Abstract
Acute schistosomiasis is a systemic hypersensitivity reaction against the migrating schistosomula and eggs. A variety of clinical manifestations appear during the migration of schistosomes in humans: cercarial dermatitis, fever, pneumonia, diarrhoea, hepatomegaly, splenomegaly, skin lesions, liver abscesses, brain tumours and myeloradiculopathy. Hypereosinophilia is common and aids diagnosis. The disease has been overlooked, misdiagnosed, underestimated and underreported in endemic areas, but risk groups are well known, including military recruits, some religious congregations, rural tourists and people practicing recreational water sports. Serology may help in diagnosis, but the finding of necrotic-exudative granulomata in a liver biopsy specimen is pathognomonic. Differentials include malaria, tuberculosis, typhoid fever, kala-azar, prolonged Salmonella bacteraemia, lymphoma, toxocariasis, liver abscesses and fever of undetermined origin. For symptomatic hospitalised patients, treatment with steroids and schistosomicides is recommended. Treatment is curative in those timely diagnosed.
acute schistosomiasis; cercarial dermatitis; FUO; dermatitis; neuroschistosomiasis; pyogenic liver abscesses
ARTICLES
Acute schistosomiasis mansoni: revisited and reconsidered
José Roberto Lambertucci+ + Corresponding author: lamber@uai.com.br
Serviço de Doenças Infecciosas e Parasitárias, Faculdade de Medicina, Universidade Federal de Minas Gerais, Belo Horizonte, MG, Brasil
ABSTRACT
Acute schistosomiasis is a systemic hypersensitivity reaction against the migrating schistosomula and eggs. A variety of clinical manifestations appear during the migration of schistosomes in humans: cercarial dermatitis, fever, pneumonia, diarrhoea, hepatomegaly, splenomegaly, skin lesions, liver abscesses, brain tumours and myeloradiculopathy. Hypereosinophilia is common and aids diagnosis. The disease has been overlooked, misdiagnosed, underestimated and underreported in endemic areas, but risk groups are well known, including military recruits, some religious congregations, rural tourists and people practicing recreational water sports. Serology may help in diagnosis, but the finding of necrotic-exudative granulomata in a liver biopsy specimen is pathognomonic. Differentials include malaria, tuberculosis, typhoid fever, kala-azar, prolonged Salmonella bacteraemia, lymphoma, toxocariasis, liver abscesses and fever of undetermined origin. For symptomatic hospitalised patients, treatment with steroids and schistosomicides is recommended. Treatment is curative in those timely diagnosed.
Key words: acute schistosomiasis - cercarial dermatitis - FUO - dermatitis - neuroschistosomiasis - pyogenic liver abscesses
Schistosomiasis is endemic in 76 countries and territories (Amaral et al. 2006). Approximately 200 million people are infected and another 600 million are at risk of infection (Chitsulo et al. 2000). In Brazil, the disease ranks higher in prevalence than HIV/AIDS. Brazil, with 25 million people living in endemic areas and 4-6 million infected, is the most affected country in the Americas.
Acute schistosomiasis is not rare in Brazil. Nevertheless, unsupported statements about it pop up in scientific meetings and medical journals: (i) it is uncommon to see patients with acute schistosomiasis in endemic areas. In fact, most people living in big cities of Brazil have never had contact with contaminated waters and if they do, may end up developing acute schistosomiasis; (ii) acute schistosomiasis is a rare event in the course of schistosomiasis. We are aware of several high-risk groups for predictable epidemics of acute schistosomiasis, including soldiers during military manoeuvres, religious organisations that baptise their followers in stream waters, fishermen, canoers and rural tourists (Fig. 1); (iii) the behaviour of schistosomiasis has changed after implementation of mass chemotherapy in Brazil associated with the migration of people from rural areas to the outskirts of large cities. It is most probable that acute cases have been overlooked, misdiagnosed, underestimated and underreported over time (Table I); (iv) there is no good surrogate marker for acute schistosomiasis. In liver tissue obtained by needle biopsy, it is easy to identify the characteristic necrotic-exudative granulomas of acute schistosomiasis. In Brazil and Africa, where differential diagnoses should include malaria, tuberculosis, typhoid fever, kala-azar, lymphoma, liver abscesses and fever of undetermined origin (FUO), it is worthwhile to consider doing a liver biopsy to be sure of the diagnosis.
Schistosome life cycle - Schistosomiasis is caused by flatworms. Adults of Schistosoma mansoni, which measure 1-2 cm in length and 0.3-0.6 mm in width, live, mate and feed on blood in the portal and mesenteric vessels. The male worm clasps the female in a gynaecophoric canal so that the pair assumes a nematoid, worm-like shape ideally suited to life in the minor vessels of the portal blood system of the definitive, vertebrate host.
The eggs, measuring 145 x 55 μm, are deposited in the venules, make their way into the faeces and hatch in fresh water, where the miracidium emerges. The miracidium penetrates the body of a snail (Biomphalaria) and multiplies asexually. Within 4-6 weeks, hundreds of motile, forked-tail cercariae 0.1-0.2 mm in length burst out. Upon encountering human skin, the cercariae penetrate and change into schistosomula. The newly transformed schistosomulum (Fig. 2) enters a nearby vein and is carried passively in the blood flow to the right heart and on to the pulmonary capillaries (Fig. 3). To develop further, the worms must next reach the liver via the splanchnic vasculature.
When the worms reach sexual maturity, pairing takes place. The muscular male folds around and embraces the female, then transports her against the blood flow in the hepatic portal vein to its branches around the intestine.
Both sexes have a weak oral sucker perforated by the mouth and a more muscular ventral sucker, which is especially well developed in the male (Fig. 4). Egg laying can begin within 25-30 days and the first eggs are detectable from day 35 onwards. Each female worm produces approximately 300 eggs per day. S. mansoni adults are normally found in the tributaries of the inferior mesenteric veins around the lower bowel, whence eggs are usually voided in the faeces.
Cercarial dermatitis - During penetration of cercariae, some previously exposed and unexposed persons experience a prickling sensation and may note a macular rash several hours later. The rash may persist up to 15 days, or even longer if scratching results in secondary infection (Table II). The rash consists of discrete erythematous raised lesions that vary in size from 1-3 cm (Fig. 5). Dermatitis is also frequently seen with avian trematode cercariae (Appleton 1984).
In immune mice (already challenged by a previous infection), it has been shown that the eosinophil-enriched accelerated dermal inflammatory response to schistosomula is best regarded as antibody-mediated and that killing of the parasites in the immune host requires contact between leukocytes and schistosomula, with eosinophils probably playing a crucial role (von Lichtenberg et al. 1977).
Cercarial dermatitis is a seasonal phenomenon. It is most prevalent during the warmer months, when the greatest numbers of people have contact with water and the rate of production of cercariae within the intermediate host is also at a peak.
Diagnosis of cercarial dermatitis in humans is difficult. Patients do not seek out medical assistance because they consider the problem to be of minor importance. Differentials include a reaction to insect bites, contact dermatitis, poison ivy, scabies and impetigo (Lambertucci 1993a).
Acute schistosomiasis mansoni
Acute schistosomiasis is a systemic hypersensitivity reaction against the migrating schistosomula and eggs and it can occur within 16-90 days after a primary infection (Lambertucci et al. 1987). A pre-egg-laying phase has been described in which the symptoms and signs of acute schistosomiasis are present, together with a non-specific hepatitis (Bogliolo & Neves 1965, Raso & Neves 1965, Lambertucci 1993b).
Pathological and immunological aspects - In the cases of acute schistosomiasis in humans in which pathological examination has been performed, the severity of the inflammatory reaction around mature eggs in tissues has been emphasised (Bogliolo 1964). The periovular granulomas are large, with predominant necrotic-exudative features and they appear as translucent granules disseminated on the serosal surface of the liver and intestines. Other organs, such as the lungs, intra-abdominal lymph nodes, brain, skin and pancreas, are also affected. All granulomas are uniformly at the same phase of formation and this is pathognomonic of acute schistosomiasis. Microscopically, they disclose central necrosis and dense eosinophilic infiltration as predominant characteristics (Fig. 6).
In S. mansoni-infected mice, the granuloma size, which may reach 100 times the volume of the egg, decreases with increasing duration of infection. With reduction in granuloma volume comes some relief of the vascular obstruction, resulting in reduced organ size and pathology. Only in the immune modulated state, as in chronic schistosomiasis, when the granulomas are smaller but still protective, do the host's immune response and the disease state maintain an acceptable balance. Some patients never effectively achieve this modulation, leaving them to respond vigorously throughout their infection. This may lead to immunopathogenic reactions that affect fibroblast reactivity and ultimately result in severe hepatic fibrosis (Neves & Raso 1965).
Schistosomal infection, like other parasitic hel-minth infections, is associated with a strong CD4+ T-helper (Th2) response. However, in the early stages (the 1st 3-5 weeks), the immunological reaction involves mainly Th1 cells, when proinflammatory cytokines like IL-2, gamma-interferon and TNF-alpha can be measured in the plasma. The Th2 response follows egg laying and causes the production of a series of cytokines, such as IL-4, IL-5, IL-10 and IL-13. The Th2 cells suppress the Th1 proinflammatory response and produce protective eosinophil-rich granulomatous lesions around newly deposited eggs, but they allow the development of fibrosis. The inability to develop a Th2 response to regulate the initial proinflammatory response can be fatal. IL-13 is known to induce not only airway hyper-responsiveness but also endothelial vascular cell adhesion molecule-1 expression, thus playing a role in asthma, acute lung injury and fibrosis (Chiaramonte et al. 1999).
Immune complexes, usually cleared by reticuloendothelial cells, are probably responsible for some manifestations of the acute phase, such as cerebral and cutaneous vasculitis (Lambertucci et al. 1997, Jauréguiberry et al. 2007), and pericardial and pleural effusions (Taliberti et al. 1978, Lawley et al. 1979, Rezende et al. 1997).
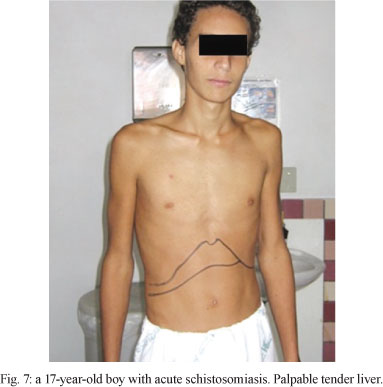
Clinical aspects - The acute phase is usually asymptomatic, but clinical signs of varying intensity may occur (Fig. 7). This stage is most marked in primary infections in non-immune individuals.
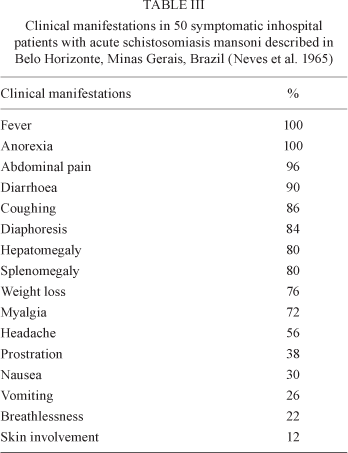
The most common manifestations are fever, chills, weakness, weight loss, headache, anorexia, nausea, vomiting, diarrhoea, dry cough, hepatomegaly, splenomegaly and skin lesions (Table III). A smaller proportion of patients also have bloody diarrhoea, urticaria, periorbital oedema and wheezing. Symptoms last for a few weeks to 2-3 months and gradually abate without therapeutic intervention.
Leukocytosis with 10-75% eosinophils is common and aids diagnosis. Immunoglobulins are elevated in the serum, especially IgE, IgG and IgM. Serum alkaline phosphatase may be increased. The absence of S. mansoni eggs in the faeces does not rule out the diagnosis. There is usually a miliary distribution of eggs in the organs of the host and laparoscopy frequently reveals whitish nodules (granulomas) on the surface of liver (Fig. 8), intestines and visceral peritoneum.
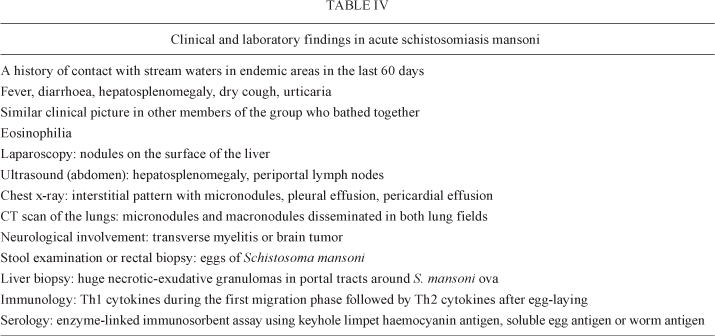
The finding of necrotic-exudative granulomas in the liver is diagnostic (Table IV). Patients with less well-defined clinical pictures, moderate eosinophilia and/or negative stool examination for parasite ova may pass unnoticed (Rocha et al. 1993).
Reports on abdominal ultrasound results in patients with acute schistosomiasis are still scarce. In a report on 26 patients with acute schistosomiasis, ultrasound showed a non-specific homogeneous size increase of the liver and spleen in all acute patients and easily identified intraabdominal lymph nodes in the periportal region in most cases (Fig. 9). Twenty-four months after successful treatment there was involution of the liver and spleen and lymph nodes, although reduced in size, were still easily recognised (Lambertucci et al. 1994, Barata et al. 1999).
Remarkable clinical presentations of acute schistosomiasis mansoni
In many cases, a particular aspect of the disease dominates the clinical picture.
Pulmonary involvement - A 17-year-old boy presented severe cercarial dermatitis after bathing in stream waters of an endemic area in Belo Horizonte (MG), Brazil. Two weeks after admission to hospital, he developed a clinical picture of bronchopneumonia (dyspnoea and mucous sputum), fever, diarrhoea, hepatosplenomegaly and eosinophilia. A chest x-ray showed micronodules and pulmonary condensations, particularly in the right lung (Fig. 10). Stool examination was repeatedly negative for S. mansoni ova, but it became positive two weeks later. A liver biopsy revealed a non-specific hepatitis. A control chest x-ray taken four weeks later was normal (Pedroso et al. 1984). This is a probable case of bronchopneumonia caused by schistosomula.
After egg laying, the importance of lung involvement has been described by many authors in different countries (Sami 1951, Bogliolo 1964, Gelfand 1966, Neves et al. 1965, Rocha et al. 1995a, Cooke et al. 1999, N'Goran 2003). Bogliolo (1964), during autopsy of four patients who died during the acute phase of schistosomiasis, found a miliary distribution of eggs in the lungs of three and one had pleural effusion. In 1995, 115 Brazilian Army recruits had contact with schistosome-infected natural waters on the outskirts of Belo Horizonte, during training military manoeuvres. Thirty recruits developed symptoms of acute schistosomiasis and 19 (63.3%) presented respiratory signs or symptoms (cough, wheezing, thoracic pain, dyspnoea and rhinorrhoea). Radiological pulmonary alterations, such as thickening of bronchial wall and beaded micronodulation, were common (Rocha et al. 1995a).
More recently, with routine computerised tomography scans of the lungs, we have been able to describe micro and macronodules in all patients with acute schistosomiasis, even in those without symptoms referred to the lungs. The presence of pleural and pericardial effusion has also been reported (Lambertucci et al. 2007a, Taliberti et al. 1978) (Fig. 11).
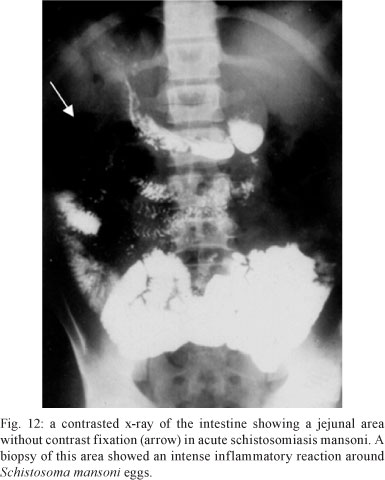
Intestinal involvement - There are few reports of small bowel involvement in acute schistosomiasis. Castro et al. (1971), using peroral biopsy of the jejunum in 13 patients with acute schistosomiasis, found ova in eight (61%). The authors concluded that, in the acute phase, eggs are laid with great frequency and continuously in the submucosa of the small intestine. Pedroso et al. (1987) reported the results of a radiological study of the small intestine in 17 untreated patients with acute schistosomiasis; 12 (70%) had jejunal alterations similar to those described for patients with malabsorption syndromes (Fig. 12). The findings described by both sets of researchers are complementary and may explain the diarrhoea reported in the course of acute schistosomiasis. In such patients, the absorption of drugs given orally may be impaired.
Neves et al. (1993) reported two cases of ischaemic necrosis of the sigmoid colon in two brothers aged seven and four years. Laparotomy disclosed, in both children, extensive necrosis of the descending colon and sigmoid. The histopathological findings were similar: extensive ischaemic necrosis extending to the muscular layer and serosa, in which granulomas in the necrotic-exudative phase were seen. Eggs and granulomas were also found in regional lymph nodes.
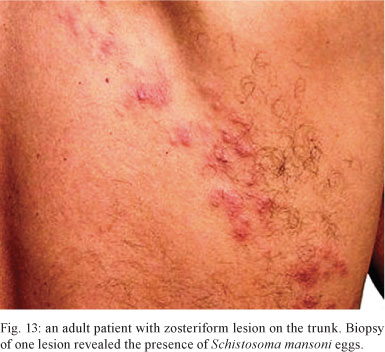
Skin involvement - Andrade Filho et al. (1998) reported two cases of ectopic cutaneous schistosomiasis, of which one had acute schistosomiasis. They also reviewed the publications of 25 other cases with skin involvement. In 19 of the 25 cases (76%), skin lesions were located on the trunk. Clinical presentation of skin lesions varied (papules, nodules and plaques), but they most frequently had a zosteriform appearance (Fig. 13). In one case, a skin biopsy unveiled the diagnosis of a patient who presented with acute schistosomiasis and neurological symptoms, which preceded the dermatitis (Wood et al. 1976). Approximately 40% of the skin lesions occurred in the acute phase (Faust 1948, Findlay & Whiting 1971, Bittencourt et al. 1979, Guimarães & Souza 1987, Miligan & Burns 1988, Rocha et al. 1995b, Andrade Filho et al. 1998).
Faust (1948) reported several cases of extragenital skin lesions caused by Schistosoma japonicum in soldiers who had fought in Asia in World War II. Ramos (1973), working in Mozambique, also found a 4% incidence of extragenital lesions in 100 patients with schistosomiasis.
A patient with several small necrotic lesions on the chest during acute schistosomiasis has been reported (Fig. 14). Skin biopsies did not find ova of S. mansoni, but vasculitis was described by a pathologist and was attributed to the deposition of circulating immune complexes. The skin lesions disappeared after treatment with steroids and oxamniquine (Lambertucci et al. 1997).
In a series of 34 patients with acute schistosomiasis, angioedema and urticaria were described in 16 (47%). The intensity of skin manifestations was mild, with only one case of generalised and lasting urticaria (Rocha et al. 1995a).
Pyogenic liver abscesses - Lambertucci et al. (1990) described the cases of two children with skin pustules and acute schistosomiasis who also developed multiple pyogenic liver abscesses caused by Staphylococcus aureus (Fig. 15). Liver abscesses were duplicated in a murine model of schistosomiasis when mice were injected with bacteria 60 days after receiving cercariae of S. mansoni (Fig. 16). Other investigators have confirmed and extended our initial findings (Teixeira et al. 1996, Mahmoud & Awad 2000, Lambertucci et al. 2001, Sánchez-Olmedo et al. 2003, Goldani et al. 2005).
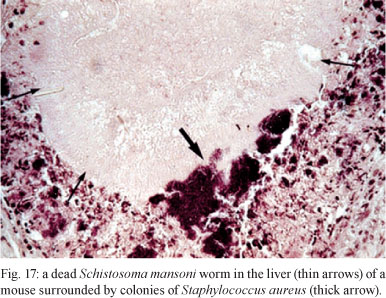
Several mechanisms have been proposed as probable explanations for the association of schistosomiasis with pyogenic abscesses: (i) liver necrosis caused by S. mansoni eggs or dead worms may be colonised by bacteria (Ottens & Dickerson 1972) (Fig. 17), (ii) there is transient impairment of cell-mediated immunity in the acute phase of schistosomiasis in animal models and (iii) the literature contains several reports of cases of recurrent infections caused by S. aureus in the presence of high serum IgE levels (Buckley et al. 1972, Lambertucci 1996).
To complete the picture, migrating larvae of other parasitic agents have been associated with pyogenic abscesses. Liver abscesses have been described in patients infected with Toxocara canis (visceral larva migrans) and intestinal nematodes (Rayes et al. 1999, 2001, Moreira-Silva et al. 2002). Tropical pyomyositis (pyogenic muscle abscesses) caused by S. aureus has also been described in association with T. canis infection (Rayes et al. 2000). Similar pathogenetic mechanisms may be operating on these cases.
Neuroschistosomiasis - As in other organs affected by schistosomal infection, the periovular granulomatous reaction in the central nervous system comprises three stages: necrotic-exudative, productive and a stage of healing by fibrosis. These stages represent a modulation of the immune response to parasite antigens as the disease evolves from the acute to the chronic stage (Pitella 1991). Perivascular inflammatory infiltration and vascular lesions (vasculitis) have been reported in patients with neuroschistosomiasis (Jauréguiberry et al. 2007).
Using magnetic resonance (MRI) of the brain, it is possible to demonstrate the presence of a conglomerate of nodules with well-defined limits (Scrimgeour & Gajdusek 1985, Lambertucci et al. 2008a, b) (Fig. 18).
Neurological symptoms over the course of acute schistosomiasis are frequently accompanied by fever and eosinophilia (Wood et al. 1976, Urban et al. 1996). Patients may become confused, develop focal or generalised seizures or become stuporose. Diagnosis is usually confirmed by surgical brain biopsy because differential diagnosis with brain tumour, based on clinical and imaging aspects, is difficult.
Schistosomal myeloradiculopathy (SMR) is caused by the inflammatory reaction accompanying the deposition of eggs in the venules located in and around the spinal cord. Analysis of the cerebral spinal fluid of SMR patients reveals slight to moderate increases in protein content, lymphocytosis and eosinophils (Neves et al. 1973, Braga et al. 2003, Silva et al. 2002, 2004, Souza-Pereira et al. 2006, Lambertucci et al. 2007b).
Characteristically, the illness starts with a burning pain in the lumbar region that radiates to the lower limbs, followed by weakness, flaccid paralysis and sensory loss. The pain usually subsides with the onset of paraplegia (Silva et al. 2002, Ahmed et al. 2008). Tendon reflexes in the legs usually cannot be elicited and dysfunction of the bladder and rectal sphincters are common. Men become impotent (Lambertucci et al. 2005, 2007b).
MRI reveals abnormalities in the spinal cord in almost all SMR cases (Fig. 19). Following treatment with schistosomicides and corticosteroids, the alterations observed by MRI disappear as the clinical condition of the patient improves (Silva et al. 2004). Occasionally, the neurological symptoms may return after stopping treatment, but as soon as treatment is re-started, the signs and symptoms of SMR disappear.
Acute over chronic infection
Katayama syndrome caused by S. japonicum infections also occurs in people living in endemic areas with a history of previous infection. At least two cases of re-infection have been reported in Brazil (Katz & Bittencourt 1965, Neves & Raso 1965).
We recently examined two male patients (18 and 19 years old) with chronic schistosomiasis who were re-infected in the stream waters of a gold mine near Belo Horizonte (unpublished observations). They were admitted to different hospitals for investigation of an FUO and both presented fever, diarrhoea and emaciation. One patient died and autopsy showed a liver with typical Symmers fibrosis but with a miliary distribution of ova of S. mansoni, as has been described in acute schistosomiasis; however, there was an interesting difference: no granulomas had a necrotic-exudative appearance (Fig. 20). The other patient, treated with steroids and oxamniquine, survived. In a fragment of his liver obtained by percutaneous ultrasound-guided biopsy, similar microscopic findings were described. Both patients probably had severe re-infection with dissemination of ova facilitated by the presence of portal hypertension and portasystemic shunts.
Serology for diagnosing acute schistosomiasis
The most common technique used in antibody detection is the enzyme-linked immunosorbent assay (ELISA). Soluble egg antigen, worm antigen and the cathionic fraction 6 display high sensitivity but low specificity. An ELISA test using keyhole limpet haemocyanin as the antigen has been shown to be efficient in differentiating acute from chronic schistosomiasis in patients living in endemic areas of Egypt and Brazil (Rabello 1995). While these tests may be useful for diagnosing patients from non-endemic areas visiting endemic areas, in general, antibody-based methods suffer from low specificity, persistence after chemotherapy, cross-reactivity and the need for reference centre to perform them.
Antigen detection - The sensitivity of antigen detection varies from 55-100%, being low in patients with low worm burdens and thus this technique offers no advantage over stool examination. Antigen capture with monoclonal antibodies is expensive and reproducibility of the method is not good.
Treatment
Two main approaches have been proposed for the treatment of acute schistosomiasis:
Schistosomicides alone - Oxamniquine and praziquantel are potent schistosomicides against mature S. mansoni worms (Lambertucci et al. 1982). However, they present low efficacy against immature worms both in man and in experimentally infected mice (Lambertucci et al. 1980, 1989a, 2000). A cure rate of 40-50% may be expected in acute schistosomiasis. Schistosomicides may be used alone in asymptomatic patients. Treatment should be repeated 2-3 months later in patients still passing eggs in the stools. A deterioration or exacerbation of the clinical picture after treatment has been reported (Chou et al. 1963, Lambertucci et al. 1982, Harries & Cook 1987, Grandière-Perez et al. 2006, Jauréguiberry et al. 2007). Asymptomatic patients may become symptomatic, emboli of dead worms may end up in the liver or lungs with rebound liver pain, pulmonary symptoms and radiological alterations and antigens liberated by dead worms may form immune complexes and cause vasculitis or urticaria. The efficacy of schistosomicides is also immune-dependent (Doenhoff et al. 1988, 1991, Lambertucci et al. 1989a).
Association of steroids and schistosomicides - Clinical and experimental evidence indicate that steroids act synergistically with schistosomicides in the treatment of acute schistosomiasis (Lambertucci et al. 1989a, Lambertucci 1993a, b). We usually give prednisone followed by oxamniquine or praziquantel (Table V). The association of steroids and schistosomicides in the treatment of symptomatic patients augments the cure rate, speeds the recovery time, prevents the recurrence of symptoms and improves the quality of medical care. Before starting steroids, however, it is good medical practice to treat patients for strongyloidiasis with ivermectin (200 μm/kg, single dose) or albendazole to prevent the development of fatal strongyloides' sepsis triggered by prednisone.
Artemisinin derivatives
These compounds are active against immature worms of all major human schistosome species (Xiao & Catto 1989). Artemisinins by themselves have been successfully used as antischistosomals in some special circumstances (e.g., in people exposed to the infection at a defined time because of a flood), but they cannot be considered as possible alternatives to other schistosomicides due to their limited activity against adult worms. Artemether seems to be a promising treatment for acute schistosomiasis because it is active against juvenile worms. Nevertheless, in humans, the efficacy against Schistosoma haematobium seems to be moderate (N'Goran et al. 2003).
CONCLUSION
There is a broad spectrum of clinical manifestations of acute schistosomiasis in humans. Physicians of different medical specialties, including neurology, lung diseases, dermatology, internal medicine and gastroenterology, must be trained to recognise acute schistosomiasis. Groups at risk for acquiring it (tourists, military personnel, religious congregations and people practicing water sports) must be alerted and advised about the disease and its complications. Additionally, physicians of endemic and non-endemic countries are not aware of the importance of acute schistosomiasis and of its multiform clinical presentation.
Received 15 May 2009
Accepted 16 October 2009
- Ahmed AF, Idris AS, Kareem AM, Dawoud TA 2008. Acute toxemic schistosomiasis complicated by acute flaccid paraplegia due to schistosomal myeloradiculopathy in Sudan. Saudi Med J 29: 770-773.
- Amaral RS, Tauil PL, Lima DD, Engels D 2006. An analysis of the impact of the Schistosomiasis Control Programme in Brazil. Mem Inst Oswaldo Cruz 101 (Suppl I): 79-85.
- Andrade Filho J de S, Lopes MS, Corgozinho Filho AA, Pena GP 1998. Ectopic cutaneous schistosomiasis: report of two cases and a review of the literature. Rev Inst Med Trop Sao Paulo 40: 253-257.
- Appleton CC 1984. Schistosome dermatitis - an unrecognized problem in South Africa? S Afr Med J 65: 467-469.
- Barata CH, Pinto-Silva RA, Lambertucci JR 1999. Abdominal ultrasound in acute schistosomiasis mansoni. Br J Radiol 72: 949-952.
- Barbosa CS, Montenegro SML, Abath FGC, Domingues ALC 2001. Specific situations related to acute schistosomiasis in Pernambuco, Brazil. Mem Inst Oswaldo Cruz 96 (Suppl.): 169-172.
- Bittencourt AL, Pinho O, Lenzi HL, Costa IM 1979. Extragenital cutaneous lesions of schistosomiasis mansoni: report of two cases. Am J Trop Med Hyg 28: 84-86.
- Bogliolo L 1964. Subsídios para o estudo da anatomia patológica da forma aguda toxêmica da esquistossomose mansônica. Gen 19: 157-236.
- Bogliolo L, Neves J 1965. Ocorrência da hepatite na forma aguda da esquistossomose mansoni, antes da maturação e da postura de ovos. An Fac Med Minas Gerais 2: 47-74.
- Braga BP, da Costa Junior LB, Lambertucci JR 2003. Magnetic resonance imaging of cerebellar schistosomiasis mansoni. Rev Soc Bras Med Trop 36: 635-636.
- Buckley RH, Wray BB, Belmaker EZ 1972. Extreme hyperimmunoglobulinemia E and undue susceptibility to infection. Pediatrics 49: 59-70.
- Castro L de P, Dani R, Alvarenga RJ, Chamone D de A, de Oliveira CA 1971. A peroral biopsy study of the jejunum in human schistosomiasis mansoni. Rev Inst Med Trop Sao Paulo 13: 103-109.
- Chiaramonte MG, Schopf LR, Neben TY, Cheever AW, Donaldson DD, Wynn TA 1999. IL-13 is a key regulatory cytokine for Th2 cell-mediated pulmonary granuloma formation and IgE responses induced by Schistosoma mansoni eggs. J Immunol 162: 920-930.
- Chitsulo L, Engels D, Montresor A, Savioli L 2000. The global status of schistosomiasis and its control. Acta Trop 77: 41-51.
- Chou HC, Huangfu-Ming, Chou HL 1963. Clinical evaluation of F30066 in the treatment of acute schistosomiasis. Chin Med J 82: 250-257.
- Cooke GS, Lalvani A, Gleeson FV, Conlon CP 1999. Acute pulmonary schistosomiasis in travelers returning from Lake Malawi, sub-Saharan Africa. Clin Infect Dis 29: 836-839.
- Coura JR, Coura LC, Kalache A, Argendo CA 1970. Esquistossomose aguda autóctone de foco na cidade do Rio de Janeiro. Estudo de 22 casos. Rev Soc Bras Med Trop 6: 387-396.
- Doenhoff MJ, Modha J, Lambertucci JR 1988. Anti-schistosome chemotherapy enhanced by antibodies specific for a parasite esterase. Immunology 65: 507-510.
- Doenhoff MJ, Modha J, Lambertucci JR, McLaren DJ 1991. The immune dependence of chemotherapy. Parasitol Today 7: 16-18.
- Enk MJ, Amorim A, Schall VT 2003. Acute schistosomiasis outbreak in the metropolitan area of Belo Horizonte, Minas Gerais: alert about the risk of unnoticed transmission increased by growing rural tourism. Mem Inst Oswaldo Cruz 98: 745-750.
- Faust EC 1948. An inquiry into the ectopic lesions in schistosomiasis. Am J Trop Med Hyg 28: 175-199.
- Ferreira H, Oliveira CA, Bittencourt D, Katz N, Carneiro LFC, Grinbaum E, Veloso C, Dias RP, Alvarenga RJ, Dias CB 1966. A fase aguda da esquistossomose mansoni. J Bras Med 11: 54-67.
- Ferreira LF, Naveira JB, Silva JR 1960. Fase toxêmica da esquistossomose mansoni. Considerações a propósito de alguns casos coletivamente contaminados em uma piscina. Rev Inst Med Trop Sao Paulo 68: 714-715.
- Findlay FH, Whiting DA 1971. Disseminated and zosteriform cutaneous schistosomiasis. Br J Dermatol 85 (Suppl. 7): 98-101.
- Gelfand M 1966. Pulmonary schistosomiasis in the early "Katayama" phase of the disease. J Trop Med Hyg 69: 143-144.
- Goldani LZ, dos Santos RP, Sugar AM 2005. Pyogenic liver abscess in patients with schistosomiasis mansoni. Trans R Soc Trop Med Hyg 99: 932-936.
- Gonçalves JF, Santana W, Barbosa CS, Coutinho A 1992. Esquistossomose aguda de caráter episódico na Ilha de Itamaracá, estado de Pernambuco. Cad Saude Publica 7: 424-425.
- Grandière-Pérez L, Ansart S, Paris L, Faussart A, Jaureguiberry S, Grivois JP, Klement E, Bricaire F, Danis M, Caumes E 2006. Efficacy of praziquantel during the incubation and invasive phase of Schistosoma haematobium schistosomiasis in 18 travelers. Am J Trop Med Hyg 74: 814-818.
- Guimarães NS, Souza APB 1987. Esquistossomose mansônica cutânea ectópica: três novos casos. An Bras Dermatol 62: 101-103.
- Harries AD, Cook GC 1987. Acute schistosomiasis (Katayama fever): clinical deterioration after chemotherapy. J Infect 14: 159-161.
- Jauréguiberry S, Ansart S, Perez L, Danis M, Bricaire F, Caumes E 2007. Acute neuroschistosomiasis: two cases associated with cerebral vasculitis. Am J Trop Med Hyg 76: 964-966.
- Katz N, Bittencourt D 1965. Sobre um provável caso de forma toxêmica no decurso da forma hepatoesplênica da esquistossomose mansônica. Hospital (Rio J) 67: 847-858.
- Lambertucci JR 1993a. Acute schistosomiasis: clinical, diagnostic and therapeutic features. Rev Inst Med Trop Sao Paulo 35: 399-404.
- Lambertucci JR 1993b. Schistosoma mansoni: pathological and clinical aspects. In P Jordan, G Webbe (eds.), Human schistosomiasis, Cab International, Wallingford, p. 195-225.
- Lambertucci JR 1996. Hyperimmunoglobulinemia E, parasitic diseases and staphylococcal infection. Rev Soc Bras Med Trop 29: 407-410.
- Lambertucci JR, da Silva RA, Gerspacher-Lara R, Barata CH 1994. Acute Manson's schistosomiasis: sonographic features. Trans R Soc Trop Med Hyg 88: 76-77.
- Lambertucci JR, dos Santos Silva LC, Andrade LM, de Queiroz LC, Carvalho VT, Voieta I, Antunes CM 2008a. Imaging techniques in the evaluation of morbidity in schistosomiasis mansoni. Acta Trop 108: 209-217.
- Lambertucci JR, Greco DB, Pedroso ER, da Costa Rocha MO, Salazar HM, de Lima DP 1982. A double blind trial with oxamniquine in chronic schistosomiasis mansoni. Trans R Soc Trop Med Hyg 76: 751-755.
- Lambertucci JR, Modha J, Curtis R, Doenhoff M 1989a. The association of steroids and schistosomicides in the treatment of experimental schistosomiasis. Trans R Soc Trop Med Hyg 83: 354-357.
- Lambertucci JR, Modha J, Doenhoff M 1989b. Schistosoma mansoni: the therapeutic efficacy of oxamniquine is enhanced by immune serum. Trans R Soc Trop Med Hyg 83: 362-363.
- Lambertucci JR, Pedroso ER, de Souza DW, de Lima DP, Neves J, Salazar HM, Marinho RP, da Costa Rocha MO, Coelho PM, de Lima Costa MF, Greco DB 1980. Therapeutic efficacy of oral oxamniquine in the toxemic form of schistosomiasis mansoni: treatment of eleven individuals from two families, and experimental study. Am J Trop Med Hyg 29: 50-53.
- Lambertucci JR, Rayes AA, Serufo JC, Nobre V 2001. Pyogenic abscesses and parasitic diseases. Rev Inst Med Trop Sao Paulo 43: 67-74.
- Lambertucci JR, Rayes AAM, Barata CH, Teixeira R, Gerspacher-Lara R 1997. Acute schistosomiasis: report on five singular cases. Mem Inst Oswaldo Cruz 92: 631-635.
- Lambertucci JR, Rocha RS, Carvalho OS, Katz N 1987. A esquistossomose em Minas Gerais. Rev Soc Bras Med Trop 20: 47-52.
- Lambertucci JR, Serufo JC, Gerspacher-Lara R, Rayes AA, Teixeira R, Nobre V, Antunes CM 2000. Schistosoma mansoni: assessment of morbidity before and after control. Acta Trop 77: 101-109.
- Lambertucci JR, Silva LC, de Queiroz LC 2007a. Pulmonary nodules and pleural effusion in the acute phase of schistosomiasis mansoni. Rev Soc Bras Med Trop 40: 374-375.
- Lambertucci JR, Silva LC, do Amaral RS 2007b. Guidelines for the diagnosis and treatment of schistosomal myeloradiculopathy. Rev Soc Bras Med Trop 40: 574-581.
- Lambertucci JR, Sousa-Pereira SR, Silva LC 2005. Myeloradiculopathy in acute schistosomiasis mansoni. Rev Soc Bras Med Trop 38: 277-278.
- Lambertucci JR, Teixeira R, Navarro MM, Coelho PM, Ferreira MD 1990. Liver abscess and schistosomiasis. A new association. Rev Soc Bras Med Trop 23: 239-240.
- Lambertucci JR, Voieta I, Silveira I dos S 2008b. Cerebral schistosomiasis mansoni. Rev Soc Bras Med Trop 41: 693-694.
- Lawley TJ, Ottesen EA, Hiatt RA, Gazze LA 1979. Circulating immune complexes in acute schistosomiasis. Clin Exp Immunol 37: 221-227.
- Leocádio J 1969. O período toxêmico da esquistossomose. Rev Soc Bras Med Trop 3: 143-164.
- Mahmoud MS, Awad AA 2000. A study of the predisposition of schistosomiasis mansoni to pyogenic liver abscess in experimentally infected mice. J Egypt Soc Parasitol 30: 277-286.
- Marques RJ 1957. A propósito da chamada "fase toxêmica" da esquistossomose mansônica. An Fac Med Recife 17: 243-256.
- Martins AV, Versiani W 1939. Plano de combate a "schistosomose mansoni" em Belo Horizonte. Hospital (Rio J) 15: 197-206.
- Milligan A, Burns DA 1988. Ectopic cutaneous schistosomiasis and schistosomal ocular inflammatory disease. Br J Dermatol 119: 793-798.
- Moreira-Silva SF, Leite AL, Brito EF, Pereira FE 2002. Nematode infections are risk factors for staphylococcal infection in children. Mem Inst Oswaldo Cruz 97: 395-399.
- Neves J, Marinho RP, de Araujo PK, Raso P 1973. Spinal cord complications of acute schistosomiasis mansoni. Trans R Soc Trop Med Hyg 67: 782-792.
- Neves J, Martins NR, Tonelli E 1965. Forma toxêmica da esquistossomose mansoni. Considerações diagnósticas em torno de 50 casos identificados em Belo Horizonte. An Fac Med Minas Gerais 22: 75-98.
- Neves J, Raso P 1965. Estudo anátomo-clínico de um caso de forma toxêmica da esquistossomose mansoni que evoluiu para a forma hepatesplênica em 130 dias (fibrose hepática tipo Symmers). Rev Inst Med Trop Sao Paulo 7: 256-266.
- Neves J, Raso P, Pinto D de M, da Silva SP, Alvarenga RJ 1993. Ischaemic colitis (necrotizing colitis, pseudomembranous colitis) in acute schistosomiasis mansoni: report of two cases. Trans R Soc Trop Med Hyg 87: 449-452.
- Neves PF, Costa PD, Oliveira C 1970. Forma toxêmica da esquistossomose mansônica no Estado da Guanabara. O Hospital 78: 5-14.
- N'Goran EK, Utzinger J, Gnaka HN, Yapi A, N'Guessan NA, Kigbafori SD, Lengeler C, Chollet J, Shuhua X, Tanner M 2003. Randomized, double-blind, placebo-controlled trial of oral artemether for the prevention of patent Schistosoma haematobium infections. Am J Trop Med Hyg 68: 24-32.
- Ottens H, Dickerson G 1972. Studies on the effects of bacteria on experimental schistosome infections in animals. Trans R Soc Trop Med Hyg 66: 85-107.
- Pedroso ER, Lambertucci JR, Rocha MO, Greco DB, Ferreira CS, Lima DP, Raso P 1984. Pulmonary schistosomiasis: broncho-pneumonitis probably due to schistosomulae. Rev Soc Bras Med Trop 17: 213-215.
- Pedroso ER, Lambertucci JR, Rocha MO, Greco DB, Ferreira CS, Rocha RS, Katz N 1987. Bowel x-ray alterations in acute human schistosomiasis. Rev Soc Bras Med Trop 20: 159-162.
- Pittella JE 1991. The relation between involvement of the central nervous system in schistosomiasis mansoni and the clinical forms of the parasitosis. A review. J Trop Med Hyg 94: 15-21.
- Rabello A 1995. Acute human schistosomiasis mansoni. Mem Inst Oswaldo Cruz 90: 277-280.
- Ramos SF 1973. Late cutaneous bilharziasis. S Afr Med J 47: 2103-2108.
- Raso P, Neves J 1965. Contribuição ao estudo da ação dos corticóides na forma toxêmica da esquistossomose humana. An Fac Med Minas Gerais 22: 167-180.
- Rayes A, Teixeira D, Nobre V, Serufo JC, Gonçalves R, Valadares L, Lambertucci JR 1999. Visceral larva migrans syndrome complicated by liver abscess. Scand J Infect Dis 31: 324-325.
- Rayes AA, Nobre V, Teixeira DM, Serufo JC, Filho GB, Antunes CM, Lambertucci JR 2000. Tropical pyomyositis and human toxocariasis: a clinical and experimental study. Am J Med 109: 422-425.
- Rayes AA, Teixeira D, Serufo JC, Nobre V, Antunes CM, Lambertucci JR 2001. Human toxocariasis and pyogenic liver abscess: a possible association. Am J Gastroenterol 96: 563-566.
- Rezende SA, Lambertucci JR, Goes AM 1997. Role of immune complexes from patients with different clinical forms of schistosomiasis in the modulation of in vitro granuloma reaction. Mem Inst Oswaldo Cruz 92: 683-687.
- Rocha MO, Greco DB, Pedroso ER, Lambertucci JR, Rocha RL, Rezende DF, Neves J 1995a. Secondary cutaneous manifestations of acute schistosomiasis mansoni. Ann Trop Med Parasitol 89: 425-430.
- Rocha MO, Pedroso ER, Neves J, Rocha RS, Greco DB, Lambertucci JR, Rocha RL, Katz N 1993. Characterization of the non-apparent clinical form in the initial phase of schistosomiasis mansoni. Rev Inst Med Trop Sao Paulo 35: 247-251.
- Rocha MO, Rocha RL, Pedroso ER, Greco DB, Ferreira CS, Lambertucci JR, Katz N, Rocha RS, Rezende DF, Neves J 1995b. Pulmonary manifestations in the initial phase of schistosomiasis mansoni. Rev Inst Med Trop Sao Paulo 37: 311-318.
- Sami AA 1951. Pulmonary manifestations of schistosomiasis. Dis Chest 19: 698-705.
- Sánchez-Olmedo JI, Ortiz-Leyba C, Garnacho-Montero J, Jiménez-Jiménez J, Travado-Soria P 2003. Schistosoma mansoni and Staphylococcus aureus bacteremia: a deadly association. Intensive Care Med 29: 1204.
- Scrimgeour EM, Gajdusek DC 1985. Involvement of central nervous system in Schistosoma mansoni and S. haematobium infection. A review. Brain 108: 1023-1038.
- Silva A, Santana LB, Jesus AR, Burattini MN, Carvalho E 2000. Aspectos clínicos de um surto de esquistossomose aguda no estado de Sergipe, Brasil. Rev Soc Bras Med Trop 33 (Suppl. 1): 376.
- Silva LC, Kill CM, Lambertucci JR 2002. Cervical spinal cord schistosomiasis. Rev Soc Bras Med Trop 35: 543-544.
- Silva LC, Maciel PE, Ribas JG, Souza-Pereira SR, Antunes CM, Lambertucci JR 2004. Treatment of schistosomal myeloradiculopathy with praziquantel and corticosteroids and evaluation by magnetic resonance imaging: a longitudinal study. Clin Infect Dis 39: 1618-1624.
- Sousa-Pereira SR, Teixeira AL, Silva LC, Souza AL, Antunes CM, Teixeira MM, Lambertucci JR 2006. Serum and cerebral spinal fluid levels of chemokines and Th2 cytokines in Schistosoma mansoni myeloradiculopathy. Parasite Immunol 28: 473-478.
- Taliberti BB, Ribeiro VL, Foscarini LG, Carvalho DG 1978. Apresentação clínica incomum de um caso de esquistossomose mansônica aguda. Rev Ass Med Minas Gerais 29: 23-27.
- Teixeira R, Ferreira MD, Coelho PM, Filho GB, Azevedo Júnior GM, Lambertucci JR 1996. Pyogenic liver abscesses and acute schistosomiasis mansoni: report on 3 cases and experimental study. Trans R Soc Trop Med Hyg 90: 280-283.
- Urban CD, Piovesan EJ, de Almeida SM, Kowacs PA, Minguetti G, Werneck LC 1996. Esquistossomose aguda com comprometimento cerebral: relato de caso. Arq Neuropsiquiatr 54: 677-682.
- von Lichtenberg F, Sher A, McIntyre S 1977. A lung model of schistosome immunity in mice. Am J Pathol 87: 105-123.
- Wood MG, Srolovitz H, Schetman D 1976. Schistosomiasis. Paraplegia and ectopic skin lesions as admission symptoms. Arch Dermatol 112: 690-695.
- Xiao SH, Catto BA 1989. In vitro and in vivo studies of the effect of artemether on Schistosoma mansoni Antimicrob Agents Chemother 33: 1557-1562.
Publication Dates
-
Publication in this collection
06 Aug 2010 -
Date of issue
July 2010
History
-
Accepted
16 Oct 2009 -
Received
15 May 2009