Abstract
It has been previously shown that the crude venom of Tityus serrulatus can cause convulsions. This study was designed to investigate the neurotoxic effects of B, C, G, and K fractions isolated from this venom. Intravenous injection of these fractions in mice (0.6 - 6.0 mg/kg body weight) showed that the C fraction is a potent convulsant and G fraction decreased the threshold for tonic hand limb extension elicited by transauricular electroshock. Unilateral injection of B, C, and K fractions, but not G fraction, into the hippocampus of rats (0.6 - 6.0 µg) caused electroencephalographic alterations consisting of spikes and epileptic discharges that began in the hippocampus and evolved to the cortex. The following motor signs were observed: movements of facial muscles, wet dog shake, immobility, myoclonus, wild-running with clonus, and in some cases, loss of postural control. Intrahippocampal injection of B, C, and K fraction, but not G fraction, caused neuronal loss at the injection site as well as in other hippocampal areas. The effect of these fractions on epileptiform activity and on neuronal loss was dose-dependent. The severity of the epileptiform activity in the ipsilateral hippocampus correlated with the severity of the neuronal loss. The electrographic, behavioral, and histological changes induced by B, C, and K fractions were similar to those obtained with other drugs that are commonly used to induce convulsion. The convulsant effects of the crude venom may be caused by the fractions studied in this work.
scorpion fractions; electroshock; seizure; neurodegeneration; EEG record; hippocampus; Tityus serrulatus
Convulsive effects of some isolated venom fractions of the Tityus serrulatus scorpion: behavioral, electroencephalographic, and neuropathological aspects
F. F. CARVALHO , A. L. A. NENCIONI
, A. L. A. NENCIONI , I. LEBRUN
, I. LEBRUN , V. A. C. DORCE
, V. A. C. DORCE , M. R. L. SANDOVAL
, M. R. L. SANDOVAL
 CORRESPONDENCE TO:
M.R.L. SANDOVAL - Laboratório de Farmacologia do Instituto Butantan, Av. Dr. Vital Brasil, 1500, CEP: 05503-900, São Paulo, SP, Brazil.
Phone: 55 11 8137222 - Extension 2299, FAX: 55 11 813 7222 - Extension 2162
E-mail:
mrlsando@usp.br
CORRESPONDENCE TO:
M.R.L. SANDOVAL - Laboratório de Farmacologia do Instituto Butantan, Av. Dr. Vital Brasil, 1500, CEP: 05503-900, São Paulo, SP, Brazil.
Phone: 55 11 8137222 - Extension 2299, FAX: 55 11 813 7222 - Extension 2162
E-mail:
mrlsando@usp.br
1 Laboratory of Pharmacology and 2 Laboratory of Biochemistry and Biophysics, Butantan Institute, São Paulo, SP, 05503 900, Brazil.
ABSTRACT. It has been previously shown that the crude venom of Tityusserrulatus can cause convulsions. This study was designed to investigate the neurotoxic effects of B, C, G, and K fractions isolated from this venom. Intravenous injection of these fractions in mice (0.6 6.0 mg/kg body weight) showed that the C fraction is a potent convulsant and G fraction decreased the threshold for tonic hand limb extension elicited by transauricular electroshock. Unilateral injection of B, C, and K fractions, but not G fraction, into the hippocampus of rats (0.6 6.0 µg) caused electroencephalographic alterations consisting of spikes and epileptic discharges that began in the hippocampus and evolved to the cortex. The following motor signs were observed: movements of facial muscles, wet dog shake, immobility, myoclonus, wild-running with clonus, and in some cases, loss of postural control. Intrahippocampal injection of B, C, and K fraction, but not G fraction, caused neuronal loss at the injection site as well as in other hippocampal areas. The effect of these fractions on epileptiform activity and on neuronal loss was dose-dependent. The severity of the epileptiform activity in the ipsilateral hippocampus correlated with the severity of the neuronal loss. The electrographic, behavioral, and histological changes induced by B, C, and K fractions were similar to those obtained with other drugs that are commonly used to induce convulsion. The convulsant effects of the crude venom may be caused by the fractions studied in this work.
KEY WORDS: scorpion fractions, electroshock, seizure, neurodegeneration, EEG record, hippocampus, Tityus serrulatus..INTRODUCTION
Neurotoxins of animal or plant origin have been of interest to several areas of biology. These substances are important tools for the study of different neurobiological phenomena, providing valuable information about the functions of the central nervous system (CNS). Scorpion venom has also been used as a source of neurotoxins. These neurotoxins are basic polypeptides with a molecular weight of 4,000 to 7,000 (12,43). They act mainly on sodium (7,15,16) and potassium (15) channels. Scorpion toxins that act on mammalian sodium channels have about 70 amino acid residues and can delay the inactivation of the sodium channel (type a) or increase channel activation (type b) (6,12,21). In the last decade, toxins of several scorpions with less than 40 amino acid residues and 3 disulfide bonds have been purified (12). These toxins act by blocking different types of potassium channels (12) and delay cell repolarization (12).
Several toxins from the venom of the Brazilian scorpion Tityus serrulatus have been isolated and characterized (17,20,25,26,34). Scorpion neurotoxins acting on sodium or potassium channels induce the same effect, as they increase the neurotransmitter release in the peripheral or central nervous system (18,27). Tityustoxin stimulates the release of norepinephrine, glutamate, g-aminobutyric acid, dopamine, and acetylcholine (27,28).
Convulsions are one of the symptoms of human (11) and animal scorpion envenomation (46). Research on scorpion neurotoxins in the CNS is poorly reported. Intracerebroventricular injection of Leiurus quinquestriatus scorpion venom (41) or a toxin isolated from Tityus serrulatus venom (30) induced seizures in rats. Intracellular recordings obtained after injection of Centruroides sculpturatus venom into the cerebral cortex induced epileptiform waves (2). In addition, cerebral hemorrhagic points were observed in animals that died after envenomation (33). To further study on the convulsive effect, we have investigated the effects of intravenous or intrahippocampal injections of Tityus serrulatus venom. In those studies, we observed behavioral and electrographic seizures (46) and changes in GABA and dopamine level in different brain areas (22). We proposed that a disarrangement of Na and K channels induced by scorpion venom might cause an excess neurotransmitter release in the area involved in the source or spread of the seizure. Thus, it may be responsible for the convulsive effect. Venom fractionation was necessary to further examine these interesting results. In this investigation, we evaluated the convulsive effects of selected fractions isolated from Tityus serrulatus venom after intravenous injection and intrahippocampal injections of these fractions using behavioral, electroencephalographic, and neurophatologic analysis.
MATERIALS AND METHODS
ANIMALS. Male Swiss-Webster mice weighing 25-30 g and male Wistar rats weighing 230-250 g were used. On their arrival at the laboratory 7 days before the experiments, the animals were housed in groups of 5-6 in wire mesh cages and maintained in a room with constant temperature (22 ± 1°C), on a 12 h light/dark cycle (lights on at 7:00 a.m.). Food and water were provided ad libitum. These animals were maintained in accordance with the guidelines of the Department of Pathology of the School of Veterinary Medicine at University of São Paulo, which are based on the guidelines for animal care prepared by the Committee on Care and Use of Laboratory Animal Resources, National Research Council, USA.
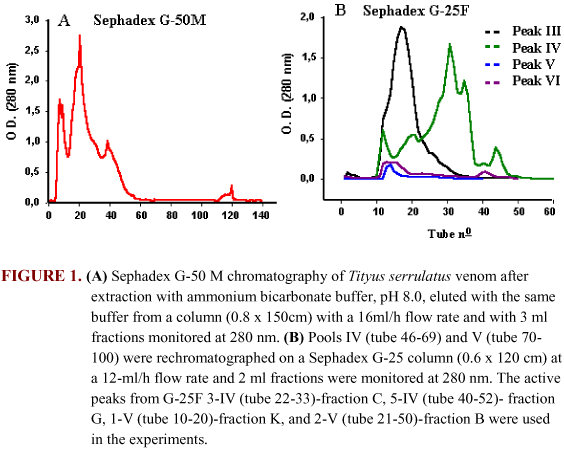
VENOM PURIFICATION. Freeze-dried fresh Tityus serrulatus venom was obtained from the Arthropod Laboratory of Butantan Institute. The venom was fractionated by gel filtration chromatography. The crude venom was eluted from a Sephadex G-50M column (0.8 x 150 cm) with 0.02 M ammonium bicarbonate (pH 8.0, flow 16 ml/h, 20°C). Fractions (3 ml each) were collected and the absorbance at 280 nm was measured (Figure 1A). Tubes 46-69 and 70-100 were pooled (pools III and IV) and rechromatographed on a Sephadex G-25 column (0.6 x 120 cm) with 0.02 M ammonium bicarbonate (pH 8.0, flow 12 ml/h, 20°C). Absorbance of the fractions (2 ml each) was measured at 280 nm (Figure 1B). Based on previous studies, the peaks from G-25F 3-IV (fraction C), 5-IV (fraction G), 1-V (fraction K), and 2-V (fraction B) were used in this study.
EFFECT OF INTRAVENOUS INJECTION OF VENOM FRACTIONS ON OPEN-FIELD ACTIVITY AND ON SEIZURE THRESHOLD. Mice were randomly assigned to a control group (injection of 0.9% NaCl into the tail vein, 10 µL/10g body weight, n=6) or to nine experimental groups (n=4-6). The animals in the experimental groups received an injection into the tail vein with either fraction C or K (0.6, 1.0 or 3.0 mg/kg) or fraction G (0.6, 3.0 or 6.0 mg/kg). All fractions were dissolved in 0.9% NaCl.
Immediately after the injection, each mouse was placed in the center of an open-field arena (10) and behavioral parameters were recorded for 15 min. Hand-operated counters and stop-watches were used to score locomotion (number of floor units entered), rearing frequency (number of times the animal stood on its hind legs), and immobility behavior (total time that the animals remained immobile).
Fifteen minutes after the end of the open-field test (30 min after toxin injection) the mice were subjected to electroshock (60 Hz, 0.3 sec, 13.75 mA) with transauricular electrodes (29). These shock parameters were obtained from a preliminary experiment. In this preliminary experiment, five groups of 10 naïve mice were subjected to shocks with a current of 10, 13.5, 14.5, 16, or 19.5 mA. Regression analysis showed that to produce tonic extension of the hind limbs in 30% of the animals, a current intensity of 13.75 mA was needed (95% confidence interval).
EFFECT OF INTRAHIPPOCAMPAL ADMINISTRATION OF VENOM FRACTIONS ON BEHAVIOR, EEG, AND NEURONAL DAMAGE. Rats were anesthetized with a mixture of pentobarbitone (1g) and chloral hydrate (4g) in 100 ml of 0.9% NaCl (3 ml/kg) for the implantation of electrodes and a brain cannula. They were placed in a stereotaxic frame. For brain injections, a stainless steel guide cannula was implanted in one side of the dorsal hippocampus (AP -4.8, L 3.5, and V 3.0 mm, according to Paxinos & Watson (42) and fixed with dental acrylate. In the contralateral hippocampus (AP -4.8, L 3.5, and V 3.5 mm), we implanted bipolar twisted electrodes to record hippocampal EEG. The electrodes were anchored to the skull with dental acrylate. For recordings of surface EEG, we inserted jeweler screws in the skull over the left and right occipital cortex, which functioned as electrodes. An additional screw placed in the frontal sinus served as a reference (indifferent) electrode. After surgery, the animals were housed individually and allowed to recover for 5 to 7 days.
On the day set for the experiment, the rats were transferred to a glass cage (30 x 30 x 39 cm). After 15 min of acclimatization, the EEG was recorded for 15 min. Then, either the vehicle (1 µL of 0.1 M phosphate buffer) or B, C, G, or K fraction solution (0.6, 1.0, or 6.0 mg/ml) was injected in the hippocampus. For these brain injections, we used a metal injector that protruded 0.5 mm beyond the tip of the guide cannula. EEG and behavior were recorded continuously for periods ranging from 2 to 5 hours after injection. Additional EEG recordings were made 24 hours after injection.
To analyze neuronal damage, as well as to verify the location of the implanted electrodes and cannulae, the brains were analyzed 7 days after toxin injection. The animals were anesthetized with ether and perfused through the heart (left ventricle) with phosphate buffered saline (PBS) followed by 10% formalin solution. The brains were removed, stored in formalin, and embedded in paraffin. Coronal sections of 10 mm were cut from a 700 mm brain block including the cannula track. Every seventh slice of 300 mm from either side of the track was mounted on a glass slide and stained with cresyl violet. Cells were counted in the hippocampal CA1, CA3, and CA4 pyramidal cell layers, and the granulate cell layer of the dentate gyrus. We magnified the slices 40 times, and counted all cells within a 100-µm grid (4). We compared identical regions of each hippocampus in each section.
grid (4). We compared identical regions of each hippocampus in each section.
STATISTICAL ANALYSIS. Data are shown as mean ± S.E.M. The maximal electrographic seizure (MES) discharges were used to analyze the EEG. Means were compared by ANOVA followed by the Dunnett or the Tukey Kramer test. The Fishers test was used to compare the number of animals with seizures in different treatment groups.
RESULTS
EFFECT OF INTRAVENOUS INJECTION OF VENOM FRACTIONS ON OPEN-FIELD ACTIVITY AND ON SEIZURE THRESHOLD. The intravenous injection of fraction C decreased mouse locomotion and rearing frequency (Table 1). Fraction C also had several other effects during the open-field test. Many mice had convulsions (4 out of 5 animals treated with the low dose, 3 out of 5 treated with the middle dose, and 6 out of 6 animals treated with the high dose). These seizures were characteristic clonic convulsions with loss of postural control preceded by wild running. One mouse treated with the high dose died. We also observed motor incoordination, irregular respiration, and increased salivary and bronchial secretion. These side effects seemed more common with the high dose. Electroshock caused no further convulsions in these mice except in a few animals that had not had a seizure. Most mice (4 out of 5) that had received the high dose of fraction C died immediately after electroshock application.
Fraction K did not alter the behavior in the open-field apparatus, but caused motor incoordination, irregular respiration, and increased salivary and bronchial secretion similar to that caused by fraction C. This fraction did not alter the electroshock effect as the incidence of tonic extension of the hind limbs was similar to mice injected with saline.
Fraction G did not alter the behavior in the open-field apparatus. This fraction potentiated the effect of electroshock, but the incidence of tonic extension of the hind limbs increased significantly only in the group treated with the low dose (4 out of 5). This tonic extension with the middle and high doses, respectively, was shown by 1out of 4 and 0 out of 5 animals, but this was not significantly different from the controls injected with saline.
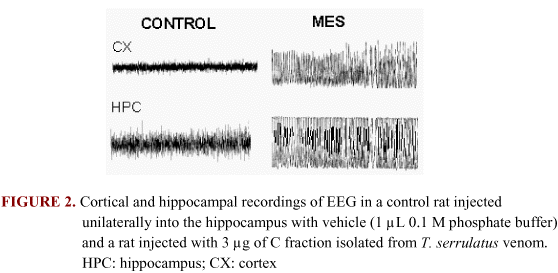
EFFECT OF INTRAHIPPOCAMPAL ADMINISTRATION OF VENOM FRACTIONS ON BEHAVIOR, EEG, AND NEURONAL DAMAGE. Injection of B, C, and K fractions, but not G fraction, into the dorsal hippocampus induced isolated and clustered spikes and maximal electrographic seizures (MES) (Figure 2 and Figure 3). The spikes started a few minutes after the toxin injection. These spikes were usually accompanied by wet dog shakes.
The interval between fraction injections and the first MES ranged from 5 to 60 min. This latency was lower for B fraction and higher for K fraction. MES first appeared at the site of injection and rapidly evolved to the cortex. From rats treated with high dose and C and K fractions, 6 out of 6 and 5 out of 6, respectively, still had MES 5 hours after the injection. The percentage of animals that had MES during the 2 hours after injection was dose dependent (Table 2). Twenty-four hours after microinjection of high dose C fraction, isolated spikes were observed in the electrographic record.
Analysis of MES frequency up to 2 hours after fraction injection showed that the 6.0 mg of B and C fractions induced a higher frequency of seizures when compared with the animals in the control group (Figure 3). Figure 3 shows the seizure duration of animals microinjected with 1.0 and 6.0 mg of B, C, and K fractions.
The injections of B, C, and K fractions in the hippocampus caused obvious behavioral changes such as immobility, wet dog shakes, movements of the facial muscles, myoclonus, wild running with clonus, and sometimes rearing and falling. The incidence of these behaviors during the first 2 hours after the injection is summarized in Table 3. Electrographic seizures did not always correspond to behavioral seizures.
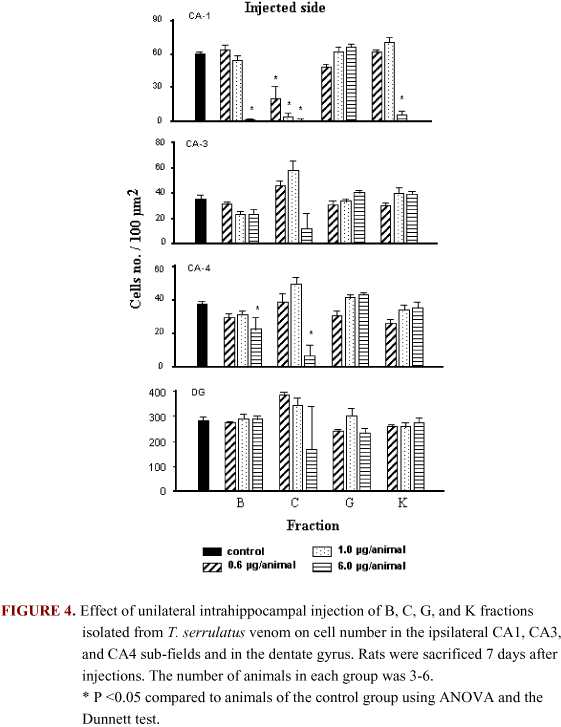
Histological analysis of the brains 7 days after treatment showed that B, C, and K fractions, but not G fraction, caused hippocampal damage (Figure 4). The area of neuronal loss showed extensive gliosis. Fraction C was especially harmful. Injection of 6.0 mg of C fraction into the CA1 area produced neuronal cell degeneration that was observed in the CA1, CA3, and CA4 pyramidal cell layers and in the DG ipisilaterally to the site of injection (Figure 4). Damage after the high dose C fraction was not limited to the ipsilateral hippocampus, but was also seen in the contralateral CA1 area.
DISCUSSION
The decrease in locomotion and rearing frequency observed after intravenous administration of the C fraction seems to be related to the presence of convulsions when the animal was in the open-field. The animals were in a post-convulsive state when electroshock was applied, and no further convulsion was observed. Fractions G and K injected intravenously were not able to induce convulsion. Fraction G had a pro-convulsive activity since it reduced the threshold to electroshock-induced convulsion. Seizure after intravenous injection of crude scorpion venom in rodents (46) and in severe human envenomation has been reported previously (11). A study designed to investigate the lung edema induced by Tityustoxin isolated from T. serrulatus venom reported a convulsive effect after intracerebroventricular administration of this toxin (30). In addition, intravenous injection of crude venom altered dopamine turnover and GAD activity (22). In this study, we observed generalized clonic convulsion with loss of postural control preceded by wild running, representing stages 4 and 5 of seizure development proposed by Racine (44).
The results obtained with B, C, and K fractions isolated from Tityus serrulatus venom clearly show that microinjections of these fractions into the dorsal hippocampus induced electroencephalographic and/or behavioral seizure and hippocampal cell damage. Only a small amount of data about the effects of Tityus serrulatus venom and toxins on the CNS is available in literature. Studies using crude venom have been published (22,46). Centruroides sculpturatus venom microinjected into the brain induced epileptiform waves in the intracellular record (2) and intracerebroventricular injection of Leiurus quinquestriatus venom induced seizure (41).
Scorpion toxins have been known to induce alterations in potassium and sodium membrane conductance (24,45) with consequent excessive neurotransmitter release (1,18). Also, the hyperexcitability of these channels may represent an intrinsic cellular mechanism, which could explain epileptogenesis (49). This mechanism may underlie the venom convulsant action, since convulsion is the last expression of a variety of pathological phenomena, resulting from an imbalance between neuronal excitation and inhibition (31,37,40,53). Thus, this process may be responsible for seizures observed in this study.
The latency for the onset of the seizures was longer for K fraction than for B or C fraction. This suggests that some toxins that compose K fraction may be acting antagonistically or have different mechanisms of action, interfering with the onset of the seizure. A similar effect was observed by Sandoval & Dorce (46), in a study in which whole venom of T. serrulatus delayed the onset of the effect of pentilenetetrazole-induced convulsion.
The frequency of seizures and the neuronal damage were dose dependent. It seems that there is a direct correlation between the frequency of seizures and the severity of hippocampal damage. This relationship has also been observed with the use of other convulsant substances like pilocarpine (51,52) and kainic acid (8).
The motor alterations observed after microinjection into the hippocampus of high doses of B, C, and K fractions, such as immobility, movements of facial muscles, unilateral or bilateral forelimb clonus, rearing, and loss of postural control in some animals were similar to those observed during stages 4 and 5 of seizure as defined by Racine (44). These behaviors are similar to but less intense than those observed after the administration of kainic acid (8), pilocarpine (51,52), or tetanic toxin (3). They are also similar to those of a-dendrotoxin, a selective K+ channel blocker isolated from Dendroaspis angusticips venom (5) and toxins from Crotalus durissus terrificus venom (36). In addition to these motor manifestations, wet dog shake (B, C, and K fractions) and wild running following clonus (B and C fractions) were observed.
It is known that electric stimulation of limbic structures (19,23) and systemic injection of kainic acid (39) can induce wet dog shake behavior and that the destruction of dentate gyrus granular cells in the hippocampus blocks it (23). In this study, the integrity of the granular cells appeared to be important for the wet dog shake manifestation.
Wild running following clonus appears to be a characteristic of audiogenic seizures (50). DBA2 mice, a strain genetically sensitive to audiogenic convulsion, show this behavior. It is known that the brain stem, cerebellum, and spinal cord (35) are involved in the manifestation of audiogenic seizures. We may assume that these brain structures are involved in the wild running observed after B, C, and K fraction administration.
Animals microinjected into the dorsal hippocampus with B, C, and K fractions showed significant neuronal loss and intense gliosis in CA1 and CA4 hippocampal subfields on the ipsilateral side of the injection. This effect was dose dependent in animals treated with C fraction.
The brain damage observed in contralateral CA1 is probably a consequence of the spread of epileptic activity rather than of diffusion of the toxin. It is unlikely that injections given slowly, in very small volumes in the CA1 sub-field can diffuse to the contralateral hippocampus (8,9). Even though the CA1 sub-field on the contralateral side showed no significant decrease in the number of pyramidal cells, several pycnotic cells were present, indicating neurodegeneration in this region.
Another fact that should be considered here is the involvement of excitatory amino acids in the convulsive effect and the neuronal loss observed with the scorpion fractions. The trisynaptic pathway is the hippocampal circuit most extensively studied in current neurobiological research (8,32). It is known that these synapses have glutamate as a neurotransmitter and that the excitation induced by glutamate can be involved in the excitotoxicity process (37). In this way, large quantities of glutamate and aspartate in the extracelular space cause an overstimulation of the NMDA and AMPA/Kainate receptors and a massive calcium influx is observed. This increase in calcium may induce neuronal death (13,47,48). We suggest that the convulsion and neuronal loss induced by B, C and K fractions could be due to an increase in the excitatory amino acids that are present in the major hippocampal pathway (14,38).
This study showed that mainly C fraction isolated from Tityus serrulatus scorpion venom can be responsible, at least in part, for convulsions observed after animal and human envenomation. In addition, it is possible that the convulsion and hippocampal neurodegeneration induced by B, C, and K fractions after intrahippocampal injection could be useful as neurobiological tools for the investigations of convulsive seizures.
ACKNOWLEDGEMENTS
This work was supported by grants from FAPESP (Fundação de Amparo à Pesquisa do Estado de São Paulo). We thank Patrícia H. C. S. Couto and Geane A. Lourenço for technical assistance. This work is part of the MSc. dissertation presented by F. F. Carvalho to the Department of Pathology, University of São Paulo, Brazil.
REFERENCES
Received 22 April 1999
Accepted 08 June 1999
- 01 ADLER-GRASCHINSKY E., PIMENTA AF., DINIZ CR. Comparison of the release of endogenous and3 H-acetylcholine from slices of rat cerebral cortex. Acta Physiol. Latinoam., 1980, 30, 860-9.
- 02 ALLON N., WOODY C.D. Epileptiform activity induced in single cells of the sensorimotor cortex of the cat by intracellular applied scorpion venom. Exp. Neurol.,1983, 80, 491-7.
- 03 BAGETTA G., NISTICO G. Tetanus toxin as a neurobiological tool to study mechanisms of neuronal cell death in the mammalian brain. Pharmacol Ther.,1994, 62, 29-39.
- 04 BAGETTA G., NISTICO G., BOWERY, NG. Prevention by the NMDA receptor antagonist MK 801 of neuronal loss produced by tetanustoxin in the rat hippocampus. Br. J. Pharmacol, 1990, 101, 776-80.
- 05 BAGETTA G., NISTICÓ G., DOLLY, O. Production of seizures and brain damage in rats by a-dendrotoxin, a selective K+ channel blocker. Neurosci. Lett.,1992, 139, 34-40.
- 06 BARHANIN J., GIGLIO J. R., LÉOPOLD, P. Tityus serrulatus venom contains two classes of toxins, Tityus g toxin is a new tool with a very high affinity for studying the Na+ channel. J. Biol. Chem.,1982, 257, 12553-8.
- 07 BECKER S., GORDON RD. Sodium channel specific neurotoxins: Recent advances in the understanding of their molecular mechanisms. In: Herken H., Hucho F. Eds. Selective neurotoxicity. Berlin: Springer-Verlag, 1992: 719-33
- 08 BEN-ARI Y., TREMBLAY E., OTTERSEN OP., MELDRUN B.S. The role of epileptic activity in hippocampal and remote cerebral lesions induced by kainic acid. Brain Res., 1980, 191, 79-97.
- 09 BEN-ARI Y., TREMBLAY E., OTTERSEN OP., MELDRUM BS. Injections of kainic acid into the amygdaloid complex of the rat: an electrographic, clinical and histological study in relation to the pathology of epilepsy. Neuroscience, 1980, 5, 515-28.
-
10BROADHURST P. Experiments in psychogenetics. In: EISENK E. Ed. Experiments in personality London, Routledge and Kegan Paul, 1960, 1:3-71.
-
11CAMPOS JA., SILVA OS., LOPES M., FREIRE-MAIA L. Signs, symptoms and treatment of severe scorpion poisoning in children. In: EABER D., WADSTOM T. Ed. In: Natural toxins. Oxford: Pergamon,1980:61-8
-
12CÉARD B., DE LIMA M-E, BOUGIS PE., MARTIN-EAUCLAIRE M-F. Purification of the main b-toxin from Tityus serrulatus scorpion venom using high-performance liquid chromatography. Toxicon, 1992, 30, 105-10.
-
13CHOI DW. Calcium: still center-stage in hypoxic-ischemic neuronal death. Trends Neurosci., 1995, 18, 58-60.
-
14CHOI DW. Glutamate neurotoxicity: a three-stage process. In: GUIDOTTI, A. Ed.Neurotoxicity of excitatory amino acids New York: Raven Press, 1990:235–42
-
15COURAD F., JOVER E. Mechanisms of action of scorpion toxins. In: TU TA., DEKKER M. Eds.Handbook of natural toxins: insect poisons, allergens and other invertebrate venoms New York : M. Dekker, 1983, 2:659-78.
-
16COURAD F., JOVER E., DUBOIS J.M., ROCHAT H. Two types of scorpion toxin receptor sites, one related to the activation, the other to the inactivation of the action potential of the sodium channel. Toxicon, 1982, 20, 9-16.
-
17COUTINHO-NETO J. Purificação e caracterização parcial da tityustoxina Ribeirão Preto: Faculdade de Medicina de Ribeirão Preto, 1975. 112p. (Tese - Doutorado).
-
18COUTINHO-NETO J., ABDUL-GHANI A.S., NORRIS PJ., THOMAS AJ., BRADFORD HF. The effects of scorpion venom toxin on the release of amino acid neurotransmitters from cerebral cortex in vivo and in vitro. J. Neurochem., 1980, 35, 558-65.
-
19DAMIANO BP., CONNOR JD. Hippocampal mediation of shaking behavior induced by electrical stimulation of the perforant path in the rat. Brain Res., 1984, 308, 383-6.
-
20DEBIN JA., MAGGIO JE., STRICHARTZ GR. Purification and characterisation of chlorotoxin, a chloride channel ligand from the venom of the scorpion. Am. J. Physiol., 1993, 264, C361-9.
-
21DORCE VAC., SANDOVAL MRL. Brazilian scorpion venoms: pharmacological aspects. Ciênc. Cult., São Paulo, 1992, 44, 187-91.
-
22DORCE VAC., SANDOVAL M.R.L. Effects of Tityus serrulatus crude venom on the GABAergic and dopaminergic systems on the rat brain. Toxicon, 1994, 32, 1641-7.
-
23FRUSH DP., MCNAMARA JD. Evidence implicating dentate granule cells in wet dog shakes produced by kindling stimulations of entohinal cortex. Exp. Neurol., 1986, 92, 102-13.
-
24GARCIA ML., KNAUS H., MUNUJOS P., SLAUGHTER RS., KACZOROWSKI, G. Charibdotoxin and its effects on potassium channels. Am. J. Physiol, 1995, 269, C1-10.
-
25GOMEZ MV. Purificação e caracterização da toxina do escorpião Tityus serrulatus. Belo Horizonte: Universidade de Minas Gerais, 1967. 68p. (Tese -Doutorado).
-
26GOMEZ MV., DINIZ CR. Separation of toxic components from the Brazilian scorpion - Tityus serrulatus - venom. Mem. Inst. Butantan, 1966, 33, 899-902.
-
27GOMEZ MV., FARRELL N. The effect of tityustoxin and ruthenium red on the release of acetylcholine from slices of cortex of rat brain. Neuropharmacology, 1985, 24: 1103-7.
-
28GOMEZ MV., ROMANO-SILVA MA., PRADO MAM. Effects of tityustoxin on central nervous system. J. Toxicol. Toxin Rev., 1995, 14, 437-56.
-
29KLEINROK Z., CZUCZWAR SJ., KOZIEKA M. Effect of dopaminergic and GABA-ergic drugs given alone or in combination on the anticonvulsant action of phenobarbital and diphenylhydantoin in the electroshock test in mice. Epilepsia, 1980, 21, 519-29.
-
30LIMA EG., ALMEIDA HO., GOMEZ MV., FREIRE-MAIA L. Acute pulmonary edema induced by injection of tityustoxin into the lateral ventricle on rats. Toxicon, 1975, 13, 205-306.
-
31LOSCHER W., SCHMIDT D. Which animal models should be used in the search for new antiepileptic drugs? A proposal based on experimental and clinical considerations. Epilepsy Res, 1988, 2, 145-81.
-
32LOTHMAN EW., BERTRAM EH., STRINGER JL. Functional anatomy of hippocampal seizures. Prog. Neurobiol., 1991, 37, 1-82.
-
33MAGALHÃES O. Escorpionismo III. Memória. Ann. Fac. Med. Univ. Minas Gerais, 1935, 1, 3-84.
-
34MARANGONI S., GHISO J., SAMPAIO SV., ARANTES EC., GIGLIO JR., OLIVEIRA S., FRANGIONE B. The complete amino acid sequence of toxin TsTx-VI isolated from the venom of the scorpion Tityus serrulatus J. Protein Chem, 1990, 9, 595-601.
-
35MAXSON SC., COWEN JS. Effects of cortical spreading depression on audiogenic seizure in C57BL/6 mice. Pharmacol. Biochem. Behav., 1977, 6, 349-50.
-
36MOREIRA VMTS. Efeitos da injeção intra-hipocampal de crotoxina, crotamina e giroxina em ratos. São Paulo: Escola Paulista de Medicina, 1993 101p. (Tese - Doutorado).
-
37OLNEY JW. Excitotoxic mechanisms of neurotoxicity. In: SPENCER PS., SCHAUMBERG NH. Eds. Experimental and clinical neurotoxicity. Baltimore: Williams and Wilkins, 1980: 272-94.
-
38OLNEY JW. Excitatory amino acids and epilepsy-related brain damage. Internat. Rev. Neurobiol, 1985, 27, 337-62.
-
39OLNEY JW., RHEE V., HO OL. Kainic acids: a powerful neurotoxic analogue of glutamate. Brain Res, 1974, 77, 507-12.
-
40OLSEN RW. The GABA postsynaptic membrane receptor-ionophore complex. Site of action of convulsant and anticonvulsant drugs. Mol. Cell Biochem, 1981, 39, 261-79.
-
41OSMAN OH., ISMAIL M., WENGER T. Hyperthermic response to intraventricular injection of scorpion venom: role of brain monoamines. Toxicon, 1973, 11, 361-8.
-
42PAXINOS G., WATSON C. The rat brain in stereotaxic coordinates Sydney: Academic Press, 1982. 1.v.
-
43POSSANI LD., ALAGON AC., FLECHTER JR PL., ERICKSON BW. Purification and properties of mammalian toxins from the venom of the Brazilian scorpion Tityus serrulatus Lutz and Mello. Arch. Biochem. Biophys, 1977,180, 394-403.
-
44RACINE R.J. Modification of seizure activity by electrical stimulation. II. Motor seizure Electromyogr. Clin. Neurophysiol, 1972, 32, 281-94.
-
45ROCHAT H., BERNARD P., COURAD F. Scorpion Toxins: chemistry and mode of action. In: CECCARELLI B., CLEMENTI F. Eds. Neurotoxins: tools in neurobiology. New York: Raven Press, 1979:325-35.
-
46SANDOVAL MRL., DORCE VAC. Behavioral and electroencephalographic effects of Tityus serrulatus scorpion venom in rats. Toxicon, 1993, 31, 205-12.
-
47SCATTON B. Pharmacology of excitatory amino acid receptors: Novel therapeutic approaches in neurodegenerative disorders. In: RACAGNI G.; BRUNELLO N., LANGER, SZ. Eds. Recent advances in the treatment of neurodegenerative disorders and cognitive dysfunction Basel: Karger, 1994:157-65.
-
48SCHOEPP DD. Novel functions for subtypes of metabotropic glutamate receptors. Neurochem. Int..,1994, 24: 439-49.
-
49SCHWARTZKROIN PA., WYLER AR. Mechanism underlying epileptiform burst discharge. Ann. Neurol., 1980, 7, 95-107.
-
50SEYFRIED TN. Audiogenic seizures in mice. Fed. Proc., 1979, 38, 2399.
-
51TURSKI W.A., CAVALHEIRO E.A., BORTOLLOTO Z.A., MELLO L.M., SCHWARZ M., TURSKI L. Seizures produced by pilocarpine in mice: a behavioral, electroencephalographic and morphological analysis. Brain Res., 1984, 321, 237-53.
-
52TURSKI L., CAVALHEIRO E.A., SIEKLUCHA-DZINKA M., IKONOMIDOU-TURSKI C., CZUCZWAR S.J., TURSKI W.A. Seizures produced by pilocarpine: Neuropathological sequelae and activity of glutamate decarboxilase in the rat forebrain. Brain Res., 1986, 398, 37-48.
-
53TURSKI L., IKONOMIDOU C., TURSKI W.A., BORTOLLOTO Z.A., CAVALHEIRO E.A. Review cholinergic mechanisms and epileptogenesis. The seizures induced by pilocarpine: A novel experimental model of intractable epilepsy. Synapse, 1989, 3, 154-71.
 CORRESPONDENCE TO:
CORRESPONDENCE TO:Publication Dates
-
Publication in this collection
22 Sept 2000 -
Date of issue
2000
History
-
Accepted
08 June 1999 -
Received
22 Apr 1999