgesic intake and the therapeutic assessement performed by the patient and physician.
As it is known, BT-A can achieve temporary chemodenervation, actively inhibiting ACh release by cleaving intracellular proteins involved in ACh exocytosis, mainly SNAP 25. This protein cleavage prevents ACh vesicle fusion with the plasma membrane and, therefore, inhibits ACh neurotransmitter release at the neuromuscular junction. The ACh inhibition prevents muscle contraction, and promotes temporary atrophy and posterior reinnervation. However, it has been described that BT-A alleviates pain associated with various neurologic conditions, with or without concomitant excess muscle contractions and before its muscle action, as observed in several types of neurological disorders7,16-18. The mechanisms by which BT-A relieves pain is not fully understood. However, experimental studies performed by Aoki et al have failed to demonstrate improvement of acute pain in rats challenged with formalin injection, but showed improvement up to 46% to chronic pain. Using the rat model, the authors observed that formalin-induced peripheral glutamate release in the rat footpad was significantly reduced by BT-A compared to saline8. Other experiments have demonstrated the presence of C-fos within lamina I and II of the dorsal horn after injection of BT-A in rats, showing CNS influence of BT-A6. As migraine has peripheral and central mechanisms, it is possible that BT-A can influence pain perception in different levels of the peripheral and central nervous system in a way not yet established.
Results of all studies published up to now have shown BT-A to be safe and well tolerated, and it its action was observed up to four months12,13, making it an alternative to patients in whom other therapeutic options to migraine have failed. However, several questions such as the dose and injection site remain unsolved. Up to now, doses of Botox® has ranged from 16 to 200 U19,20 and those of Dysport® from 200 to 500 U21,22. It seems that higher doses offer better results than lower, but the study of Silberstein et al. demonstrated the opposite, although methological problems, such as uneven groups for comparison, can be observed. In this last paper, the group receiving 25 U of Botox® had a better result than the 75 U group. Both of them had better response than the placebo group23.
The selected protocol for injections and injection application techniques sometimes are regarded as important causes of lack of efficacy of BT-A. Regarding the site of injection, all authors use BT-A in the frontal and temporal region, and the majority of them also inject in two nuchal points, close to the occipital implant of the trapezius muscle. It seems clear that there is no standardized point in the head for the injections, but a larger distribution of points seems compatible with better results.
Received 1 December 2006, received in final form 5 March 2007. Accepted 25 April 2007.
Dr. Ailton Melo - Avenida Magalhães Neto 735 / 802 - 41820-020 Salvador BA - Brasil.
- 1. Collins A, Jankovic J. Botulinum toxin injection for congenital muscular torticollis presenting in children and adults. Neurology 2006;67:1083-1085.
- 2. Jankovic J.Treatment of dystonia. Lancet Neurol 2006;5:864-872.
- 3. Cardoso ES, Rodrigues BM, Barroso M, et al. Botulinum toxin type A for the treatment of the spastic equinus foot in cerebral palsy. Pediatr Neurol 2006;34:106-109.
- 4. Heckmann M, Plewig G. Hyperhidrosis Study Group. Low-dose efficacy of botulinum toxin A for axillary hyperhidrosis: a randomized, side-by-side, open-label study. Arch Dermatol 2005;141:1255-1259.
- 5. Nóbrega AC, Mota B, Torres AC, Enzo A, Melo A. Does botulinum toxin improves sialorrhea in Parkinson´s disease patients? J Neurol Sci (in press).
- 6. Ondo WG, Gollomp S, Galvez-Jimenez N. A pilot study of botulinum toxin A for headache in cervical dystonia. Headache 2005;45:1073-1077.
- 7. Dressler D, Saberi FA, Barbosa ER. Botulinum toxin: mechanisms of action. Arq Neuropsiquiatr 2005;63:180-185.
- 8. Aoki KR. Evidence for antinociceptive activity of botulinum toxin type A in pain management. Headache 2003;43(Suppl 1):S9-S15.
- 9. Aoki KR. Pharmacology and immunology of botulinum toxin serotypes. J Neurol 2001;248(Suppl 1):S3-S10.
- 10. Tepper SJ, Bigal ME, Sheftell FD, Rapoport AM. Botulinum neurotoxin type A in the preventive treatment of refractory headache: a review of 100 consecutive cases. Headache 2004;44:794-800.
- 11. Behmand RA, Tucker T, Guyuron B. Single-site botulinum toxin type a injection for elimination of migraine trigger points. Headache 2003;43: 1085-1089.
- 12. Mathew NT, Frishberg BM, Gawel M, Dimitrova R, Gibson J, Turkel C, BOTOX CDH Study Group. Botulinum toxin type A (BOTOX) for the prophylactic treatment of chronic daily headache: a randomized, double-blind, placebo-controlled trial. Headache 2005;45:293-307.
- 13. Silberstein SD, Gobel H, Jensen R, et al. Botulinum toxin type A in the prophylactic treatment of chronic tension-type headache: a multicentre, double-blind, randomized, placebo-controlled, parallel-group study. Cephalalgia 2006;26:790-800.
- 14. Rodrigues BM, Menezes C, Rosseti Jr A, Cardoso ES, Melo A. Treatment of chronic tension-type headache with botulinum toxin type A: a meta-analysis. J Clin Pharm Ther (in press).
- 15. The International Classification of Headache Disorders. 2.Ed. Cephalalgia 2004;24(Suppl 1):S8-S160.
- 16. Ghosh B, Das SK. Botulinum toxin: a dreaded toxin for use in human being. J Indian Med Assoc 2002;100:607-614.
- 17. Jankovic J, Brin MF. Botulinum toxin: historical perspective and potential new indications. Muscle Nerve 1997;(Suppl 6):S129-S145.
- 18. Middlebrook JL, Franz DR. Botulinum toxins. In Medical aspects of chemical and biological warfare, Maryland: 1996.
- 19. Behmand RA, Tucker T, Guyuron B. Single-site botulinum toxin type a injection for elimination of migraine trigger points. Headache 2003;43: 1085-1089.
- 20. Silberstein S, Mathew N, Saper J, Jenkins S.Botulinum toxin type A as a migraine preventive treatment. For the BOTOX Migraine Clinical Research Group. Headache 2000;40:445-450.
- 21. Rollnik JD, Tanneberger O, Schubert M, Schneider U, Dengler R. Treatment of tension-type headache with botulinum toxin type A: a double-blind, placebo-controlled study. Headache 2000;40:300-305.
- 22. Schulte-Mattler WJ, Krack P, BoNTTH Study Group. Treatment of chronic tension-type headache with botulinum toxin A: a randomized, double-blind, placebo controlled multicenter study. Pain 2004;109: 110-114.
- 23. Silberstein S. Botulinum preventive pathway as a migraine preventive treatment Headache 2000;40:445-450.
Botulinum toxin type A in refractory chronic migraine: an open-label trial
Publication Dates
-
Publication in this collection
10 Sept 2007 -
Date of issue
Sept 2007
History
-
Received
05 Mar 2007 -
Accepted
25 Apr 2007