Abstract
Human Papillomavirus (HPV) infection is the most prevalent sexually-transmitted virus worldwide. It is known to be the etiological agent of cervical cancer and cervical intraepithelial neoplasia (CIN). Consequently, there is strong motivation to evaluate HPV testing in cervical cancer screening. Recently developed, the second generation of the hybrid capture test (HCA II) is a non-radioactive, relatively rapid, hybridization assay, designed to detect 18 HPV types divided into high and low-risk groups. We evaluated 7,314 patients (5,833 women and 1,481 men) for HPV infection by HCA II. Among them, 3,008 (41.1%) presented HPV infection: 430 (14.2%) had HPV DNA of low risk for cancer, 1,631 (54.2%) had high risk HPV types and 947 (31.5%) had both types. The prevalence in females was 44.9%. The prevalence of HPV DNA in the group for which cytological results were available was slightly higher: 55.3% (1007/1824). Significant differences were detected in the frequency of HPV infection of the cervix between normal cases and those with high-grade squamous-intraepithelial lesions (HSIL)(P<0.0001). Among males, the prevalence was 26.2%, composed of 9.1% in Group A, 9.7% in Group B and 7.4% with multiple infections. We observed that male prevalence was lower and that low-risk types were more frequent than in females. HPV viral load was significantly greater in SILs than in normal or inflammatory cases (P<0.0001), suggesting an association between high viral load values and risk of SIL. Because of high costs, the HCA II test cannot be recommended for routine mass screening for cervical infection in poor countries. Nevertheless, it was found to be a useful tool, when combined with cytology, discovering high-risk infections in apparently normal tissues and revealing silent infections that may be responsible for the maintenance of HPV in the general population. These findings point to the need for close and careful management of patients, thereby reducing overtreatment, allowing analysis of both sexual partners and finally contributing to the control of genital infections associated with a risk for cancer.
HPV; SIL; cancer; hybrid capture
ORIGINAL PAPERS
Prevalence of human papillomavirus infection in the genital tract determined by hybrid capture assay
Fernanda N. Carestiato; Katia C Silva; Trude Dimetz; Ledy H. S. Oliveira; Silvia M. B. Cavalcanti
Department of Microbiology and Parasitology, Biomedical Institute of Universidade Federal Fluminense and Department of Molecular Biology, Laboratórios Sergio Franco; RJ, Brazil
Address for correspondence Address for correspondence: Dr. Silvia Maria Baeta Cavalcanti Dep. de Microbiologia e Parasitologia, Instituto Biomédico-UFF rua Prof. Ernani Melo, 101, 3º andar (Virologia) Zip code: 24210-130, Niterói, RJ E-mail: silviacavalcante@vm.uff.br.
ABSTRACT
Human Papillomavirus (HPV) infection is the most prevalent sexually-transmitted virus worldwide. It is known to be the etiological agent of cervical cancer and cervical intraepithelial neoplasia (CIN). Consequently, there is strong motivation to evaluate HPV testing in cervical cancer screening. Recently developed, the second generation of the hybrid capture test (HCA II) is a non-radioactive, relatively rapid, hybridization assay, designed to detect 18 HPV types divided into high and low-risk groups. We evaluated 7,314 patients (5,833 women and 1,481 men) for HPV infection by HCA II. Among them, 3,008 (41.1%) presented HPV infection: 430 (14.2%) had HPV DNA of low risk for cancer, 1,631 (54.2%) had high risk HPV types and 947 (31.5%) had both types. The prevalence in females was 44.9%. The prevalence of HPV DNA in the group for which cytological results were available was slightly higher: 55.3% (1007/1824). Significant differences were detected in the frequency of HPV infection of the cervix between normal cases and those with high-grade squamous-intraepithelial lesions (HSIL)(P<0.0001). Among males, the prevalence was 26.2%, composed of 9.1% in Group A, 9.7% in Group B and 7.4% with multiple infections. We observed that male prevalence was lower and that low-risk types were more frequent than in females. HPV viral load was significantly greater in SILs than in normal or inflammatory cases (P<0.0001), suggesting an association between high viral load values and risk of SIL. Because of high costs, the HCA II test cannot be recommended for routine mass screening for cervical infection in poor countries. Nevertheless, it was found to be a useful tool, when combined with cytology, discovering high-risk infections in apparently normal tissues and revealing silent infections that may be responsible for the maintenance of HPV in the general population. These findings point to the need for close and careful management of patients, thereby reducing overtreatment, allowing analysis of both sexual partners and finally contributing to the control of genital infections associated with a risk for cancer.
Key Words: HPV, SIL, cancer, hybrid capture.
Human Papillomavirus (HPV) is the most prevalent virus involved in sexually-transmitted diseases worldwide, being an important public health challenge [1]. HPV is also considered the main cause of most cervical cancers and of cervical intraepithelial neoplasias (CIN) [2].
The diagnosis of cervical disease, through the finding of abnormal cervical epithelial cells, is usually obtained by microscopic examination of Papanicolaou-stained (PAP) smears. This has been the method of choice since the 1950s; it proved to be valuable for mass screening and for enabling detection of lesions early enough to be treated effectively. However, the PAP smear has some problems. The most important is its limited sensitivity for detecting cancer precursors, along with the subjective interpretation of results. As a consequence, false-negative rate range from 20% to 30%, and women who have been given the false-negative result may eventually develop cervical cancer. Hence, complementary methods that could improve the diagnosis of cervical disease have been studied during the past two decades. Molecular detection of HPV provides a different approach to screening and patient management, allowing identification of HPV infection in patients at risk for disease [3]. Also, the search for epidemiological sources of HPV infection demonstrated the essential role of male infection, usually involving subclinical lesions, which might thus silently spread to female partners [1].
In men, productive HPV infection can result in simple condyloma acuminata, giant condyloma, or Buschke-Löwenstein tumor, mainly caused by HPV genotypes 6 and 11. However, visible genital warts are detectable only in about 1% of this population, representing only the tip of the HPV iceberg [4]. HPV-associated penis intraepithelial neoplasia are found in the great majority of cases, but they are inconspicuous lesions caused by high-risk HPV types, especially HPV 16 and 18, histologically showing low, moderate, or severe dysplasia (PIN grades 1, 2 and 3) [5]. Less frequently, high-risk HPV infection can progress to penile carcinoma, also associated with HPV 16 and 18 in 30 to 50% of the cases [6]. Associated lesions are detected in 50 to 70% of male partners of infected women, and partners of men having penile cancer had a cervical cancer incidence eight times higher than penile cancer incidence [7]. These data could explain the vicious circle of infection and recurrence and treatment failure in females, due to reinfection by male partners with subclinical HPV lesions. Sensitive methods to diagnose male infection with HPV could contribute to control this route of dissemination of HPV, in women who are chronically reinfected.
Consequently, there has been strong motivation to develop HPV testing for both male and female genital lesions, with a need for improvements in and standardization of testing methods. HPV detection has generally been conducted by hybridization and PCR methods. But neither research assays nor commercial kits (dot blot or in situ hybridization) have been found to be adequate for clinical use. An assay for routine clinical use requires reliable and accurate detection of a broad range of pathogenic HPV types that infect the genital tract [8].
Recently developed, the second generation of the Hybrid Capture System HPV DNA detection test from Digene Diagnostics (Silver Spring, Md.) is a non-radioactive, relatively rapid, hybridization assay designed to detect 18 HPV types divided into high and low-risk groups. As a possibly unique advantage compared with other available HPV test kits, the hybrid capture test is also designed to provide quantitative estimates of viral load, which can correlate with the grade and the natural history of cervical pathology [9].
Given the fact that various clinical laboratories are currently using this method, we examined the new epidemiological data on HPV infection in both female and male patients, correlating the prevalence of HPV with its respective viral load and with cytological diagnosis, in order to develop tools for the interpretation of this newly available procedure, to monitor natural infection in both sexes and hence contribute to the control of HPV dissemination and evolution to cancer.
Material and Methods
Study population and specimen collection
The study population included 7,314 patients (5,833 women and 1,481 men) attended at Laboratórios Sérgio Franco, Rio de Janeiro, from January 2000 to December 2002. The women had come in for routine exams, while specimens from men were obtained after medical suspicion of HPV infection, mainly because of acetowhite lesions. The smears were collected with a cervical cytobrush and transported in Digene Specimen Transport Medium (Digene Diag, Md).
Cytological test
The Papanicolaou test was developed and smears were classified as NORMAL for normal epithelium, INFLAMMATORY for minor alterations of cervical cells, LSIL for low grade squamous intraepithelial lesions and HSIL for high-grade squamous intraepithelial lesions/in situ carcinoma. No cases of invasive cancer were recorded. Data from the men's cytological exams were not available, but most data indicated a search for diagnosis of acetowhite lesions.
HPV testing
The assay kit detects the high-risk HPV types 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59 and 68. The low-risk group assay detects the types most commonly associated with condyloma acuminatum: HPV types 6, 11, 42, 43 and 44. Following the kit protocol, specimens were treated with sodium hydroxide to hydrolyse specimen RNA and to denature the DNA. The liberated single-strand DNA was hybridized in solution with an RNA probe mix consisting of high-risk or low-risk HPV types. Each reaction mixture, containing any RNA-DNA hybrids that formed, was transferred to a capture tube coated with antibodies to the hybrids, immobilizing them. Bound RNA-DNA hybrids were then reacted with an alkaline phosphatase-conjugated antibody directed against the hybrids. Unreacted material was removed by washing, and a dioxetane-based chemiluminescent compound, Lumi-Phos 530, was added as a substrate for alkaline phosphatase. The light produced by the ensuing reaction was measured with a luminometer. Light measurements were expressed as relative light units (RLUs). As a negative control, sonicated herring sperm DNA in Digene transporting medium (100mg/mL) was used. Triplicate specimens of HPV 16 or HPV 11 DNAs at 1.0pg/mL served as the positive controls for the high and low-risk probes, respectively.
All RLU measurements for specimens were divided by the mean RLU of the three positive controls (PCs) to give a ratio of specimen RLU/PC. A ratio of 1.0 or greater was regarded as positive for HPV DNA, and a ratio of less than 1.0 was regarded as negative. Since the amount of the light produced by the hybrid capture assay should be proportional to the amount of target HPV DNA, the results can be viewed as quantitative.
Statistical analysis
The statistical significance of the results was analyzed with the Fisher Exact test for heterogeneity, with Yates continuity correction. All analyses were done using SSPS 8.0.
Results
The 7,314 female and male genital samples were investigated to detect HPV DNA. The average age of participants was 28.8 years. Three thousand and eight (41.1%) cases presented HPV infection, as detected by hybrid capture. Half of the patients were from 21-30 years old; this age interval was the most commonly affected by HPV infection (Table 1). Mean female age was slightly lower than the mean male age (27.2 and 31.1 years old, respectively).
The HPV prevalence rates varied according to gender; 5,833 samples were from female cervical smears, of which 44.9% were positive for HPV with 5.1% of the cases showing low-risk infection, 25.5% had high risk HPV and 14.3% had mixed infections (Table 2). The other 1,481 samples were from male lesions, with 9.1% low risk HPV, 9.7% in the high-risk group and 7.4% with mixed infections, giving a total prevalence of 26.2%.
Female HPV prevalence rates differed with cytological diagnosis (Table 3); the overall prevalence of HPV DNA was 55.3% (1,007/1,824), ranging from 9.9% (15/151) in NORMAL to 90.4% (113/125) in HSIL. There were significant differences in frequency of HPV infection of the cervix among these groups (P<0.0001). The prevalence of the different HPV types are varied (Table 3); among the women with HPV, 88 (8.7%) had HPV DNA of low risk for cancer, 644 (63.9%) had high-risk HPV types and 276 (27.4%) had both types. Group A (low-risk HPV types) had low rates of prevalence, with the highest rates in inflammatory, HPV and LSIL cases; they were absent from HSIL lesions. Group B (high risk) HPV types were detected in most of the cases, alone or mixed with low-risk types, with increasing prevalence according to the severity of the cytological diagnosis, reaching 100% of the detected viruses in HSIL. Patients with normal cytology had significantly less prevalence of HPV than did the altered cytology groups (P<0.00001).
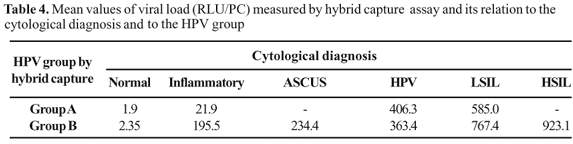
The mean values of HPV Viral Load (RLU) measured by HCA II varied significantly between subjects with normal, low risk and high-risk HPV, indicating an association between viral load and risk of SIL (P<0.001).
Discussion
The World Health Organization reported that cervical cancer is the second cause of malignant neoplasia and death in women worldwide [2]. In poor countries, cervical cancer is still the most frequent cause of death from cancer; this disease has presented earlier in the female population during the last two decades [10]. Also, based on prevalence rates, HPV is the most widely disseminated sexually-transmitted viral disease [4]. Although there have been various studies of the natural history of HPV in the female genital tract, male infections have not received much attention. The few epidemiological data show that nearly 50% of sexually active men and women between the 15 and 49 years old present infections with at least one, and sometimes several, genital HPV types [5]. Male infections are frequently subclinical, allowing the virus to spread silently. The male urethra has been postulated to be a reservoir for HPV, with prevalence rates ranging from 17% to 50% [6,7,11].
We evaluated the infection in both sexes, comparing prevalence of oncogenic and benign viruses. The average age of the patients was 28.8 years. Half of them were from 21-30 years old; this age interval had the highest frequency of HPV infection not only in women but also in male cases (Table 1). This age range coincided with the peak of sexual activity. Female mean age was slightly lower than the male mean (27.2 x 31.1 years old), but was not significantly different (P > 0.05). There was a high percentage of positive cases in the age interval under 20 years. These high levels of infection could be due to young cervical tissue, with high transformation activity, making it vulnerable to STDs, such as HPV disease. The decrease in the rate of infection observed with increase in age can be explained by the reduction in sexual activity; probably, older cases are persistent infections. Hence, several authors have proposed DNA test screening at age intervals over 30 or 35 years old as an important tool for reducing cancer worldwide [12].
Hybrid capture assay has proven to be a reliable, accurate and reproducible method for HPV testing in routine clinical practice [13,14]. We found 44.9% of tested women to be infected by HPV, similar to previously-described prevalences [8], using the same method. (Table 2). We had cytological data for part of our patient group. In this subgroup, the prevalence increased to 55.3% (Table 3). In the comparison with the cytological diagnosis, the hybrid capture assay results were strongly associated with a likelihood of SIL in concurrently obtained cervical smears (P<0.0001). We detected nearly 10% normal or inflammatory cytological results unrelated to HPV that were positive for HPV infection. Koutsky et al. [12] described women with no abnormality presenting HPV DNA, and they observed that 30% of the women developed CIN within two years. Some cases of disagreement between DNA testing and cytology could also be due to error in the cytological diagnoses. Nearly 10% of both LSIL and HSIL presented negative results in HCA II. These inconsistent results could be due to low copy number of the HPV genome, infection by untested types or unidentified reasons, such as DNA testing errors [15]. We also found that HSIL was exclusively associated with high-risk HPV (Table III). No low-risk virus was found alone in these smears. Recent reports using HCA II also indicated no severe dysplasia in women with low-risk HPV [15]. High-risk HPV was present in all cases of HSIL, showing a 100% of correlation between high-risk HPV infection and risk of cancer.
The HCA results from males showed 26.2% infection by HPV, with the highest prevalence among young men. More than 65% of the men were infected with oncogenic HPV types. Rosenblatt [5] obtained 16.7% HPV DNA in men, among which 46.7% were low-risk and 53.3% were high-risk. Based on our results, there may be a higher risk of cancer progression; in fact, Brazil has a high incidence of genital cancer [16]. Cavalcanti et al. [17] found a 67.1% prevalence of HPV infection by in situ hybridization. HPV DNA was detected in 75% of condylomas and papules, 65% of acetowhite lesions and 50% of carcinomas. Medical misinformation and sampling problems could explain the low prevalence and the reduced viral loads, perhaps giving false negative results. No practical sampling technique has been established to easily identify HPV infection in the penis, and relatively few cells are recovered by swabbing the urethra or the glans penis [18].
Analysis of male samples also showed an HPV profile of infection different from the one obtained for females [19]. The number of HPV-positive cases in the male study was smaller than in the female study (26.2% x 44.9%, P<0.05), but with a higher prevalence of infections caused by low-risk HPV types in men compared to women (135/388 low-risk types in males (34.8%) against 295/2620 low-risk in female cases (11.3%, P<0.001)). The reduced number of infected male patients in relation to female patients could be explained by the different kind of epithelial tissue that covers the penis and the uterine cervix. The penile shaft and the outer surface of the foreskin are covered by a keratinized stratified squamous epithelium that provides a natural protective barrier against HPV infection [20]. While, in woman, besides the epithelium being non-keratinized throughout most of the cervix, there is a squamous-columnar junction, called the transformation zone, which exposes the epithelial basal layer to HPV infection. Also, it is accepted that the more frequent infections of low-risk types in men are probably due to differences in cell tropism characteristics inherent to HPV types [20].
The two groups that we studied came from the same geographical region and were attended during the same period at the same laboratory. Hence, we assume that they are similar populations, sharing socio-economic conditions and customs. We postulate that male cases are intimately related to female infection, being the reservoir for their partners, making it difficult to control these sexually-transmitted-disease epidemics.
In fact, Neves et al. [21] proposed that HPV infection and natural history leading to penile cancer is a multi-factorial process similar to that found in cervical squamous cell carcinoma. Fernandes [22] also showed important parallels of vulvar and cervical cancer with penile cancer. Also, Rubin [23] and Gross & Pfister [5] found a 40%-45% prevalence of HPV-DNA in penile carcinoma, which is similar to the detection rate of HPV-DNA in vulvar carcinoma (50%), with the histological sub-types of penile neoplasia being identical to those described for the vulva.
We also examined the viral loads demonstrated by HCA tests. As found by other researchers [14], the quantitative information provided by the hybrid capture test had good reliability. We found that high viral loads paralleled increases in SILs (Table 4). Other investigators have shown that a high viral load predicts an increased probability of histological/cytological confirmation of SIL [24] and a higher risk of high-grade SIL [15]. Women waho had a high viral load were at significantly greater risk for SIL and carcinoma in Taiwan, based on the HCA II test. There was a distinct upward trend of high-risk HPV DNA levels parallel with histological grade of the lesion (P < 0.001), especially for HSIL [25]. Other studies also showed that the amount of HPV DNA is a useful predictor of progression to cervical carcinoma and concluded that the risk of cancer increased in the patients [26]. But some reports showed conflicting results [27]. Thus, the quantitative aspect of the hybrid capture test merits further evaluation.
In conclusion, the scientific evidence supporting the value of HPV testing as an additional option is now abundant and consistent. Molecular screenings based on HCA showed that HPV types are not always absolutely consistent with the clinical type of HPV-associated genital lesion, showing diverse prevalence rates in different countries and cities; high-risk HPV types, such as 16 and 18, can also be isolated from "benign" HPV-associated genital lesions more than is usually expected [28,29] These oncogenic types that infect the male population with a benign profile may be a risk for the female population, which is exposed to high-risk viruses. These variations in virological data have implications for vaccine testing, choice of diagnostic methods, and epidemiological studies involving disease control. Because of high costs, the HPV test for routine cervical, mass screening cannot be recommended in poor countries. Nevertheless, it is a useful tool when combined with cytology, demonstrating high-risk infections in apparently normal tissues, which might indicate a need for closer and careful management of patients by medical doctors, to reduce the risk of cancer. This new methodology merits further evaluation in order to establish its cancer prevention potential in at-risk groups.
Received on 24 May 2006; revised 26 August 2006.
Financial support: grant from Conselho Nacional de Pesquisa - CNPq /CAPES Brazil.
- 1. Skerlev M., Giri M., Skerlev H.S. Human Papillomavirus male genital Infections: Clinical variations and the Significance of DNA Typing. Clinics Dermatol 2002;20:173-78.
- 2. Muñoz N., Bosh X., Sanjose S., et al. Epidemiologic classification of human papillomavirus types associated to cervical cancer. New England Journal Medicine 2003;348:518-27.
- 3. Molijn A., Kleth B., Quint W., van Doorn L.J. Molecular diagnosis of human papillomavirus infection. J Clin Virology 2005;23S:43-51.
- 4. Gilbert G. Human papillomavirus and human cancer. Int J Cancer 2003;133:121-6.
- 5. Gross G., Pfister H. Role of human papillomavirus in penile cancer, penile intraepithelial squamous cell neoplasias and in genital warts. Med. Microbiol. Immunol 2004;193:35-44.
- 6. Rosenblatt C., Lucon A.M., Pereyra E.A.G., et al. HPV prevalence among partners of women with cervical intraephitelial neoplasia. Int. J. of Gynecology and Obstetrics 2004;l84:156-61.
- 7. Aynaud O., Ionesco M., Barrado R. Cytologic Detection of Human Papillomavirus DNA in Normal Male Urethral Samples. Urology 2003;61:1098-101.
- 8. Callaghan J., Karim S., Mortlock S., et al. Hybrid capture as a means of detecting human papillomavirus DNA from liquid-based cytology specimens: a preliminary evaluation. Brit J Biomed Sci 2001;58:184-9.
- 9. Cox J.T., Lorincz A.T., Schiffman M.H., et al. Human papillomavirus testing by hybrid capture appears to be useful in triaging women with a cytologic diagnosis of ASCUS. Am J Obstet Gynecol 1995;172:946-54.
- 10. Câmara G.N.L., Cerqueira D.M., Oliveira A.P.G., et al. Prevalence of human papillomavirus types in women with pre-neoplastic and neoplastic cervical lesions in the Federal District of Brazil. Mem Inst Oswaldo Cruz 2003;98:879-83.
- 11. Hippelainen M., Syrjanen S., Hippelainen M.J., et al. Diagnosis of genital human papillomavirus (HPV) lesions in the male: Correlation of peniscopy, histology and in situ hybridization. Genitourin Med 1993;69:346-51.
- 12. Koutsky L.A., Holmes K.K., Critlow C.W., et al. A cohort study of the risk of cervical neoplasia grade 2 or 3 in relation to papillomavirus infection. N Engl J Med 1992;327:1272-8.
- 13. Schiffman M.H., Kiviat N.B., Burk R.D., et al. Accuracy and interlaboratory reliability of human papillomavirus DNA testing by hybrid capture. J Clin Microbiol 1995;33:545-50.
- 14. Castle P.E., Lorincz A.T., Lohnas I.M., et al. Results of human papillomavirus DNA testing with the hybrid capture II assay are reproducible. J Clin Microbiol 2002;40:1088-90.
- 15. Morrison H. Human papillomavirus absence predicts normal cervical histopathologic findings with abnormal papanicolaou smears. J Hum Virol 1993;4:283-7.
- 16. Inca Instituto Nacional do Câncer.Ministério da Saúde. Câncer de pênis. (2004) Available at: http://www.inca.gov.br/conteudo_view.asp?id=338 Accessed 14 August, 2006
- 17. Cavalcanti S.M.B., Gouvea T., Passos M.R.L., Oliveira L.H.S. Diagnóstico de lesões causadas por papilomavirus humanos no trato genital masculino: correlação da escopia genital, histologia e hibridização in situ Rev Bras Patol Clin 1994;30:120-4.
- 18. Bleeker M.C.G., Hogewoning C.J.A., van de Brule A.J.C., et al. Penile lesions and human papillomavirus in male sexual partners of women with cervical intraepithelial neoplasia. J Am Acad Dermatol 2002;47:351-7.
- 19. Carestiato F.N., Carvalho M.O.O., Ribeiro M.O., et al. Estudo das infecções causadas por papilomavírus humanos em pacientes do sexo feminino detectadas pela técnica de captura do híbrido: levantamento de casos. J Bras Doenças Sex Transm 2002;14:9-12.
- 20. Castellsagué X., Bosh F.X., Muñoz N., et al. Male circumcision, penile human papillomavirus infection, and cervical cancer in female partners. N Engl J Med 2002;346:1105-12.
- 21. Neves D., Camara G., Alencar T.R., et al. Prevalence of Human Papillomavirus in Penile Carcinoma. Brazil J Urology 2002;l(28):221-6.
- 22. Fernandes M.G.M., Ferreira F.V.A., Ferreira S.N.H., et al. MIB1 and p53 in penile intraepithelial and invasive squamous HPV related lesions. Rev Brasil Cancerologia 2002;48:29-37.
- 23. Rubin M.A., Kleter B., Zhou M., et al. Detection and Typing of Human Papillomavirus DNA in Penile Carcinoma. Evidence for Multiple Independent Pathways of Penile Carcinogenesis. American J Pathol 2001;159:1211-8.
- 24. Cusick J., Terry G., Ho L., et al. Human papillomavirus type 16 DNA in cervical smears as a predictor of high-grade cervical intraepithelial neoplasia. Lancet 1992;339:959-60.
- 25. Sun C.A., Liu J.F., Wu D.M., et al. Viral load of high risk human papillomavirus in cervical squamous intraepithelial lesions. Int J Gynecol Obstet 2002;76:41-7.
- 26. Josefsson A.M., Magnusson P.K.E., Ylitalo N., et al. Viral load of human papillomavirus 16 as a determinant for development of cervical carcinoma in situ: a nested case-control study. Lancet 2000;355:2189-93.
- 27. Yamasaki H., Sasagawa T., Basha W., et al. Hybrid capture-II and LCR-E7 for HPV typing in cervical cytologic samples. Int J Cancer 2002;94:222-7.
- 28. Cavalcanti S.M.B., Zardo L.G., Passos M.R.L., Oliveira L.H.S. Epidemiological aspects of Human papillomavirus infection and cervical cancer in Brazil. J Infection 2000;40:80-7.
- 29. Schiffman M.H., Brinton L.A. The epidemiology of cervical carcinogenesis. Cancer 1995;76:1888-901.
Publication Dates
-
Publication in this collection
31 Jan 2007 -
Date of issue
Oct 2006
History
-
Reviewed
26 Aug 2006 -
Received
24 May 2006