Abstract
The effect of Aloe vera on the reduction of plaque and gingivitis was evaluated in a randomized, parallel and double-blind clinical trial. Subjects were randomly allocated to the test group (n=15) - dentifrice containing Aloe vera - or the control group (n=15) - fluoridated dentifrice. Plaque index (PI) and gingival bleeding index (GBI) were assessed at days 0 and 30. Subjects were asked to brush their teeth with the control or test dentifrice, three times a day, during a 30-day period. There was a significant reduction on plaque and gingivitis in both groups, but no statistically significant difference was observed among them (p>0.01). The dentifrice containing Aloe vera did not show any additional effect on plaque and gingivitis control compared to the fluoridated dentifrice.
Dental plaque; Gingivitis; Aloe vera
ORIGINAL ARTICLES
Effect of a dentifrice containing aloe vera on plaque and gingivitis control. A double-blind clinical study in humans
Sílvia Morgana Araújo de OliveiraI; Ticiana Carneiro TorresI; Sérgio Luís da Silva PereiraII; Olívia Morais de Lima MotaIII; Márlio Ximenes CarlosIV
IGraduate Student, University of Fortaleza, Ceará, Brazil
IIPhD in Periodontics, University of Fortaleza, Ceará, Brazil
IIIMsC in Periodontics, University of Fortaleza, Ceará, Brazil
IVSpecialist in Periodontics, University of Fortaleza, Ceará, Brazil
Corresponding address Corresponding address: Dr. Sérgio Luís da Silva Pereira Av. Engenheiro. Leal Lima Verde, 2086 Alagadiço Novo - 60833-520 Fortaleza, CE, Brazil Phone: +55-85-3474-4108 Fax: +55-85-3477-3059 e-mail: luiss@unifor.br
ABSTRACT
The effect of Aloe vera on the reduction of plaque and gingivitis was evaluated in a randomized, parallel and double-blind clinical trial. Subjects were randomly allocated to the test group (n=15) - dentifrice containing Aloe vera - or the control group (n=15) - fluoridated dentifrice. Plaque index (PI) and gingival bleeding index (GBI) were assessed at days 0 and 30. Subjects were asked to brush their teeth with the control or test dentifrice, three times a day, during a 30-day period. There was a significant reduction on plaque and gingivitis in both groups, but no statistically significant difference was observed among them (p>0.01). The dentifrice containing Aloe vera did not show any additional effect on plaque and gingivitis control compared to the fluoridated dentifrice.
Key words: Dental plaque. Gingivitis. Aloe vera.
INTRODUCTION
Plaque-induced gingivitis is one of the most frequent periodontal diseases, affecting more than 90% of the population, regardless of age, sex or race20. Brazilian epidemiologic studies show a high prevalence of gingival inflammation, ranging from 74% to 100%, although the mean individual percentage of gingival bleeding varies from 28% to 35%5.
Mechanical plaque control is the most effective method of controlling plaque and gingivitis2,8. However, the inability of the normal adult population to perform adequate toothbrushing has led to the search for chemotherapeutic agents in order to improve plaque control12. These chemicals, mainly triclosan and chlorhexidine, have been used as mouthrinses or added to dentifrices to avoid plaque formation and development of gingivitis10,12,14,22. As some of these substances may have undesirable side effects, such as tooth staining and taste alteration, phytotherapic agents with antimicrobial and antiinflammatory properties have been investigated7,16,17.
The use of natural products in the prevention and treatment of oral conditions has increased recently and could be of benefit to low-socioeconomic level urban and rural communities4. Among the various currently available herbal agents, Aloe vera, popularly known as "babosa", is a plant commonly found the Northeast of Brazil. Its foliage, extract and resin present antimicrobial, antiinflammatory and healing properties and are indicated to hepatic and stomach diseases9.
The antimicrobial effect of a dentifrice containing Aloe vera has been demonstrated in an in vitro study, in which this phytotherapic agent inhibited the growth of diverse oral microorganisms, such as S. mutans, S. sanguis, A. viscosus and C. albicans7. The only study available evaluating the clinical effects of Aloe vera showed a significant reduction of gingivitis and plaque accumulation after use of a mouthrinse containing this natural product20.
To the present date, there is no reported controlled trial evaluating the efficacy of a dentifrice containing Aloe vera in the control of plaque and gingivitis. Therefore, the purpose of the present study was to assess the antiplaque and antigingivitis effects of this phytotherapic agent compared to a fluoridated dentifrice.
MATERIAL AND METHODS
Thirty adult subjects from the University of Fortaleza, Brazil (15 male and 15 female, aged 35 to 43 years) were enrolled in this double-blind, parallel, controlled clinical trial. All randomly screened participants were informed about the nature of the study and signed an informed consent form in compliance with the guidelines of the Brazilian National Health Council. The protocol was approved by the institutional Ethics Committee (Report Coética/Unifor: 407/2006).
The subjects were entered in the study if they had gingival bleeding index (GBI)1>40%, presence of at least 20 natural teeth and absence of supragingival calculus and other plaque retentive factors. Subjects with medical disorders or probing depth >3 mm, individuals under antimicrobial therapy at least 1 month prior to the study and using mouthrinses or dentifrices containing substances with antiinflammatory properties, as well as smokers and pregnant women were excluded from the trial.
The participants were assigned to either the test group (n=15) or the control group (n=15) by random permutation of three. The test group used a herbal dentifrice containing Aloe vera (Forever BrightÒ, Forever Living Products, Tempe, AZ, USA) and the control group used a fluoridated dentifrice (Sorriso Dentes BrancosÒ, Kolynos do Brasil, São Paulo, SP, Brazil), with no antiinflammatory properties, but with color and taste similar to those of the test dentifrice.
The volunteers were examined for plaque and gingivitis at baseline and after 30 days. A single, previously calibrated examiner3 scored the gingival bleeding index (GBI)1 and the plaque index (PI)19, which were recorded on the buccal, mesial, distal and lingual surfaces of all teeth. The values of 4 sites of each tooth were averaged to determine the GBI and PI for each subject. In addition to this examination, the hard and soft oral tissues were visually inspected for the presence of any adverse reaction by the same examiner.
After the initial examination, all teeth of each subject were polished with pumice and flossed to eliminate plaque remnants. A personal "kit" containing a new toothbrush (LeaderÒ; Facilit Odontológica e Perfumaria Ltda, Rio de Janeiro, RJ, Brazil) and the test or control dentifrice was given to all participants. They were instructed to brush their teeth for 1 min, three times a day, using the Bass technique, and to refrain from any other oral hygiene procedures throughout the period of the clinical trial. Verbal and written instructions about the correct use of dentifrice were given to all subjects as well. The tubes containing the dentifrices were previously coded to warrant that neither the examiner nor the volunteers knew their content, which was revealed only after completion of the study. The subjects were asked to return their dentifrice tubes, that were weighted by a digital balance (Filizola, modelo BP6, Indústrias Filizola S.A., São Paulo, SP, Brazil), previously and after the trial, so that compliance could be indirectly evaluated.
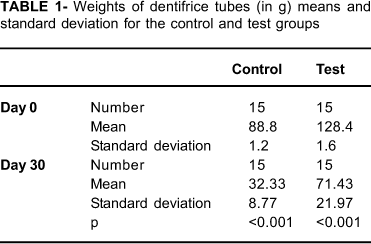
Student's t-test was used to evaluate statistical differences between the weights of dentifrice tubes on days 0 and 30 ( α=0.01). Mann-Whitney test was performed to evaluate statistical differences between control and test groups on days 0 and 30 (α=0.01). In each group, the mean scores of GBI and PI were compared between baseline and the end of the trial by the Wilcoxon test (α=0.01). Results are presented as mean and standard deviation.
RESULTS
The test dentifrice had a good acceptance and did not show adverse effects, such as formation of abscess and ulcerations or allergic reactions. Only one subject in the test group reported unpleasant taste, but he did not drop out the clinical trial.
There was a significant reduction of dentifrice tube weights between days 0 and 30 in both groups (p<0.001), which indirectly indicated that the volunteers actually used the dentifrices (Table 1).
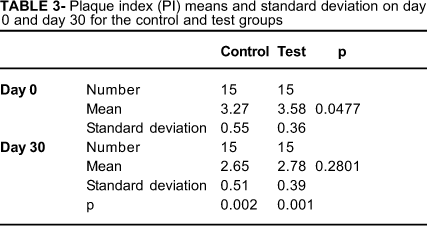
On day 0, there was no statistically significant difference between the control and test groups with respect to GBI (p=0.8774) and PI (p=0.0477) means. These results indicated that both groups were well balanced at baseline (Tables 2 and 3). At the 30th day, plaque (p=0.2801) and gingival bleeding (p=0.9346) were present in both groups, but the difference between them was not significant statistically (Tables 2 and 3).
Comparing the means between baseline and day 30 in each group, there was statistically significant difference in both the GBI (p=0.002, control group and p=0.001, test group) and PI indexes (p=0.002, control group and p=0.001, test group) (Tables 2 and 3).
DISCUSSION
Aloe vera is a natural product contained in herbal dentifrices with commercial appeal on the control of plaque and gingivitis. Despite its free commercial use, this phytotherapic agent does not have sufficient data to support its antigingivitis and antiplaque claims21.
To the best of our knowledge, the present study is the first report about effect of a dentifrice containing Aloe vera on gingivitis. The results showed that both toothpastes were efficient on plaque reduction (23% in test group and 19% in control group). This percent difference was not significant at the end of the trial. In spite of a reported in vitro inhibitory effect of Aloe vera against microorganisms from supragingival biofilm7, its in vivo antiplaque effect in the present study was not satisfactory. It is interesting to note that this previous study7 evaluated a dentifrice that also contained other antiplaque agents, which probably masked the effect of Aloe vera.
The test and control dentifrices reduced gingivitis significantly, although the fluoridated dentifrice presented a higher percent reduction on bleeding areas (56% versus 53%). The GBI is a generally used dichotomous index to evaluate gingivitis, but it does not access the severity of gingival inflammation. Studies evaluating the reduction of gingivitis by a grading index could be interesting to complement these results. Gingival index uses a scale in which color changes in the gingival tissues precede bleeding on probing; however this parameter is not necessarily an accurate indicator of gingivitis18.
The findings of the present study are in agreement with those of Villalobos, et al.20 (2001), who observed a significant reduction on plaque and gingivitis after a 30-day use of mouthrinses containing Aloe vera associated to toothbrushing. This study20 also showed an additional antiplaque and antigingivitis effect of this phytotherapic agent, which was not observed in the present clinical trial.
Villalobos, et al.20 (2001) used a higher concentration of Aloe vera (50%), which could explain the better effect of this phytotherapic agent when compared to our findings. Although the manufacturer does not inform the concentration of Aloe vera in the product used in the present study, the percentage of therapeutic agent in a dentifrice usually range from 0.4% to 1.0% of the total formulation6, which was probably the concentration used in our study and could explain those results. Furthermore, Villalobos, et al.20 (2001) used a mouthrinse containing only Aloe vera as the active agent, favoring its action without interference of other components. The test dentifrice used on the present trial contains other agents that can promote a moderate antiplaque effect, such as menthol and sodium lauryl sulfate12,21. Since the last two components are also present in the control dentifrice and considering that no difference was found between groups, one can conclude that the herbal agent had no influence on the results. The antagonism of diverse substances with similar effects, in the same product, should be considered as well. This fact has been highlighted by Wua and Savitt21 (2002) and confirmed by other clinical trials comparing fluoridated and herbal dentifrices11,15.
Home-use dentifrice studies are often influenced by a number of factors which can mask the superiority of a test agent over the controls. Participants in clinical trials may experience some improvement associated not specifically to the therapeutic properties of the test agent but rather related to a behavior change - Hawthorne effect15. Subjects enrolled in oral hygiene studies usually improve their toothbrushing, irrespective of the product they receive11,13,15.
Although the volunteers of the present study were not aware of which dentifrice they were using, another important factor is the Novelty effect, which is the motivation of oral hygiene practice by the use of a new substance. On the other hand, lack of compliance in the correct use of dentifrice can occur as well15. In order to minimize its occurrence, the participants were asked to bring the dentifrice tubes at the end of the trial to be weighed, so we could evaluate indirectly subject compliance. The significant difference between weights in both groups, before and after the study, indicates that the participants used the products, but does not confirm if they were used correctly. Compliance was also reinforced by reduction on gingivitis in both groups.
The experimental period of 30 days was chosen for permitting comparison to other studies. However, it may not be sufficient to show the superiority of the test dentifrice over the control toothpaste15. Further long-term studies must be performed to evaluate the antigingivitis effect of this herbal dentifrice. If its real benefit is confirmed, the use of Aloe vera should be advantageous in cases where patients have little motor skills and toothbrushing is compromised.
CONCLUSION
Within the limits of this clinical study, it may be concluded that the dentifrice containing Aloe vera did not show any additional effect on plaque and gingivitis control compared to the fluoridated dentifrice.
Received: October 5, 2007
Accepted: March 27, 2008
- 1. Ainamo J, Bay I. Problems and proposals for recording gingivitis and plaque. Int Dent J. 1975;25(4):229-35.
- 2. Axelsson P, Lindhe J, Nystrom B. On the prevention of caries and periodontal disease: results of a 15-year longitudinal study in adults. J Clin Periodontol. 1991;18(3):182-9.
- 3. Blieden TM, Caton JG, Proskin HM, Stein SH, Wagener CJ. Examiner reliability for an invasive gingival bleeding index. J Clin Periodontol. 1992;19(4):262-7.
- 4. Botelho MA, Nogueira NAP, Bastos GM, Fonseca SGC, Lemos TLG, Matos FJA, et al. Antimicrobial activity of the essential oil from Lippia Sidoides, carvacrol and thymol against oral pathogens. Braz J Med Biol Res. 2007;40(3):349-56.
- 5. Cardoso L, Rösing CK, Kramer PF. Doença periodontal em crianças: levantamento epidemiológico através dos índices de placa visível e de sangramento gengival. JBP J Bras Odontopediatr Odontol Bebe. 2000;3(11):55-61.
- 6. Galan J Jr. Materiais dentários: o essencial para o estudante e o clínico geral. São Paulo: Editora Santos; 1999.
- 7. Lee SS, Zhang W, Li Y. The antimicrobial potential of 14 natural herbal dentifrices: results of an in vitro diffusion method study. J Am Dent Assoc. 2004;135(5):1133-41.
- 8. Löe H, Theilade E, Jensen SB. Experimental gingivitis in man. J Periodontol. 1965;36(3):177-87.
- 9. Matos FJA, Sousa MP, Craveiro AA, Matos MEO. Constituintes químicos ativos e propriedades biológicas de plantas medicinais brasileiras. 2. ed. Fortaleza: Editora UFC; 2004.
- 10. Moran J, Addy M, Newcombe RG, Marlow I. A study to asses the plaque inhibitory action of a newly formulated triclosan toothpaste. J Clin Periodontol. 2001;28(1):86-9.
- 11. Mullaly BH, James JA, Coulter WA, Linden GJ. The efficacy of a herbal based toothpaste on the control of plaque and gingivitis. J Clin Periodontol. 1995;22(9):686-9.
- 12. Nogueira-Filho GR, Toledo S, Cury JA. Effect of 3 dentifrices containing triclosan and various additives: an experimental gingivitis study. J Clin Periodontol. 2000;27(7):494-8.
- 13. Owens J, Addy M, Faulkner J. An 18-week home-use study comparing the oral hygiene and gingival benefits of triclosan and fluoride toothpastes. J Clin Periodontol. 1997;24(9):626-31.
- 14. Palomo F, Wantland L, Sanchez A, Volpe AR, McCool J, DeVizio W. The effect of three commercially available dentifrices containing triclosan on supragingival plaque formation and gingivitis: a six month clinical study. Int Dent J. 1994;44(Suppl. 1):75-81.
- 15. Pannuti CM, Mattos JP, Ranoya PN, Jesus AM, Lotufo RFM, Romito GA. Clinical effect of a herbal dentifrice on the control of plaque and gingivitis: a double-blind study. Pesqui Odontol Bras. 2003;17(4):314-8.
- 16. Salgado ADY, Maia JL, Pereira SLS, Lemos TLG, Mota OML. Antiplaque and antigingivitis effects of a gel containing Punica Granatum Linn extract: a double-blind clinical study in humans. J Appl Oral Sci. 2006;14(3):162-6.
- 17. Sastravaha G, Gassmann G. Sangtherapitikul P, Grimm WD. Adjunctive periodontal treatment with Centella asiatica and Punica granatum extracts in supportive periodontal therapy. J Int Acad Periodontol. 2005;7(3):70-9.
- 18. Soukoulis S, Hirsch R. The effects of a tea tree oil-containing gel on plaque and chronic gingivitis. Aust Dent J. 2004;49:78-83.
- 19. Turesky S, Gilmore ND, Glickman I. Reduced plaque formation by the chloromethyl analogue of Victamin C. J Periodontol. 1970;41(1):41-3.
- 20. Villalobos OJ, Salazar CR, Sánchez GR. Efecto de un enjuague bucal compuesto de aloe vera en la placa bacteriana e inflamación gingival. Acta Odontol Venez. 2001;39(2):16-24.
- 21. Wu CD, Savitt ED. Evaluation of the safety and efficacy of over-the-counter oral hygiene products for the reduction and control of plaque and gingivitis. Periodontol 2000. 2002;28(1):91-105.
- 22. Yates R, Jenkins S, Newcombe RG, Wade WG, Moran J, Addy M. A 6-month usage trial of a 1% chlorhexidine toothpaste (1): effects on plaque, gingivitis, calculus and toothstaining. J Clin Periodontol. 1993;20(2):130-8.
Publication Dates
-
Publication in this collection
14 July 2008 -
Date of issue
Aug 2008
History
-
Received
05 Oct 2007 -
Accepted
27 Mar 2008