Abstract
PURPOSE:
To investigate the effects of occupational exposure to waste anesthetic gases on genetic material and antioxidant status in professionals during their medical residency.
METHODS:
The study group consisted of 15 medical residents from Anesthesiology and Surgery areas, of both genders, mainly exposed to isoflurane and to a lesser degree to sevoflurane and nitrous oxide; the control group consisted of 15 young adults not exposed to anesthetics. Blood samples were drawn from professionals during medical residency (eight, 16 and 22 months of exposure to waste anesthetic gases). DNA damage was evaluated by comet assay, and antioxidant defense was assessed by total thiols and the enzymes glutathione peroxidase (GPX), superoxide dismutase (SOD) and catalase (CAT).
RESULTS:
When comparing the two groups, DNA damage was significantly increased at all time points evaluated in the exposed group; plasma thiols increased at 22 months of exposure and GPX was higher at 16 and 22 months of exposure.
CONCLUSION:
Young professionals exposed to waste anesthetic gases in operating rooms without adequate scavenging system have increased DNA damage and changes in redox status during medical residency. There is a need to minimize exposure to inhalation anesthetics and to provide better work conditions.
Anesthetics; Occupational Exposure; Toxicity
Introduction
Worldwide, healthcare professionals who work in environments contaminated by waste anesthetic gases (surgeons, anesthesiologists, nurses, technicians, dentists, and dental assistants) besides students are occupationally exposed to halogenated anesthetics and nitrous oxide (N2O). Occupational exposure may result in adverse health effects such as fatigue, irritability and headache11. Plummer JL, Sandison CH, Ilsley AH, Cousins MJ. Attitudes of anaesthetists and nurses to anaesthetic pollution. Anaesth Intensive Care. 1987;15:411-20. , 22. Saurel-Cubizolles MJ, Estryn-Behar M, Maillard MF, Mugnier N, Masson A, Monod G. Neuropsychological symptoms and occupational exposure to anaesthetics. Br J Ind Med. 1992;49:276-81.; abnormalities of the liver, kidneys and hematopoietic system33. Green CJ. Anaesthetic gases and health risks to laboratory personnel: a review. Lab Anim. 1981;15:397-403. , 44. Franco G, Marraccini P, Santagostino G, Filisetti P, Preseglio I. Behaviour of urinary D-glucaric acid excretion in surgical patients and anaesthesiology staff acutely exposed to isoflurane and nitrous oxide. Med Lav. 1991;82:527-32.; and neurobehavioral changes55. Lucchini R, Placidi D, Toffoletto F, Alessio L. Neurotoxicity in operating room personnel working with gaseous and nongaseous anesthesia. Int Arch Occup Environ Health. 1996;68:188-92.. Furthermore, exposure to waste anesthetic gases are also related to an increased abortion/miscarriage incidence66. Rowland AS, Baird DD, Shore DL, Weinberg CR, Savitz DA, Wilcox AJ. Nitrous oxide and spontaneous abortion in female dental assistants. Am J Epidemiol. 1995;141:531-8. , 77. Boivin JF. Risk of spontaneous abortion in women occupationally exposed to anaesthetic gases: a meta-analysis. Occup Environ Med. 1997;54:541-8., reduced fertility88. Ahlborg Jr G, Axelsson G, Bodin L. Shift work, nitrous oxide exposure and subfertility among Swedish midwives. In t J Epidemiol. 1996;25:783-90., and birth defects, which are especially related to N2O99. Bodin L, Axelsson G, Ahlborg Jr G. The association of shift work and nitrous oxide exposure in pregnancy with birth weight and gestational age. Epidemiology . 1999;10:429-36..
Although healthcare workers are exposed to much lower anesthetic concentrations than patients, such exposure can extend over many years. Studies have shown increased DNA damage, assessed by sister chromatid exchange (SCE), chromosomal aberration (CA) and micronuclei (MN) tests in operating room personnel exposed for several years to waste anesthetic gases1010. Sardas S, Aygün N, Gamli M, Unal Y, Unal N, Berk N, Karakaya AE. Use of alkaline comet assay (single cell gel electrophoresis technique) to detect DNA damages in lymphocytes of operating room personnel occupationally exposed to anaesthetic gases. Mutat Res. 1998;418:93-100.
11. Chinelato AR, Froes ND. Genotoxic effects on professionals exposed to inhalation anesthetics. Rev Bras Anestesiol. 2002;52:79-85.
12. Bilban M, Jakopin CB, Ogrinc D. Cytogenetic tests performed on operating room personnel (the use of anaesthetic gases). Int Arch Occup Environ Health. 2005;78:60-4.
13. Chandrasekhar M, Rekhadevi PV, Sailaja N, Rahman MF, Reddy JP, Mahboob M, Grover P. Evaluation of genetic damage in operating room personnel exposed to anaesthetic gases. Mutagenesis. 2006;21:249-54.
14. Eroglu A, Celep F, Erciyes N. A comparison of sister chromatid exchanges in lymphocytes of anesthesiologists to nonanesthesiologists in the same hospital. Anesth Analg. 2006;102:1573-7.
15. Rozgaj R, Kasuba V, Brozovic G, Jazbec A. Genotoxic effects of anaesthetics in operating theatre personnel evaluated by the comet assay and micronucleus test. Int J Hyg Environ Health. 2009;212:11-7.
16. Wrońska-Nofer T, Palus J, Krajewski W, Jajte J, Kucharska M, Stetkiewicz J, Wasowicz W, Rydzyński K. DNA damage induced by nitrous oxide: study in medical personnel of operating rooms. Mutat Res. 2009;666:39-43.
-
1717. El-Ebiary A, Abuelfadl A, Sarhan N, Othman M. Assessment of genotoxicity risk in operation room personnel by the alkaline comet assay. Hum Exp Toxicol. 2013;32:563-70.. However, there is still no report about the effect of waste anesthetic gases in professionals occupationally exposed for a shorter time.
Free radicals and reactive oxygen species (ROS) are important molecules known to defend the organism against microorganisms, but their exacerbate release can lead to nucleic acid, lipid and protein damages1818. Ferguson LR, Philpott M, Karunasinghe N. Oxidative DNA damage and repair: significance and biomarkers. J Nutr. 2006;136:2687S-9S.. Oxidative stress is defined as the imbalance between ROS formation and antioxidant defense. Additionally, ROS are formed during the metabolism of drugs, including anesthetics. Only few studies have linked exposure to waste anesthetic gases with oxidative stress. Nonetheless, increased plasma lipid peroxidation, but no changes in antioxidant capacity, has been observed in health professionals exposed to anesthetic gases1919. Malekirad AA, Ranjbar A, Rahzani K, Kadkhodaee M, Rezaie A, Taghavi B, Abdollahi M. Oxidative stress in operating room personnel: occupational exposure to anesthetic gases. Hum Exp Toxicol. 2005;24:597-601.. Antioxidant enzymes and trace elements in operating room personnel exposed to a mixture of volatile anesthetics have been reported to be lower than in a non-exposed group2020. Türkan H, Aydin A, Sayal A. Effect of volatile anesthetics on oxidative stress due to occupational exposure. World J Surg. 2005;29:540-2.. Moreover, anesthesiologists, nurses and surgeons exposed to halothane, isoflurane, sevoflurane, desflurane and N2O for a mean of seven years have shown increased DNA damage and oxidative status2121. Baysal Z, Cengiz M, Ozgonul A, Cakir M, Celik H, Kocyigit A. Oxidative status and DNA damage in operating room personnel. Clin Biochem. 2009;42:189-93..
Long-term exposure to anesthetics causes toxic effects. However, the effects on DNA breaks and oxidative stress in medical residents exposed to inhaled anesthetics have not yet been investigated. Therefore, this study evaluated the DNA damage and antioxidant status in professionals exposed to waste anesthetic gases during their medical residency.
Methods
This study was approved by the Human Research Ethics Review Board, Federal University of Amazonas - UFAM (0044.0.115.000-10), in accord with the Helsinki Declaration of 1975 as revised in 2008. Written informed consent was obtained from all subjects, who were asked to respond to a standardized questionnaire on lifestyle, health status and previous exposure to environmental pollutants. Thirty adults, of both genders, aged 25-32 years, were enrolled in the study. The exposed group consisted of 15 medical residents in the fields of Anesthesiology, General surgery, Neurosurgery and Orthopedics mainly exposed to isoflurane and to a lesser degree to sevoflurane and N2O, from eight months up to 22 months of exposure. The control group comprised 15 volunteers not exposed to waste anesthetic gases or other pollutants, who did not work in a hospital area. The UFAM Hospital in Manaus (Northern, Brazil) has seven operating rooms with no active scavenging system.
Subjects with any disease, smokers, and alcoholics, those recently exposed to radiation, under medication or vitamin supplements/antioxidants, and those with any kind of occupational exposure other than waste anesthetic gases (exposed group) were excluded from the study.
Blood sampling
Venous blood samples were drawn eight, 16 and 22 months of exposure to waste anesthetic gases from the exposed residents, and once from the controls. All samples were coded, and subjected to blind analysis. Each step was carried out under indirect light to prevent additional damage. Whole blood was used for glutathione peroxidase (GPX) detection, erythrocytes for the assessment of superoxide dismutase (SOD) and catalase (CAT) activities, lymphocytes for comet assay, and plasma for total thiols measurement. Butylated hydroxytoluene (BHT) was used as an antioxidant preservative for biochemical analysis.
DNA damage
Lymphocytes were separated from whole blood and freshly used for the comet assay as previously described2222. Singh NP, McCoy MT, Tice RR, Schneider EL. A simple technique for quantitation of low levels of DNA damage in individual cells. Exp Cell Res. 1998;175:184-91., with some modifications. Lymphocytes (20 µL) were mixtured with 90 µL of low-melting point agarose, and embedded in agarose gel on microscope slides, and kept in lysing solution. The slides were left in a high-pH buffer for 20 min to allow DNA unwinding. Electrophoresis was conducted at 25 V and 300 mA for 20 min. Following neutralization, the slides were fixed with ethanol and stained with 60 µL SYBR® green and immediately analyzed under a fluorescent microscope (Leica DMI 6000, Switzerland) by visual scoring as previously described2323. Collins AR. The comet assay for DNA damage and repair: principles, applications, and limitations. Mol Biotechnol. 2004;26:249-61.. One hundred nucleoids were randomly analyzed and assigned to one of five classes, from 0 (no tail) to 4 (almost all DNA in tail). The total score followed the calculation formula 1 x n1 + 2 x n2 + 3 x n3 + 4 x n4, where nrepresents nucleoid number attributable to each damage class (score). Thus, the total score was between 0 and 400 arbitrary units.
Biochemical analyses
Total thiols interacted with 5, 5'-dithiobis-(2-nitrobenzoic acid) (DTNB), forming a yellow complex with absorbance measured at 412 nm in spectrophotometer2424. Ellman GL. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959;82:70-7..
Antioxidant defense was also evaluated through the most important enzymes. CAT activity was evaluated as previously described2525. Aebi H. Catalase in vitro. Methods Enzymol. 1984;105:121-6.. SOD activity was determined by using a commercially available assay - Ransod kit (Randox Laboratories, UK), according to instructions, and GPX was evaluated using the Ransel kit according to the manufacturer's protocol (Randox Laboratories, UK). For biochemical analysis, the DTX 800 Multimode Detector (Beckman Couter, Germany) and UV/Visible Spectrophotometers model T70 (PG Instruments Limited, UK) were used.
Statistics
The characteristics of the study subjects and biochemical data are expressed as means and standard deviations (X ± SD). Student's t-test was used to compare groups and ANOVA was applied in the analysis of exposure times (exposed group), followed by Tukey's test. The distribution of gender in each group was compared using the Chi-square test. For comet assay data (expressed as median, 25% and 75%), the non-parametric Mann-Whitney test was used to compare the groups, while the test of Friedman was applied to compare exposure times within the same group. Pearson's correlation was used to correlate DNA damage with thiols and GPX only at 22 months of exposure, since this time point presented the most significant alterations. p<0.05 was considered statistically significant.
Results
Demographic characteristics of subjects are shown in Table 1. There were no significant differences between the groups in age, gender, weight, height or body mass index (p>0.05). The average length of exposure to waste anesthetic gases among medical residents was 30h/week.
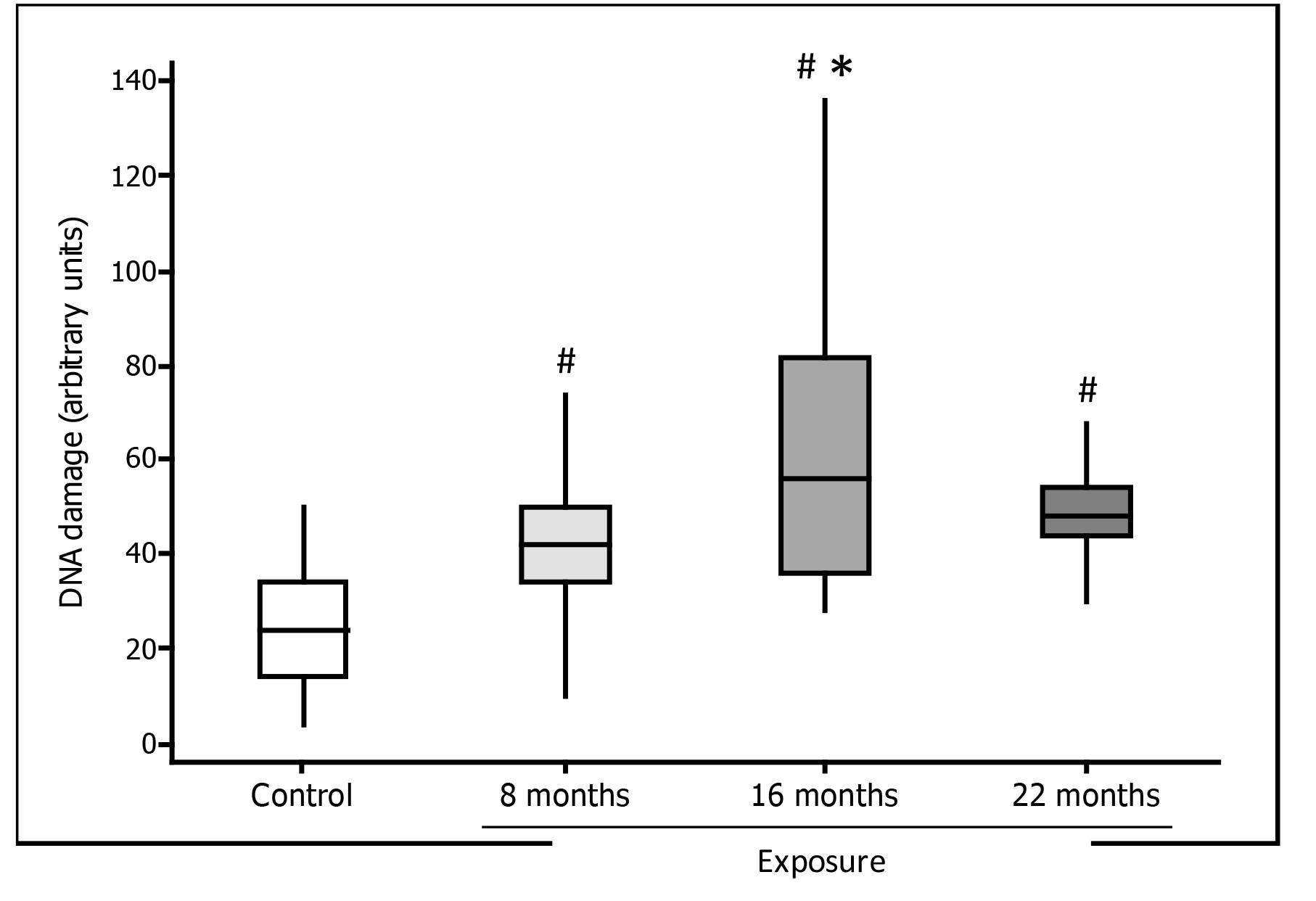
Lymphocyte DNA damage assessed by comet assay in medical residents exposed to waste anesthetic gases was significantly increased at all time points evaluated, in comparison with controls. In the exposed group, DNA damage scores were significantly higher at 16 months than at eight months of exposure (Figure 1).
Lymphocyte DNA damage detected by the comet assay (boxplot) in residents exposed to waste anesthetic gases and in unexposed volunteers (control). # p=0.001 compared to the control group; * p<0.01 in relation to eight months of exposure.
Thiols content was significantly higher at 22 months of exposure in medical residents than in controls. In the exposed group, plasma thiol values were increased at 22 months of exposure compared with the other previous samplings (p<0.05) (Figure 2).
Plasma thiols-SH (X ± SD) in both groups. # p<0.001 versus control group. * p<0.001 compared to eight and 16 months.
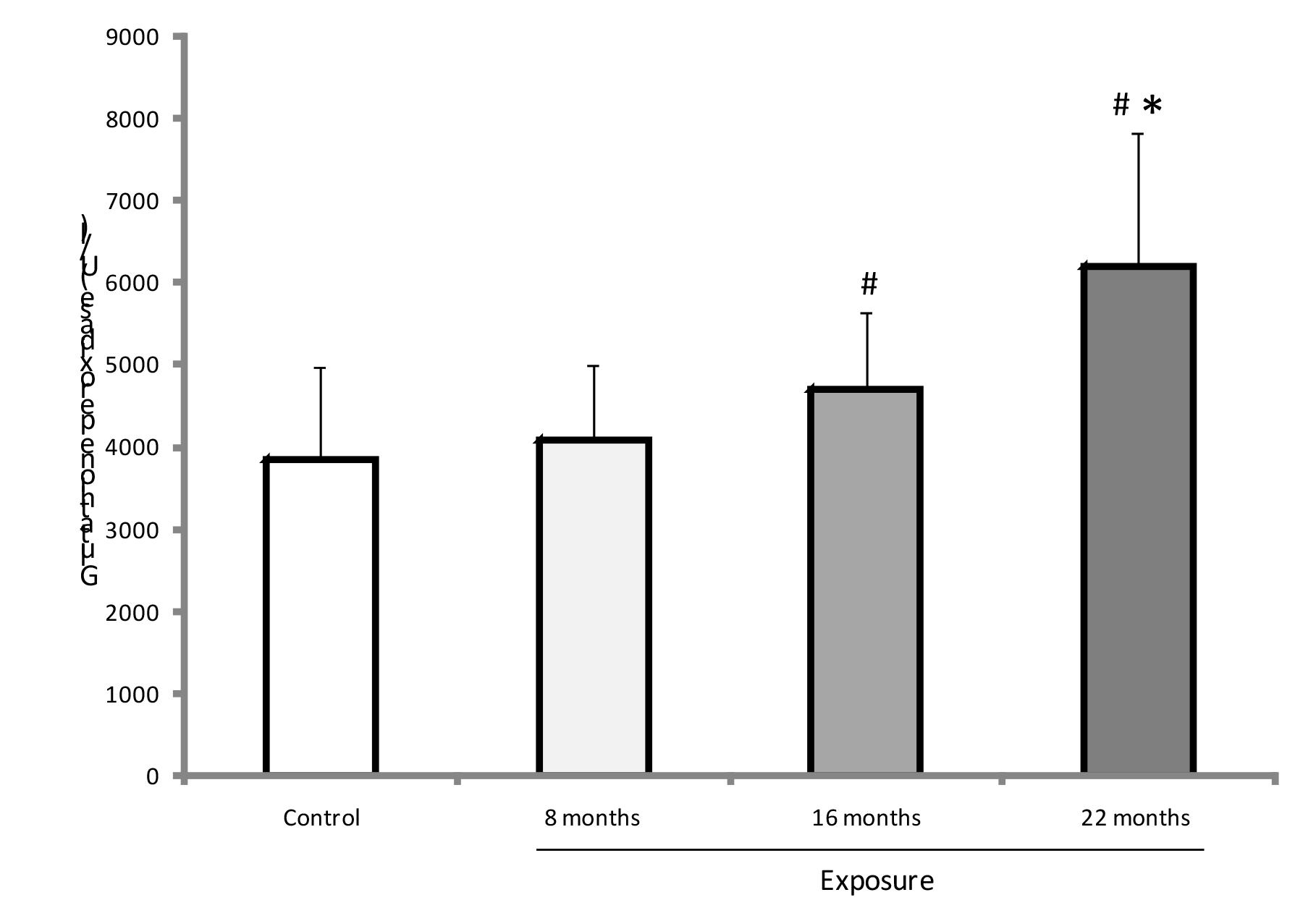
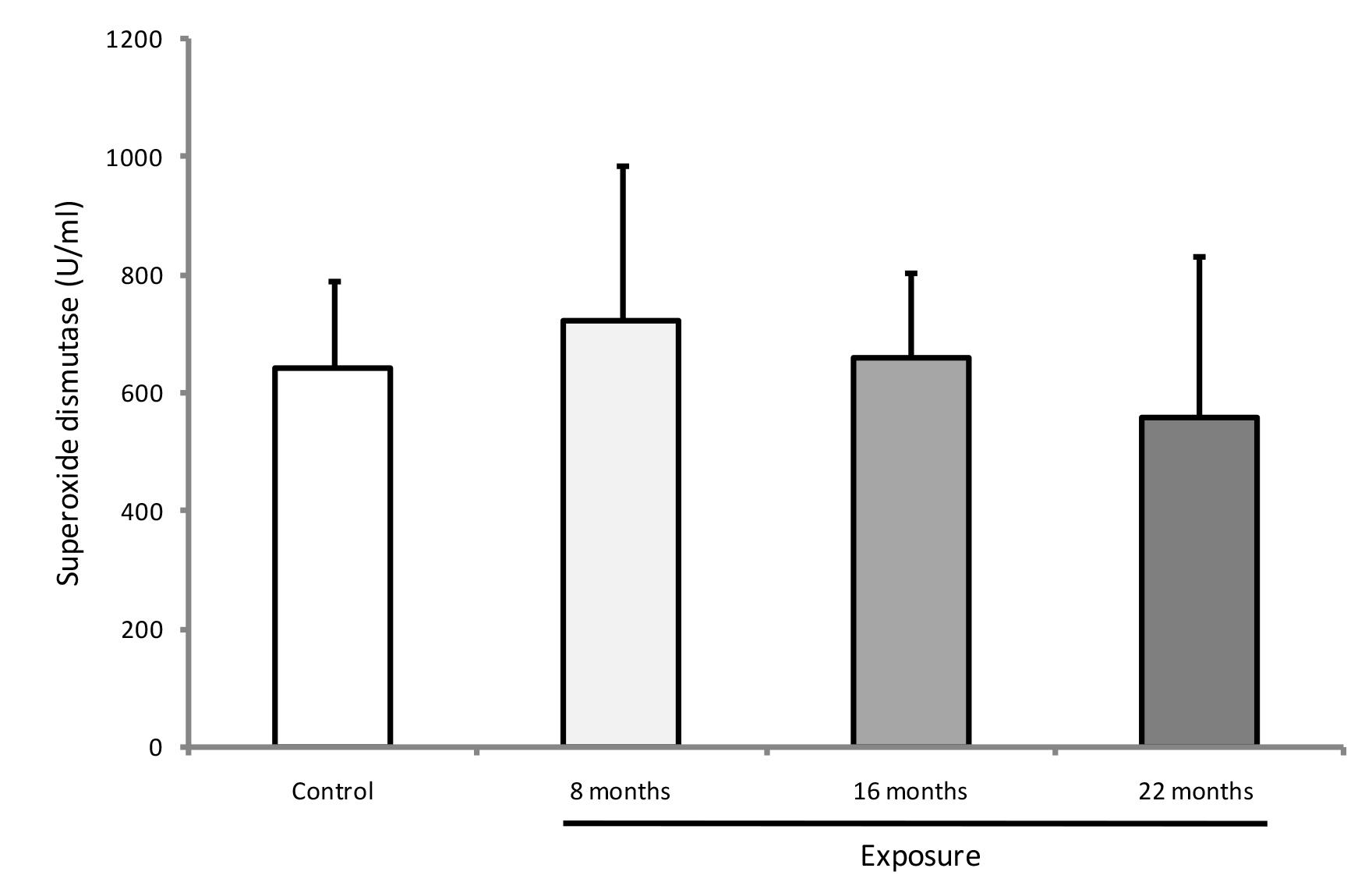
GPX was higher at 16 and 22 months of exposure among residents than in the non-exposed group (p<0.01) (Figure 3). At 22 months of exposure, GPX activity was significantly enhanced in the exposed group compared with the other two samplings (p<0.001). CAT enzyme activity showed no difference between groups (p>0.05), but it was lower at 22 months of exposure when compared with other exposure times in the resident group (p=0.012) (Figure 4). SOD activity did not significantly differ between groups or among exposure times (p>0.05) (Figure 5).
Antioxidant GPX (X ± SD) in both groups. # p<0.05 versus control group; * p<0.001 compared to eight and 16 months.
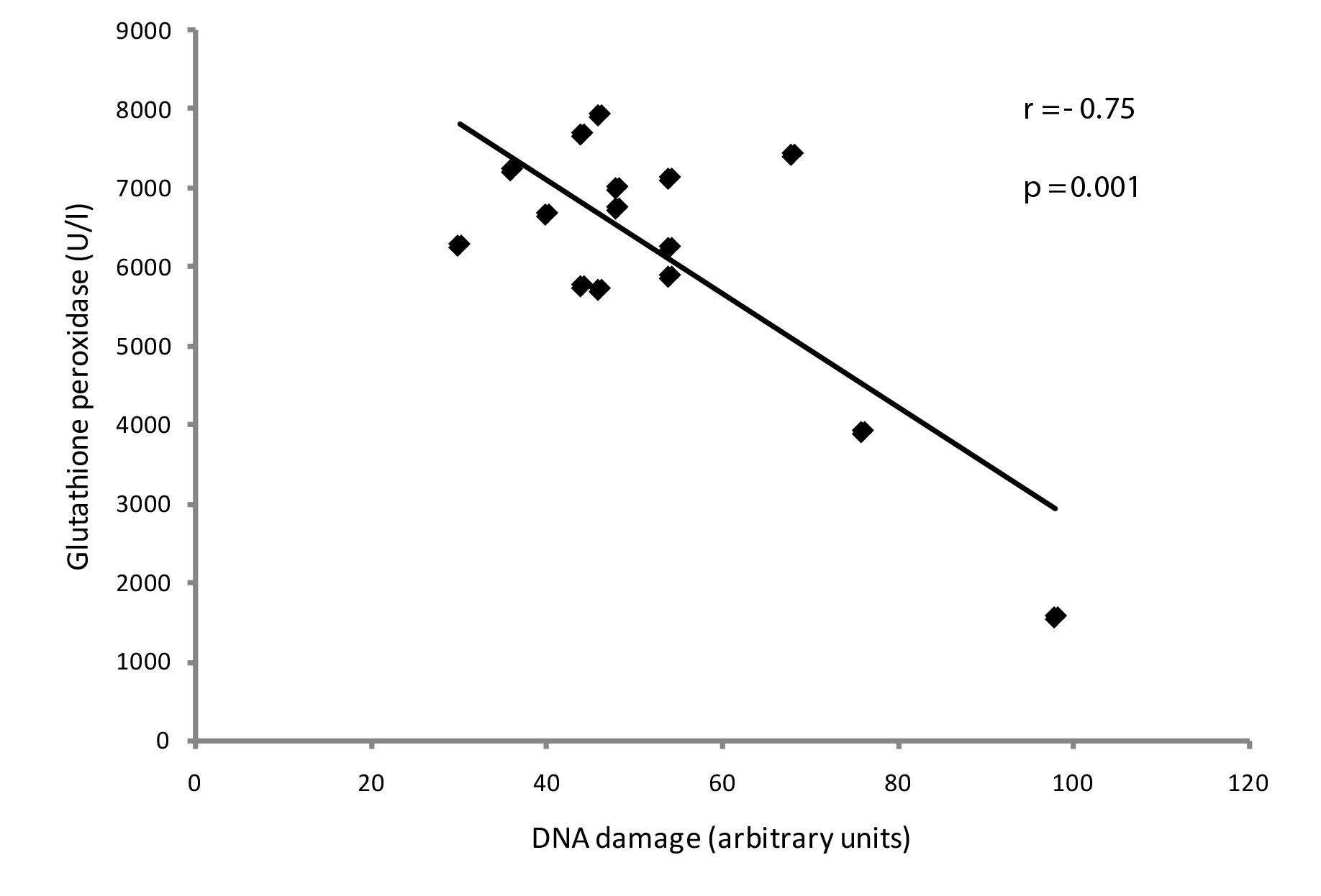
The correlations of genotoxicity with antioxidant protection were determined at 22 months of exposure in the resident group. There was a significant negative correlation between DNA damage and thiols (p=0.03) and also between DNA damage and GPX (p=0.001) (Figures 6 and 7, respectively). There were no significant correlations between DNA damage and CAT (p=0.7) or SOD (p=0.2).
Negative correlation between genetic damage and total thiols in the exposed group (residents) at 22 months of exposure (p<0.05).
Negative correlation between DNA damage and GPX activity in medical residents exposed to waste anesthetic gases at 22 months (p<0.05).
Discussion
Our study is the first to show that young professionals exposed to waste anesthetic gases already during medical residency have increased DNA damage and altered antioxidant protection.
A large number of studies conducted in professionals who worked for several years in operating rooms with no active scavenging system have related exposure to waste anesthetic gases with genetic modifications. Thus, increased DNA damage has been observed in professionals occupationally exposed for an average of 19 years to volatile anesthetics and N2O1717. El-Ebiary A, Abuelfadl A, Sarhan N, Othman M. Assessment of genotoxicity risk in operation room personnel by the alkaline comet assay. Hum Exp Toxicol. 2013;32:563-70.. Operating room personnel exposed for 12 years to halothane and N2O have shown increased frequency of MN and CA2626. Rozgaj R, Kasuba V, Jazbez A. Preliminary study of cytogenetic damage in personnel exposed to anesthetic gases. Mutagenesis. 2001;16:139-43.. Occupational exposure to halothane, enflurane, isoflurane, sevoflurane, desflurane and N2O for around 11 years has been reported in professionals with increased DNA damage levels, MN formation and CA1313. Chandrasekhar M, Rekhadevi PV, Sailaja N, Rahman MF, Reddy JP, Mahboob M, Grover P. Evaluation of genetic damage in operating room personnel exposed to anaesthetic gases. Mutagenesis. 2006;21:249-54.. Anesthetits/nurses/technicians exposed for 15 years to waste anesthetic gases have had more genetic damage including SCE, structural CA and MN than female workers not occupationally exposed or even female radiologists1212. Bilban M, Jakopin CB, Ogrinc D. Cytogenetic tests performed on operating room personnel (the use of anaesthetic gases). Int Arch Occup Environ Health. 2005;78:60-4.. Exposure to halothane has shown clastogenic effect in lymphocytes from anesthesiologists1111. Chinelato AR, Froes ND. Genotoxic effects on professionals exposed to inhalation anesthetics. Rev Bras Anestesiol. 2002;52:79-85.. Our results indicate that occupational exposure to anesthetic gases in operating rooms without an adequate scavenging system is genotoxic, even during a lower exposure time. We have observed that DNA lesions were increased from 8 months up to 22 months of exposure, with the highest levels at 16 months when subjects were exposed most of the time to halogenated and N2O anesthetics. It is noteworthy that among the residents evaluated in our study the length of exposure time was considerably long.
To minimize potential health risks, public authorities have recommended threshold values to waste anesthetic gases. Thus, the exposure limits recommended by the National Institute of Occupational Safety and Health (NIOSH) are 2 ppm for volatile anesthetics and 25 ppm for N2O. The value of halogenated anesthetic is reduced to 0.5 ppm when used concomitantly with N2O2727. NIOSH: National Institute of Occupational Safety and Health (1977) Criteria for a recommended standard: occupational exposure to waste anesthetic gases and vapors, Cincinnati, Ohio, USA: United States Department of Health, Education, and Welfare (DHEW), 77-140.. Low-level exposure to halogenated anesthetics (< 0.5 ppm) and N2O (12 ppm) does not cause MN in operating room personnel, but high-level exposure to halogenated (4 ppm) and N2O (170 ppm), for 20 h/week, causes genotoxic effects2828. Wiesner G, Hoerauf K, Schroegendorfer K, Sobczynski P, Harth M, Ruediger HW. High-level, but not low-level, occupational exposure to inhaled anesthetics is associated with genotoxicity in the micronucleus assay. Anesth Analg. 2001;92:118-22.. However, among individuals occupationally exposed for 8 h/day to anesthetic gases, even if values were within the normal range of N2O and halogenated, the frequency of SCE was increased2929. Hoerauf KH, Wiesner G, Schroegendorfer KF, Jobst BP, Spacek A, Harth M, Sator-Katzenschlager S, Rüdiger HW. Waste anaesthetic gases induce sister chromatid exchanges in lymphocytes of operating room personnel. Br J Anaesth. 1999;82:764-6..
The possible mechanisms underlying the genotoxic effects of halogenated anesthetics have been speculated. These drugs, including isoflurane, can directly damage DNA or be metabolized, giving rise to reactive metabolites3030. Jaloszynski P, Kujawski M, Wasowicz M, Szulc R, Szyfter K. Genotoxicity of inhalation anesthetics halothane and isoflurane in human lymphocytes studied in vitro using the comet assay. Mutat Res. 1999;439:199-206.. Another suggested mechanism is that anesthetic gases could act similarly to radiomimetic drugs, such as S-independent compound, inducing damage in all cell cycle phases1111. Chinelato AR, Froes ND. Genotoxic effects on professionals exposed to inhalation anesthetics. Rev Bras Anestesiol. 2002;52:79-85.. Besides that, N2O promotes reduction of cyanocobalamin (vitamin B12) molecule of methionine synthase followed by formation of superoxide and hydroxyl radicals and inactivation of the enzyme that catalyzes the remethylation of homocysteine to methionine3131. Drummond JT, Matthews RG. Nitrous oxide degradation by cobalamin-dependent methionine synthase: characterization of the reactants and products in the inactivation reaction. Biochemistry. 1994;33:3732-41..
Operating room personnel exposed to halothane and N2O showed increased lipid peroxidation, decreased thiols content, with no antioxidant capacity changes, compared to non-exposed individuals1919. Malekirad AA, Ranjbar A, Rahzani K, Kadkhodaee M, Rezaie A, Taghavi B, Abdollahi M. Oxidative stress in operating room personnel: occupational exposure to anesthetic gases. Hum Exp Toxicol. 2005;24:597-601.. In our study, medical residents were mainly exposed to isoflurane, but not to halothane, and only sometimes to sevoflurane and N2O. The antioxidant enzymes, such as SOD and CAT activities remained unchanged in the medical residents compared with control group. Within the exposed group, CAT was consumed, especially at 22 months of exposure, showing increased oxidative stress. On the other hand, thiols started to increase at 16 months of exposure, with significant elevation at 22 months. Similarly, GPX activities enhanced at 16 and 22 months of exposure to anesthetics. Thus, there is a negative correlation between DNA damage and antioxidant status, especially after 22 months of exposure. This antioxidant mechanism is likely to be important as DNA damage occurred throughout exposure. Differently, decreased plasma and erythrocyte SOD and GPX, as well as trace elements, such as selenium, have been observed in anesthesia and surgery personnel working at least 6 hours daily for at least 3 years in operating theaters with no active scavenging systems2020. Türkan H, Aydin A, Sayal A. Effect of volatile anesthetics on oxidative stress due to occupational exposure. World J Surg. 2005;29:540-2.. Cytochrome P450 2E1 is the most important enzyme for phase 1 metabolism of halogenated anesthetics. ROS, such as superoxide and hydrogen peroxide, are generated during this stage3232. Cederbaum AI. CYP2E1-biochemical and toxicological aspects and role in alcohol-induced liver injury. Mt Sinai J Med. 2006;73:657-72. , 3333. Bezerra FJ, do Vale NB, Macedo BO, Rezende AA, Almeida MG. Evaluation of antioxidant parameters in rats treated with sevoflurane. Rev Bras Anestesiol. 2010;60:93-7.. The binding of reactive intermediates to macromolecules may adversely affect cellular metabolism, protein synthesis, nucleic acids and lipids, producing a variety of injuries such as mutagenesis and carcinogenesis3434. Baden JM, Rice SA. Metabolism and toxicity of inhaled anesthetics. In: Miller RD, ed. Anesthesia. Philadelphia: Churchill Livingstone; 2000 .147-73.. Thus, our data indicate that both GPX and CAT activities, but not SOD, are directly related to hydrogen peroxide metabolism, and that exposure to waste anesthetic gases affects this antioxidant defense system. Our findings suggest that damage on DNA stimulated antioxidant defense, such as thiols and GPX to defend the organism against the aggression. Depletion of glutathione, which can promote injury leading to cell death, has been reported in professionals exposed to waste anesthetic gases for several years2020. Türkan H, Aydin A, Sayal A. Effect of volatile anesthetics on oxidative stress due to occupational exposure. World J Surg. 2005;29:540-2. , 3535. Wrońska-Nofer T, Nofer JR, Jajte J, Dziubaltowska E, Szymczak W, Krajewski W, Wasowicz W, Rydzyński K. Oxidative DNA damage and oxidative stress in subjects occupationally exposed to nitrous oxide (N2O). Mutat Res. 2012;731:58-63.. In this study, however, the subjects evaluated were young healthy adults newly exposed to waste anesthetic gases, who were still capable of providing adequate antioxidant defense responses.
Technical anesthesiology staff presented a higher level of DNA breaks than controls. Even after a12-week supplementation with ascorbic acid (500 mg/day) and tocopherol (300 mg/day), the exposed group, although with a reduction of DNA damage, still showed more damage than the controls3636. Sardas S, Izdes S, Ozcagli E, Kanbak O, Kadioglu E. The role of antioxidant supplementation in occupational exposure to waste anaesthetic gases. Int Arch Occup Environ Health. 2006;80:154-9.. Nurses have been reported to have increased oxidative DNA damage, lipoperoxidation and decreased GPX activity without alteration of alpha-tocopherol levels, when exposed from five to 27 years to high levels of N2O3535. Wrońska-Nofer T, Nofer JR, Jajte J, Dziubaltowska E, Szymczak W, Krajewski W, Wasowicz W, Rydzyński K. Oxidative DNA damage and oxidative stress in subjects occupationally exposed to nitrous oxide (N2O). Mutat Res. 2012;731:58-63.. Based on that, the same authors suggest that increased oxidative stress may represent a mechanism link between chronic exposure to N2O and genotoxicity.
Considering the outcome of this study, i.e. an increase of genetic material damage/oxidative stress caused by waste anesthetic gases, it is necessary an awareness of professionals to minimize the occupational exposure. In light of this, an active scavenging system is the most important asset for operating theaters. Additionally, better anesthesia equipment under constant maintenance, as well as the use of low fresh gas flow and total intravenous anesthesia can reduce the risks from occupational exposure.
Conclusion
The professionals occupationally exposed to waste anesthetic gases, such as isoflurane, sevoflurane and N2O, in operating rooms without adequate scavenging system may have DNA damage and changes in antioxidant defense already during medical residency.
To Prof. Lídia Raquel de Carvalho (Department of Bioestatistics, Institute of Biosciences - UNESP) for statistical advice.
References
-
1Plummer JL, Sandison CH, Ilsley AH, Cousins MJ. Attitudes of anaesthetists and nurses to anaesthetic pollution. Anaesth Intensive Care. 1987;15:411-20.
-
2Saurel-Cubizolles MJ, Estryn-Behar M, Maillard MF, Mugnier N, Masson A, Monod G. Neuropsychological symptoms and occupational exposure to anaesthetics. Br J Ind Med. 1992;49:276-81.
-
3Green CJ. Anaesthetic gases and health risks to laboratory personnel: a review. Lab Anim. 1981;15:397-403.
-
4Franco G, Marraccini P, Santagostino G, Filisetti P, Preseglio I. Behaviour of urinary D-glucaric acid excretion in surgical patients and anaesthesiology staff acutely exposed to isoflurane and nitrous oxide. Med Lav. 1991;82:527-32.
-
5Lucchini R, Placidi D, Toffoletto F, Alessio L. Neurotoxicity in operating room personnel working with gaseous and nongaseous anesthesia. Int Arch Occup Environ Health. 1996;68:188-92.
-
6Rowland AS, Baird DD, Shore DL, Weinberg CR, Savitz DA, Wilcox AJ. Nitrous oxide and spontaneous abortion in female dental assistants. Am J Epidemiol. 1995;141:531-8.
-
7Boivin JF. Risk of spontaneous abortion in women occupationally exposed to anaesthetic gases: a meta-analysis. Occup Environ Med. 1997;54:541-8.
-
8Ahlborg Jr G, Axelsson G, Bodin L. Shift work, nitrous oxide exposure and subfertility among Swedish midwives. In t J Epidemiol. 1996;25:783-90.
-
9Bodin L, Axelsson G, Ahlborg Jr G. The association of shift work and nitrous oxide exposure in pregnancy with birth weight and gestational age. Epidemiology . 1999;10:429-36.
-
10Sardas S, Aygün N, Gamli M, Unal Y, Unal N, Berk N, Karakaya AE. Use of alkaline comet assay (single cell gel electrophoresis technique) to detect DNA damages in lymphocytes of operating room personnel occupationally exposed to anaesthetic gases. Mutat Res. 1998;418:93-100.
-
11Chinelato AR, Froes ND. Genotoxic effects on professionals exposed to inhalation anesthetics. Rev Bras Anestesiol. 2002;52:79-85.
-
12Bilban M, Jakopin CB, Ogrinc D. Cytogenetic tests performed on operating room personnel (the use of anaesthetic gases). Int Arch Occup Environ Health. 2005;78:60-4.
-
13Chandrasekhar M, Rekhadevi PV, Sailaja N, Rahman MF, Reddy JP, Mahboob M, Grover P. Evaluation of genetic damage in operating room personnel exposed to anaesthetic gases. Mutagenesis. 2006;21:249-54.
-
14Eroglu A, Celep F, Erciyes N. A comparison of sister chromatid exchanges in lymphocytes of anesthesiologists to nonanesthesiologists in the same hospital. Anesth Analg. 2006;102:1573-7.
-
15Rozgaj R, Kasuba V, Brozovic G, Jazbec A. Genotoxic effects of anaesthetics in operating theatre personnel evaluated by the comet assay and micronucleus test. Int J Hyg Environ Health. 2009;212:11-7.
-
16Wrońska-Nofer T, Palus J, Krajewski W, Jajte J, Kucharska M, Stetkiewicz J, Wasowicz W, Rydzyński K. DNA damage induced by nitrous oxide: study in medical personnel of operating rooms. Mutat Res. 2009;666:39-43.
-
17El-Ebiary A, Abuelfadl A, Sarhan N, Othman M. Assessment of genotoxicity risk in operation room personnel by the alkaline comet assay. Hum Exp Toxicol. 2013;32:563-70.
-
18Ferguson LR, Philpott M, Karunasinghe N. Oxidative DNA damage and repair: significance and biomarkers. J Nutr. 2006;136:2687S-9S.
-
19Malekirad AA, Ranjbar A, Rahzani K, Kadkhodaee M, Rezaie A, Taghavi B, Abdollahi M. Oxidative stress in operating room personnel: occupational exposure to anesthetic gases. Hum Exp Toxicol. 2005;24:597-601.
-
20Türkan H, Aydin A, Sayal A. Effect of volatile anesthetics on oxidative stress due to occupational exposure. World J Surg. 2005;29:540-2.
-
21Baysal Z, Cengiz M, Ozgonul A, Cakir M, Celik H, Kocyigit A. Oxidative status and DNA damage in operating room personnel. Clin Biochem. 2009;42:189-93.
-
22Singh NP, McCoy MT, Tice RR, Schneider EL. A simple technique for quantitation of low levels of DNA damage in individual cells. Exp Cell Res. 1998;175:184-91.
-
23Collins AR. The comet assay for DNA damage and repair: principles, applications, and limitations. Mol Biotechnol. 2004;26:249-61.
-
24Ellman GL. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959;82:70-7.
-
25Aebi H. Catalase in vitro. Methods Enzymol. 1984;105:121-6.
-
26Rozgaj R, Kasuba V, Jazbez A. Preliminary study of cytogenetic damage in personnel exposed to anesthetic gases. Mutagenesis. 2001;16:139-43.
-
27NIOSH: National Institute of Occupational Safety and Health (1977) Criteria for a recommended standard: occupational exposure to waste anesthetic gases and vapors, Cincinnati, Ohio, USA: United States Department of Health, Education, and Welfare (DHEW), 77-140.
-
28Wiesner G, Hoerauf K, Schroegendorfer K, Sobczynski P, Harth M, Ruediger HW. High-level, but not low-level, occupational exposure to inhaled anesthetics is associated with genotoxicity in the micronucleus assay. Anesth Analg. 2001;92:118-22.
-
29Hoerauf KH, Wiesner G, Schroegendorfer KF, Jobst BP, Spacek A, Harth M, Sator-Katzenschlager S, Rüdiger HW. Waste anaesthetic gases induce sister chromatid exchanges in lymphocytes of operating room personnel. Br J Anaesth. 1999;82:764-6.
-
30Jaloszynski P, Kujawski M, Wasowicz M, Szulc R, Szyfter K. Genotoxicity of inhalation anesthetics halothane and isoflurane in human lymphocytes studied in vitro using the comet assay. Mutat Res. 1999;439:199-206.
-
31Drummond JT, Matthews RG. Nitrous oxide degradation by cobalamin-dependent methionine synthase: characterization of the reactants and products in the inactivation reaction. Biochemistry. 1994;33:3732-41.
-
32Cederbaum AI. CYP2E1-biochemical and toxicological aspects and role in alcohol-induced liver injury. Mt Sinai J Med. 2006;73:657-72.
-
33Bezerra FJ, do Vale NB, Macedo BO, Rezende AA, Almeida MG. Evaluation of antioxidant parameters in rats treated with sevoflurane. Rev Bras Anestesiol. 2010;60:93-7.
-
34Baden JM, Rice SA. Metabolism and toxicity of inhaled anesthetics. In: Miller RD, ed. Anesthesia. Philadelphia: Churchill Livingstone; 2000 .147-73.
-
35Wrońska-Nofer T, Nofer JR, Jajte J, Dziubaltowska E, Szymczak W, Krajewski W, Wasowicz W, Rydzyński K. Oxidative DNA damage and oxidative stress in subjects occupationally exposed to nitrous oxide (N2O). Mutat Res. 2012;731:58-63.
-
36Sardas S, Izdes S, Ozcagli E, Kanbak O, Kadioglu E. The role of antioxidant supplementation in occupational exposure to waste anaesthetic gases. Int Arch Occup Environ Health. 2006;80:154-9.
-
1
Research performed at Faculty of Pharmaceutical Sciences, Federal University of Amazonas (UFAM) and UFAM Hospital, Manaus-AM, Brazil. Part of Fellow PhD degree thesis, Postgraduate Program in Anesthesiology, Sao Paulo State University (UNESP). Tutor: Prof. José Reinaldo Cerqueira Braz.
-
Financial source: Coordination of Improvement for Higher Academic Staff (CAPES).
Publication Dates
-
Publication in this collection
Apr 2014
History
-
Received
12 Dec 2013 -
Reviewed
12 Feb 2014 -
Accepted
12 Mar 2014